Abstract
As brain and bone disorders represent major health issues worldwide, substantial clinical investigations demonstrated a bidirectional crosstalk on several levels, mechanistically linking both apparently unrelated organs. While multiple stress, mood and neurodegenerative brain disorders are associated with osteoporosis, rare genetic skeletal diseases display impaired brain development and function. Along with brain and bone pathologies, particularly trauma events highlight the strong interaction of both organs. This review summarizes clinical and experimental observations reported for the crosstalk of brain and bone, followed by a detailed overview of their molecular bases. While brain-derived molecules affecting bone include central regulators, transmitters of the sympathetic, parasympathetic and sensory nervous system, bone-derived mediators altering brain function are released from bone cells and the bone marrow. Although the main pathways of the brain-bone crosstalk remain ‘efferent’, signaling from brain to bone, this review emphasizes the emergence of bone as a crucial ‘afferent’ regulator of cerebral development, function and pathophysiology. Therefore, unraveling the physiological and pathological bases of brain-bone interactions revealed promising pharmacologic targets and novel treatment strategies promoting concurrent brain and bone recovery.
Keywords: brain, bone, interaction, clinical and experimental studies, molecular signaling
1. Introduction
While the brain is regarded as the principal coordinator of body homeostasis by regulating organ activity and their crosstalk, bone features hematopoietic, endocrine metabolic and storage functions along with its predominant mechanical role. Although brain and bone seem apparently unrelated, exceptional clinical and experimental evidence propose a bilateral dependence of both organs [1,2]. The effect of brain on bone homeostasis and regeneration, transmitted via the ‘efferent’ nervous system, is well established [3,4,5] whereas the understanding of the ‘afferent’ effect of bone on brain function and development is still evolving [6,7,8]. Therefore, multiple stress, mood and neurodegenerative brain pathologies were previously correlated with bone loss, while only a limited number of genetic skeletal diseases was associated with the modulation of brain development and function. Trauma in particular was discovered to effect brain and bone concurrently.
In this review, we first recapitulate clinical observations and confirming experimental studies, demonstrating brain-bone interconnection. Thereafter, we provide a detailed overview of the molecular bases regarding these bilateral interactions. Based on this mechanistic understanding, we review promising therapeutic targets for disorders affecting brain and bone.
2. Clinical Observations
In the clinical setting, the ‘efferent’ effect of brain on bone remodeling is eventually reflected in bone gain or loss, the latter being the most common. Loss of bone density, strength and microarchitecture leads to the degenerative skeletal disorder osteoporosis, which occurs when the physiological homeostasis is disturbed and bone resorption of osteoclasts exceeds bone formation by osteoblasts. Osteoporosis represents the most common cause for fractures in the aging population, posing a major clinical issue and a significant socioeconomic burden [9]. Bone mineral density (BMD) is assessed through dual-energy x-ray absorptiometry (DXA) in which osteoporosis is defined at T-score −2.5 or less standard deviations below the average of young and healthy adults [10]. Osteoporosis has been associated with a great variety of brain dysfunctions such as epilepsy [11], schizophrenia [12], shift work [13], post-traumatic stress disorder [14], depression [15] as well as major neurodegenerative diseases including stroke [16], Alzheimer’s [17] and Parkinson’s disease [18]. Interestingly, trauma to the central nervