Abstract
Background: The Anaplastic Lymphoma Kinase (ALK) gene is known to be affected by several genetic alterations, such as rearrangement, amplification and point mutation. The main goal of this study was to comprehensively analyze ALK amplification (ALK-A) and ALK gene copy number gain (ALK-CNG) in a large cohort of non-small-cell lung cancer (NSCLC) patients in order to evaluate the effects on mRNA and protein expression. Methods: ALK locus number status was evaluated in 578 NSCLC cases by fluorescence in situ hybridization (FISH). In addition, ALK immunohistochemistry and ALK mRNA in situ hybridization were performed. Results: Out of 578 cases, 17 cases showed ALK-A. In addition, 14 cases presented ALK-CNG and 72 cases presented chromosome 2 polyploidy. None of those carrying ALK-A and -CNG showed either ALK immunohistochemical expression or ALK mRNA expression through in situ hybridization. We observed a high frequency of extra copies of the ALK gene. Conclusions: Our findings demonstrated that ALK-A is not involved in mRNA production and consequently is not involved in protein production; these findings support the hypothesis that ALK-A might not play a role in the pathogenesis of NSCLC, underlining the absence of a specific clinical application.
Keywords: ALK, amplification, copy number gain, non-small-cell lung cancer
1. Introduction
Anaplastic lymphoma kinase (ALK) is a transmembrane tyrosine kinase receptor. During embryogenesis, ALK is expressed in the nervous system, but its expression decreases after birth [1]. In anaplastic large cell lymphoma (ALCL), ALK was originally characterized as a nucleophosmin fusion partner [2].
ALK rearrangement (ALK-R) has been also reported in 3–7% of all non-small-cell lung cancers (NSCLCs). ALK fusion proteins activate many different overlapping pathways, such as the Ras/Raf/MEK/ERK1/2 pathway, the Janus-activated kinase (JAK)/signal transducer and activator of transcription (STAT) pathway, the phosphatidylinositol 3-kinase (PI3K)/Akt (PKB) pathway and the phospholipase C (PLC)-γ pathway [3,4].
NSCLC patients harboring ALK-R present a unique subgroup of clinical and pathological features that are responsive to treatment with specific inhibitors [5].
The ALK gene could be a fusion partner of several genes in chromosomal rearrangements, but it could also be involved in other genetic alterations such as mutations and amplification; in specific clinical settings, it is responsible for gene deregulation and thus constitutive activation of the receptor [6].
Among these alterations, ALK gene amplification (ALK-A) has been identified in various cancers such as ALCL, rhabdomyosarcoma, carcinoma of the esophagus, adult renal cell carcinoma and hepatocellular carcinoma [7].
ALK-A is a frequent additional genetic mechanism in the tumorigenesis of neuroblastoma [8]. In addition, evidence from in vitro studies has shown that cell lines with ALK-A are sensitive to specific inhibitors, suggesting a potential therapeutic application in ALK-A patients [9].
ALK-A was also found to be a common aberration in inflammatory breast cancer (IBC) cell lines and tissue samples [10]. In vivo studies have reported sensitivity of IBC xenograft models with ALK-A to crizotinib; on the basis of these preclinical data, clinical trials for ALK-A IBC patients are currently in progress [11].
Previous results have shown that ALK-A is also a common occurrence in esophageal cancer, with comparable rates in both squamous cell carcinoma and adenocarcinoma. However, ALK immunohistochemical expression was not observed in such series [12].
In NSCLC, the data reported for ALK-A are scant, although ALK deregulation through gene rearrangement represents the basis for targeted therapy. In a large series of NSCLC patients, a high frequency of ALK-A has been reported, showing a significant correlation with epidermal growth factor receptor (EGFR) gene amplification [13].
Furthermore, ALK-A has also been described in lung sarcomatoid carcinomas as an early, not random, clonal event closely associated with polysomy of chromosomes 7 and 17 [14]. On the basis of this biological background, ALK-A could represent a clinical frontier to extend the spectrum of NSCLC patients sensitive to specific ALK inhibitors.
The main aim of this study was to comprehensively analyze ALK-A and ALK gene copy number gain (ALK-CNG) in a large cohort of NSCLC patients to identify a possible association with RNA expression and protein overexpression.
2. Results
2.1. Clinical and Pathological Characteristics of Patients
Overall, 578 NSCLC patients were analyzed. Among these, 398 had adenocarcinomas (ADCs), 139 had squamous cell carcinomas (SQs), 5 had adenosquamous lung carcinomas (AdSqLCs) and 36 had large-cell carcinomas. The clinical and pathological features of these patients are summarized in Table 1.
Table 1.
Clinical and pathological features of patients.
| Characteristics | No. of Cases (%) |
|---|---|
| All cases | 578 |
| Age | |
| ≥65 years | 317 (54.8%) |
| <65 years | 261 (45.2%) |
| Gender | |
| Male | 397 (68.7%) |
| Female | 181 (31.3%) |
| Smoking Habit | |
| Yes | 330 (57.1%) |
| No | 83 (14.4%) |
| ex-smokers | 32 (5.5%) |
| NA | 133 (23.0%) |
| Histotype | |
| ADCs | 398 (68.9%) |
| SQs | 139 (24.0%) |
| AdSqLCs | 5 (0.9%) |
| Other | 36 (6.2%) |
| Disease Stage | |
| IA | 132 (22.8%) |
| IB | 120 (20.8%) |
| IIA | 188 (32.6%) |
| IIB | 23 (4.0%) |
| III | 2 (0.3%) |
| IV | 81 (14.0%) |
| NA | 32 (5.5%) |
| Grade | |
| I | 37 (6.4%) |
| II | 262 (45.3%) |
| III | 210 (36.3%) |
| IV | 2 (0.3%) |
| NA | 67 (11.6%) |
NA: not available; ADCs: adenocarcinomas; SQs: squamous cell carcinomas; AdSqLCs: adenosquamous lung carcinomas.
As many as 330 of the 578 patients were current smokers, while 83 had never smoked and 32 were ex-smokers; no information on smoking habits was available for 133 patients (23.0%). Postoperative pathological stages of lung carcinomas were IA in 132 cases (22.8%), IB in 120 (20.8%), IIA in 188 (32.6%), IIB in 23 (4.0%), III in 2 (0.3%) and IV in 81 (14.0%); the pathological stage was not available for 32 cases (5.5%).
Of the 578 NSCLCs, 37 were grade I (6.4%), 262 were grade II (45.3%), 210 were grade III (36.3%) and 2 were grade IV (0.3%); grades were not available for 67 cases (11.6%).
2.2. Fluorescence In Situ Hybridization
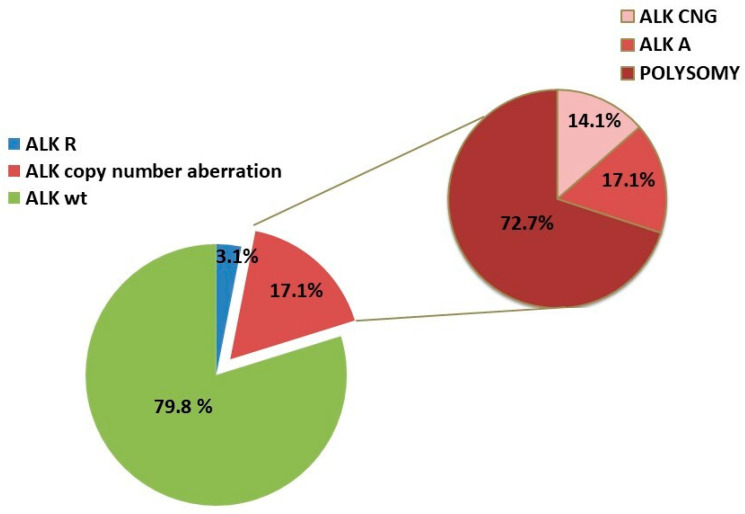
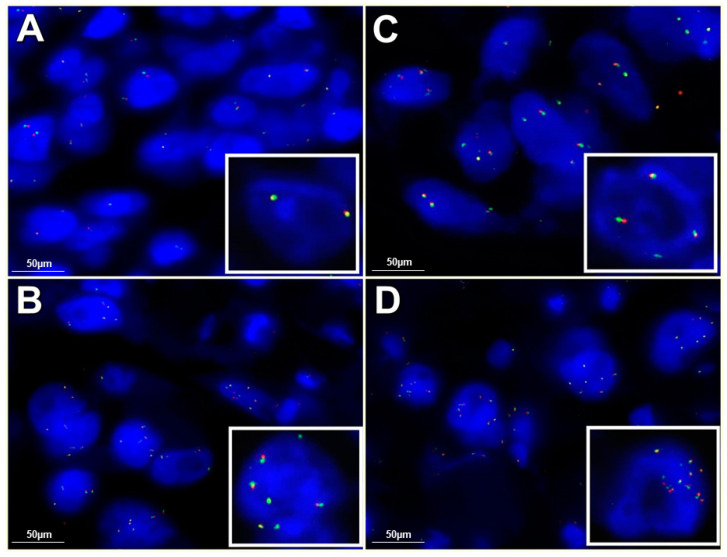
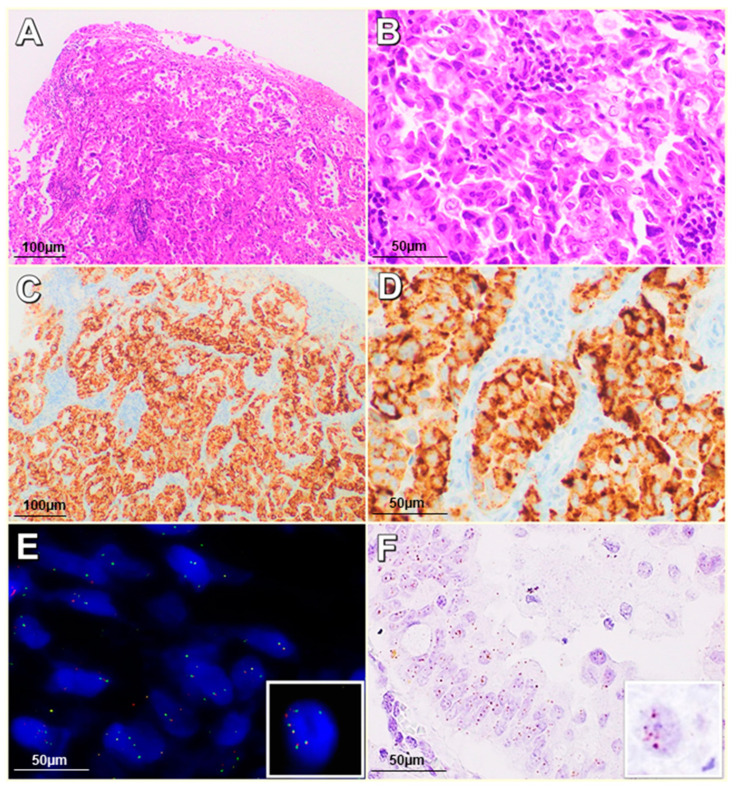
ALK-R was harbored by 18 patients (3.1%) (Figure 1). ALK-A was shown in 17 of the 578 patients; in particular, 12 patients showed 6 signals and 5 patients showed a number of signals ranging from 8 to 12 per nucleus (Figure 2). Only 1 patient showed concomitant ALK-R and ALK-A (Figure 3).
Figure 1.
Anaplastic Lymphoma Kinase (ALK) and centromeric probe 2 (CEP2) fluorescence in situ hybridization (FISH) results in our series.
Figure 2.
FISH ALK results: (A) FISH ALK wild-type (100× magnification); (B) FISH ALK gene copy number gain (ALK-CNG) (100× magnification); (C) FISH ALK amplification (ALK-A) with ≥6 signals/cell (100× magnification); (D) FISH ALK-A with 8–12 signals/cell (100× magnification).
Figure 3.
Case harboring ALK rearrangement (ALK-R) and ALK-A: (A,B) Hematoxylin and Eosin (H&E) staining (20–40× magnification); (C,D) ALK immunohistochemistry (IHC) positive (20–40× magnification); (E) ALK FISH with ALK-R and ALK-A (63× magnification); (F) ALK ISH mRNA expression, average 4–6 dots/cell (60× magnification).
The clinical and pathological features of all patients carrying ALK-A are detailed in Table 2. No statistical correlation between ALK amplification and patients’ clinical and pathological features was found.
Table 2.
Clinical and pathological features of patients harboring ALK amplification.
| Pz ALK-A |
Gender | Age | Histotype | Stage | Grade | Smoker Status | ALK ISH |
ALK IHC |
ALK-R |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 60 | SQ | IIA | G3 | NA | − | − | WT |
| 2 | M | 74 | ADC | IIA | G2 | Y | − | − | WT |
| 3 | M | 64 | SQ | IIA | G2 | Y | − | − | WT |
| 4 | M | 63 | ADC | IB | NA | Y | − | − | WT |
| 5 | F | 66 | ADC | IIA | G2 | NA | − | − | WT |
| 6 | M | 77 | SQ | IIA | G3 | Y | − | − | WT |
| 7 | M | 55 | SQ | IIA | G2 | EX | − | − | WT |
| 8 | F | 71 | ADC | IIA | G2 | EX | + | + | R |
| 9 | M | 59 | SQ | IIA | G2 | EX | − | − | WT |
| 10 | M | 49 | ADC | IIA | G2 | NA | − | − | WT |
| 11 | M | 63 | ADC | IA | G3 | NA | − | − | WT |
| 12 | F | 64 | ADC | IV | G3 | NA | − | − | WT |
| 13 | M | 63 | ADC | IB | G2 | Y | − | − | WT |
| 14 | M | 67 | ADC | IIA | G2 | Y | − | − | WT |
| 15 | F | 70 | SQ | IV | G3 | EX | − | − | WT |
| 16 | M | 63 | ADC | IA | G3 | Y | − | − | WT |
| 17 | M | 70 | ADC | IIA | G2 | NA | − | − | WT |
Anaplastic lymphoma kinase (ALK); NA: not available; ADCs: adenocarcinomas; SQs: squamous cell carcinomas.
Of the 578 cases (17.1%) 99 showed an increased number of ALK gene copies; however, after corrections with a centromeric probe 2 (CEP2) control probe, 72 cases (12.5%) with mean copy number for the ALK locus (ALK LSI)/CEP2 ratio of <2 were considered to have chromosome 2 polyploidy. Therefore, there were 14 real ALK-CNG cases (2.4%). No statistically significant association was found between ALK-CNG and clinical or pathological parameters. As many as 506 out of 578 cases presented a disomy of chromosome 2.
2.3. Immunohistochemistry Analysis of ALK Protein Expression
Specimens from ALK-R patients were immunostained by the anti-ALK D5F3 clone. None of the patients carrying ALK-CNG and ALK-A displayed ALK expression, regardless of the anti-ALK clone utilized (D5FE3, ALK1, 5A4). Only the case carrying concomitant ALK-R and ALK-A showed ALK expression, likely due to the rearrangement rather than the amplification (Table 3).
Table 3.
Cases harboring ALK aberrations in FISH.
| ALK FISH Status | ALK ISH+ | ALK IHC+ |
|---|---|---|
| 18 ALK-R | 18/18 | 18/18 |
| 17 ALK-A | 1/17 | 1/17 |
| 14 ALK-CNG | 0/14 | 0/14 |
| 72 ALK-CNG and Polisomy Chr2 | 0/72 | 0/72 |
ALK-R: ALK rearrangement; ALK-A: ALK amplification; ALK-CNG: ALK copy number gain, 3 to 5 fusion signals; FISH: fluorescence in situ hybridization; ISH: in situ hybridization; IHC: immunohistochemistry.
2.4. In Situ Hybridization (ISH) Assay for ALK RNA Detection
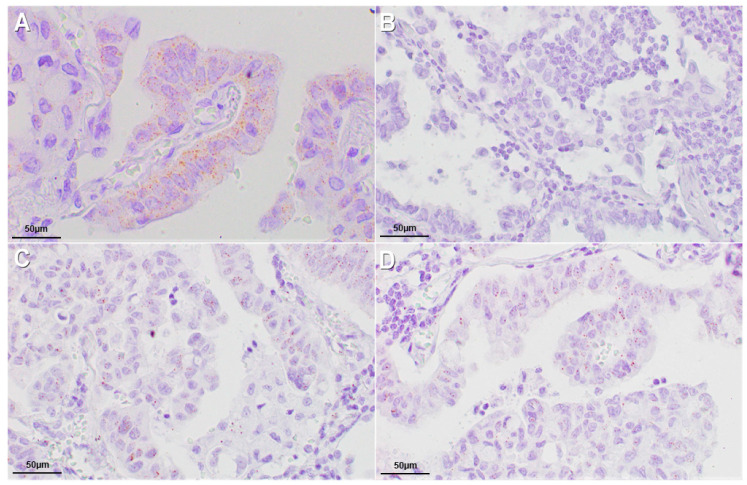
All ALK-R cases showed ALK RNA expression analyzed through in Situ Hybridization assay. None of the cases carrying ALK-CNG and ALK-A showed ALK RNA expression (Figure 4). Only the case carrying concomitant ALK-R and ALK-A showed ALK RNA expression, likely due to the rearrangement rather than the amplification (Table 3).
Figure 4.
ALK ISH results: (A) ALK mRNA expression >6 dots/cell in positive control (60× magnification); (B) ALK mRNA negative (60× magnification); (C) ALK mRNA expression, >2 dots/cell in 10% of neoplastic cells (60× magnification); (D) ALK mRNA expression, average 4–6 dots/cell (60× magnification).
3. Discussion
ALK-R in NSCLC is a target for directed therapy with specific tyrosine kinase inhibitors. Other ALK gene alterations, including mutations and amplification, have been identified in various cancer types, although they do not currently represent druggable aberrations in clinical practice. In vitro studies have shown that in NSCLC cell lines carrying ALK-CNG are sensitive to ALK inhibitors, although, to the best of our knowledge, a possible role of ALK-A has not yet been fully clarified in NSCLC patients. ALK-A and -CNG have been studied in different tumor types, not always related to ALK protein overexpression or increased downstream signaling [7,15].
In our study, the potential association between amplification and CNG was analyzed at two levels, particularly through the evaluation of both protein product and mRNA expression.
In our comprehensive evaluation, ALK-A was not associated with specific clinical and pathological features or histotype, unlike ALK-R, which is generally related to adenocarcinoma, never-smoker status and young age. Moreover, in our cohort, ALK-A occurred in NSCLC patients regardless of ALK-R, with only one case having concomitant ALK-R and ALK-A.
The copy number gain of the ALK gene in NSCLC can be due to either ALK-A or aneusomy for chromosome 2. Therefore, we stratified NSCLC patients in whom ALK-CNG was a result of chromosome 2 aneusomy against those with a true ALK-gene copy gain.
As many as 3% of the patients in our series harbored ALK-A. Overall, we observed two molecular patterns of ALK-A that distinguished cases; i.e., one group had a gene copy number that was increased by an average of 5–6 signals per nucleus (2% of cases), while another group showed an average of 8–12 signals per nucleus (1% of cases). Previous studies have reported the presence of ALK-A in NSCLC. Camidge et al. screened 1426 NSCLC clinical specimens (174 ALK rearranged and 1252 ALK not rearranged) and found an increased native ALK copy number (≥3 copies/cell in ≥40% cells) in 19% of ALK rearranged cells and 62% of ALK non-rearranged cells [16]. Another study with 170 patients reported a frequency as high as 10% [13]; a more recent study evaluating ALK gene status by fluorescence in situ hybridization (FISH) reported that only 2 out of 205 cases harbored ALK-A [17].
The variability of the ALK-A frequency in previous literature could be ascribable to both the different score systems used for assessing ALK -CNG and the concomitant evaluation of chromosome 2 polyploidy. Salido et al. considered gain to occur when a mean copy number from 3 to 5 fusion signals was observed in more than 10% of cells, and they considered amplification to occur when more than 6 copies of ALK were observed in ≥10% of the cells analyzed [13]. Camidge et al. used a unique cut-off of ≥3 copies per cell in ≥40% of cells [16]. Caliò et al. considered gene amplification as the presence of ≥8 copies of ALK per cell [17]. In our series, 72 out of 578 cases firstly showed ALK-CNG, although after corrections by the CEP2 control probe, only 14 cases confirmed a real ALK-CNG, excluding the chromosome 2 polyploidy cases. In agreement with previous results [16,17], we found that ALK-CNG occurred mostly because of chromosome 2 polyploidy (12.5% of cases) rather than as a result of locus-specific gene copy number gains (2.4% of cases). Previously published data reported that the increased copy numbers of the ALK gene could also be associated with chromosome 2 aneusomy in IBC, with a frequency of 64% [18].
These findings suggest that chromosome 2 polyploidy is a frequent event in NSCLC, making FISH CEP2 an indispensable tool to discriminate true extra ALK gene copies.
Chromosomal instability is a well-known phenomenon in NSCLC biology and is associated with tumor progression [19]. Previous molecular cytogenetic analysis of NSCLC tumors and cell lines by spectral karyotyping and comparative genomic hybridization (CGH) reported that chromosome 2p is frequently involved in gains [19,20,21]. Chromosome 2p gains have been identified through CGH assays in both squamous cell carcinoma (27.5%) and adenocarcinoma (20%) [21]. Moreover, amplifications at chromosome 2p23-p24—the locus where the ALK gene maps—have been described in NSCLC tumors and cell lines [20]. Our findings support previous data reported by Caliò et al. [17] confirming that the increase of the ALK-CNG is more frequently due to the polyploidy of chromosome 2.
For ALK IHC expression, all cases harboring ALK-A were negative regardless of the clones used. Our results are in accordance with previous findings observed in other series of NSCLCs and esophageal cancer reporting negative ALK IHC in ALK-A cases [12,13,14]. Despite the high specificity and sensibility of the D5F3 clone in detecting ALK-R cases, compared to other common ALK1 and 5A4 clones, the results obtained through IHC and FISH were discordant in some cases, with an unpredictable response to ALK inhibitors [22].
It is worth noting the different sensibility and specificity observed for these clones in detecting ALK-R in NSCLC and other tumors, which were higher for D5F3. According to this observation, the use of the clone D5F3 is recommended in clinical practice to select patients harboring ALK-R for targeted therapy [23]. The differences observed with the use of the different clones could be related to different rearrangements occurring in NSCLC which are responsible for the loss of specific antigenicity detected by the clones. Thus, the negativity described in our ALK-A and ALK-CNG cases could be related to the loss of all antigenicities detected by the available clones. There are four ALK antibody clones that have been evaluated for NSCLC. In our study, we also examined ALK mRNA expression in cases of NSCLCs carrying ALK-A in order to evaluate the status of gene transcription in ALK-A and ALK-CNG cases. An intriguing result was that all cases carrying ALK-A were negative not only to immunostaining for anti-ALK clones but also to mRNA expression in the ISH assay. The ability to visualize individual transcript molecules within a single cell represents a powerful tool for clarifying the possible value of ALK-A in NSCLC. Finally, the absence of ALK mRNA supports the negative ALK IHC results observed in ALK-A cases.
4. Materials and Methods
4.1. Patients and Specimens
A series of 578 NSCLC tumor tissue samples from surgical resections performed between 2006 and 2011 at the Istituto Nazionale per lo Studio e la Cura dei Tumori, IRCCS “Fondazione Pascale”, the Fondazione IRCCS “Ca’ Granda”—Ospedale Maggiore Policlinico and the AORN Vincenzo Monaldi were collected. Sections of 4 µm were obtained from at least 3 blocks per tumor for each case and stained with hematoxylin and eosin. All 578 cases were reviewed according to the new IASLC/ATS/ERS classification [24].
In this study, we analyzed various clinical and pathological parameters, including age of the patient at initial diagnosis, smoking habits, tumor stage and histologic grade and the eventual recurrence or metastasis.
After careful explanation, informed consent was obtained from all patients, which authorized the reexamination of biological samples for research purposes, as approved by the General Directors of the involved hospitals (Istituto Nazionale per lo Studio e la Cura dei Tumori, IRCCS “Fondazione Pascale” and Monaldi Hospital, number 15, approval date 15 January 2016, establishing and regulating our Biobank, the Fondazione IRCCS “Ca’ Granda”—Ospedale Maggiore Policlinico Institutional Review Board 179/2013; approval date 19 March 2013).
4.2. Tissue Microarray Construction
All 578 NSCLCs were used for building 14 tissue microarrays (TMAs) after careful re-evaluation of tumoral and nontumoral areas by two experienced pathologists. The tissue microarrays were built using three cores of 1 mm from different areas and, whenever possible, one core of normal tissue of the same block for each case, collected in a recipient paraffin block (3 × 2.5 cm) using a semi-automated tissue arrayer (Galileo TMA; Integrated Systems Engineering, Milano, Italy) [25].
4.3. Fluorescence In Situ Hybridization
FISH analysis was performed on unstained 3-μm-thick TMA slides to detect ALK status and CEP2. Two types of probes used: (i) an ALK probe-Vysis LSI ALK Dual Color, Break Apart Rearrangement Probe (Abbott Molecular, Abbott Park, Illinois, USA) that is constituted by two different fluorescent probes that flank the ALK breakpoint, where one probe is labeled with a fluorochrome in Spectrum orange and the other is labeled with a fluorochrome in Spectrum green, and (ii) a centromeric alpha-satellite specific for chromosome 2-Vysis CEP 2 (D2Z1) ((Abbott Molecular, Abbott Park, IL, USA)) that was used as a control probe to detect polysomy. After deparaffinization of sections, denaturation and hybridization of the probes were carried out following previously described protocols [25]. The experimental results were evaluated through an epifluorescence microscope (Olympus Corporation of the Americas Headquarters Corporate Parkway Center Valley, PA, USA); the images were acquired through a CCD microscopy camera.
4.4. ALK Gene Rearrangement Interpretation
FISH for ALK-R was considered positive, as previously described, when (i) one fusion signal and two separated orange and green signals, as a classic break-apart pattern, or (ii) a single red signal without a corresponding green signal in addition to fused signals, as an atypical pattern, were observed. A case was interpreted as positive when more than 15% of the cells were positive [25].
4.5. ALK Copy Number Gain/Amplification Interpretation
The mean copy number for the ALK locus (LSI) was evaluated first. The mean copy number of CEP2 was then detected by performing the FISH assay on adjacent serial TMA sections. In order to screen for polyploidy, the ratio between ALK gene mean copy number and CEP2 mean copy number was finally evaluated.
The criteria for copy number aberrations of ALK gene were the cutoffs proposed by Salido et al.: ALK-CNG with a mean copy number of 3 to 5 fusion signals in ≥10% of cells and ALK-A with the presence of ≥6 copies of ALK per cell in ≥10% of analyzed cells [13]. When clusters were observed, we interpreted cases where more than 10% of cells showed ALK clusters as cases of amplification.
Amplification of the ALK locus gene was considered to occur when the ALK LSI/CEP2 ratio was ≥2; meanwhile, a case carrying ALK-CNG that showed an ALK LSI/CEP2 ratio of <2 after corrections by control probes was interpreted as having chromosomal gains due to polyploidy [17].
4.6. Centromeric Alpha-Satellite Specific for Chromosome 2 Interpretation
In a normal interphase nucleus, two orange signals associated to the disomic status of chromosome 2 are expected; an increase in signals per nucleus indicates the polyploidy of the chromosome.
4.7. Immunohistochemistry Analysis of ALK Protein Expression
Immunohistochemical staining was performed on 4-μm-thick TMA sections. IHC assays were performed using three different anti-ALK antibodies: ALK1 Ventana, 5A4 Abcam and D5F3 Ventana. IHC using anti-ALK clones ALK1 and 5A4 was performed with ultraView (Ventana Medical Systems Inc., Tucson, AZ, USA), and the scoring was based on the cytoplasmic and/or membrane-staining intensity as follows: strong staining, 3+; moderate staining, 2+; weak staining, 1+ [26].
IHC assays using the VENTANA anti-ALK (D5F3) rabbit monoclonal primary antibody were performed according to the manufacturer’s protocol. The staining results were evaluated according to a binary scoring system (positive or negative) [23].
4.8. In Situ Hybridization (ISH) Assay for ALK RNA Detection
In situ detection of ALK RNA was performed using the RNAscope (RNAscope 2.5 HD Detection Reagent—BROWN User Manual, (Advanced Cell Diagnostics USA, Newark, CA) according to the manufacturer’s instructions. The assay was performed using two controls: peptidylprolyl isomerase B (cyclophilin B) (PPIB) mRNA, a positive control and duplex negative control probe (dapB), a negative control. The TMA sections were pretreated using the target retrieval solution (slides were boiled at 95 °C for 30 min) and the protease treatment (slides were treated at 40 °C for 30 min). Then, we hybridized the ALK probe for 2 h at 40 °C. The detection kit (BROWN) was used to amplify and reveal the signal, according to the manufacturer’s instructions. The evaluation of the signals was independently carried out by two different observers.
ALK ISH was interpreted as positive considering brown punctate dot-like signals present in the nucleus and/or cytoplasm. The analysis was carried out on at least 60 tumor cells.
The expression of ALK was analyzed in every core of the TMAs. ALK had an expression level varying between 0 to >6 copies per cell. We sorted the cases into positive (>2 dots/cell in 10% cells) or negative (no staining or 1 dot/cell in 10% cells).
4.9. Statistical Analysis
In order to determine the association of clinical and pathological features with ALK protein and/or mRNA expression and ALK-CNG or amplification, a Pearson chi-square test was conducted using SPSS 20.0 for Mac (SPSS Inc., Chicago, IL, USA).
5. Conclusions
In conclusion, we found that extra copies of the ALK gene occurred most frequently because of unspecific chromosome 2. Thus, FISH analysis of chromosome 2 centromeric probes is indispensable in defining true ALK copy number gain status and amplification compared to chromosome 2 polysomy.
In neuroblastomas, high ALK expression was found to be associated with ALK amplification, as well as copy number gain of the ALK locus, supporting the hypothesis that ALK expression depends upon increasing the DNA dose; thus, we could expect a superimposable feature in NSCLC. However, in NSCLC cases harboring ALK-A, ALK immunostaining and ALK mRNA ISH are negative. This supports the hypothesis that ALK-A is not a productive mutational event in the biological setting of NSCLC because such genetic occurrence is related to the absence of gene transcription rather than to an undetectable protein expression through the common clones used in immunohistochemical routine. Thus, as ALK-A has no critical impact on clinical behavior and could not be considered a possible target for specific biological therapy, our results should be confirmed in prospective clinical studies.
Abbreviations
| ALK | anaplastic lymphoma kinase |
| NSCLC | non-small cell lung cancer |
| ALK-CNG | ALK gene copy number gain |
| ALK-R | ALK gene rearrangement |
| ALK-A | ALK gene amplification |
| FISH | fluorescence in situ hybridization |
| ALCL | anaplastic large-cell lymphoma |
| JAK/STAT | Janus-activated kinase/signal transducer and activator of transcription |
| EGFR | epidermal growth factor receptor |
| ADC | adenocarcinoma |
| SQ | squamous carcinoma |
| AdSqLC | adenosquamous lung carcinomas |
| TMA | tissue microarrays |
| CEP2 | centromeric probe 2 |
Author Contributions
Conceptualization, F.Z.M. and R.F.; methodology, R.F., F.Z.M., G.B. and G.A.; validation, R.D.C., S.F. and G.G.; formal analysis, P.M. and M.C.M.; investigation, D.R.; data curation, A.P. and R.S.; writing—original draft preparation R.F.; writing—review and editing, G.R. and A.G.; visualization, A.M.; supervision, A.M.; project administration, D.R.; funding acquisition, G.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Italian Ministry of Health (Ricerca Corrente).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Motegi A., Fujimoto J., Kotani M., Sakuraba H., Yamamoto T. ALK receptor tyrosine kinase promotes cell growth and neurite outgrowth. J. Cell Sci. 2004;117:3319–3329. doi: 10.1242/jcs.01183. [DOI] [PubMed] [Google Scholar]
- 2.Morris S.W., Kirstein M.N., Valentine M.B., Dittmer K.G., Shapiro D.N., Saltman D.L., Look A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science. 1994;263:1281–1284. doi: 10.1126/science.8122112. [DOI] [PubMed] [Google Scholar]
- 3.Chiarle R., Voena C., Ambrogio C., Piva R., Inghirami G. The anaplastic lymphoma kinase in the pathogenesis of cancer. Nat. Rev. Cancer. 2008;8:11–23. doi: 10.1038/nrc2291. [DOI] [PubMed] [Google Scholar]
- 4.Roskoski R., Jr. Anaplastic lymphoma kinase (ALK): Structure, oncogenic activation, and pharmacological inhibition. Pharmacol. Res. 2013;68:68–94. doi: 10.1016/j.phrs.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 5.Franco R., Rocco G., Marino F.Z., Pirozzi G., Normanno N., Morabito A., Sperlongano P., Stiuso P., Luce A., Botti G., et al. Anaplastic lymphoma kinase: A glimmer of hope in lung cancer treatment? Expert Rev. Anticancer Ther. 2013;13:407–420. doi: 10.1586/era.13.18. [DOI] [PubMed] [Google Scholar]
- 6.Palmer R.H., Vernersson E., Grabbe C., Hallberg B. Anaplastic lymphoma kinase: Signalling in development and disease. Biochem. J. 2009;420:345–361. doi: 10.1042/BJ20090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marino F.Z., Rocco G., Morabito A., Mignogna C., Intartaglia M., Liguori G., Botti G., Franco R. A new look at the ALK gene in cancer: Copy number gain and amplification. Expert Rev. Anticancer Ther. 2016;16:493–502. doi: 10.1586/14737140.2016.1162098. [DOI] [PubMed] [Google Scholar]
- 8.Subramaniam M.M., Piqueras M., Navarro S., Noguera R. Aberrant copy numbers of ALK gene is a frequent genetic alteration in neuroblastomas. Hum. Pathol. 2009;40:1638–1642. doi: 10.1016/j.humpath.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 9.McDermott U., Iafrate A.J., Gray N.S., Dowell L., Ulkus L.E., Kuhlmann G., Greninger P., Christensen J.G., Haber D.A., Settleman J. Alterations of Anaplastic Lymphoma Kinase May Sensitize Tumors to Anaplastic Lymphoma Kinase Inhibitors. Cancer Res. 2008;68:3389–3395. doi: 10.1158/0008-5472.CAN-07-6186. [DOI] [PubMed] [Google Scholar]
- 10.Robertson F.M., Petricoin E.F., III, Van Laere S.J., Bertucci F., Chu K., Fernandez S.V., Mu Z., Alpaugh K., Pei J., Circo R., et al. Presence of anaplastic lymphoma kinase in inflammatory breast cancer. Springerplus. 2013;2:497. doi: 10.1186/2193-1801-2-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuma R.S. ALK gene amplified in most inflammatory breast cancers. J. Natl. Cancer Inst. 2012;104:87–88. doi: 10.1093/jnci/djr553. [DOI] [PubMed] [Google Scholar]
- 12.Schoppmann S.F., Streubel B., Birner P. Amplification but not translocation of anaplastic lymphoma kinase is a frequent event in oesophageal cancer. Eur. J. Cancer. 2013;49:1876–1881. doi: 10.1016/j.ejca.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Salido M., Pijuan L., Martínez-Avilés L., Galván A.B., Cañadas I., Rovira A., Zanui M., Martínez A., Longarón R., Sole F., et al. Increased ALK gene copy number and amplification are frequent in non-small cell lung cancer. J. Thorac. Oncol. 2011;6:21–27. doi: 10.1097/JTO.0b013e3181fb7cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelosi G., Gasparini P., Cavazza A., Rossi G., Graziano P., Barbareschi M., Perrone F., Barberis M., Takagi M., Kunimura T., et al. Multiparametric molecular characterization of pulmonary sarcomatoid carcinoma reveals a nonrandom amplification of anaplastic lymphoma kinase (ALK) gene. Lung Cancer. 2012;77:507–514. doi: 10.1016/j.lungcan.2012.05.093. [DOI] [PubMed] [Google Scholar]
- 15.Miyake I., Hakomori Y., Shinohara A., Gamou T., Saito M., Iwamatsu A., Sakai R. Activation of anaplastic lymphoma kinase is responsible for hyperphosphorylation of ShcC in neuroblastoma cell lines. Oncogene. 2002;21:5823–5834. doi: 10.1038/sj.onc.1205735. [DOI] [PubMed] [Google Scholar]
- 16.Camidge D.R., Skokan M., Kiatsimkul P., Helfrich B., Lu X., Barón A.E., Schulte N., Maxson D., Aisner D.L., Franklin W.A., et al. Native and rearranged ALK copy number and rearranged cell count in NSCLC: Implications for ALK inhibitor therapy. Cancer. 2013;119:3968–3975. doi: 10.1002/cncr.28311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caliò A., Bria E., Pilotto S., Gilioli E., Nottegar A., Eccher A., Cima L., Santo A., Pedron S., Turri G., et al. ALK gene copy number in lung cancer: Unspecific polyploidy versus specific amplification visible as double minutes. Cancer Biomark. 2017;18:215–220. doi: 10.3233/CBM-161680. [DOI] [PubMed] [Google Scholar]
- 18.Krishnamurthy S., Woodward W., Yang W., Reuben J.M., Tepperberg J., Ogura D., Niwa S., Huo L., Gong Y., El-Zein R., et al. Status of the anaplastic lymphoma kinase (ALK) gene in inflammatory breast carcinoma. Springerplus. 2013;2:409. doi: 10.1186/2193-1801-2-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Testa J.R., Siegfried J.M. Chromosome abnormalities in human non-small cell lung cancer. Cancer Res. 1992;52:2702s–2706s. [PubMed] [Google Scholar]
- 20.Luk C., Tsao M.S., Bayani J., Shepherd F., Squire J.A. Molecular cytogenetic analysis of non-small cell lung carcinoma by spectral karyotyping and comparative genomic hybridization. Cancer Genet. Cytogenet. 2001;125:87–99. doi: 10.1016/S0165-4608(00)00363-0. [DOI] [PubMed] [Google Scholar]
- 21.Shen H., Gao W., Wu Y.J., Qiu H.R., Shu Y.Q. Multicolor fluorescence in situ hybridization and comparative genomic hybridization reveal molecular events in lung adenocarcinomas and squamous cell lung carcinomas. Biomed. Pharmacother. 2009;63:396–403. doi: 10.1016/j.biopha.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Cabillic F., Hofman P., Ilie M., Peled N., Hochmair M., Dietel M., Von Laffert M., Gosney J.R., Lopez-Rios F., Erb G., et al. ALK IHC and FISH discordant results in patients with NSCLC and treatment response: For discussion of the question to treat or not to treat? ESMO Open. 2018;3:e000419. doi: 10.1136/esmoopen-2018-000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uruga H., Mino-Kenudson M. ALK (D5F3) CDx: An immunohistochemistry assay to identify ALK-positive NSCLC patients. Pharmgenom. Pers. Med. 2018;11:147–155. doi: 10.2147/PGPM.S156672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Travis W.D. Pathology of lung cancer. Clin. Chest Med. 2011;32:669–692. doi: 10.1016/j.ccm.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Marino F.Z., Liguori G., Aquino G., La Mantia E., Bosari S., Ferrero S., Rosso L., Gaudioso G., De Rosa N., Scrima M., et al. Intratumor Heterogeneity of ALK-Rearrangements and Homogeneity of EGFR-Mutations in Mixed Lung Adenocarcinoma. PLoS ONE. 2015;10:e0141521. doi: 10.1371/journal.pone.0139264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsao M.S., Hirsh F.R., Yatabe Y. IASLC Atlas of ALK and ROS1 Testing in Lung Cancer. 2nd ed. Fort Meyers Editorial Rx Press; Florida, FL, USA: 2016. [Google Scholar]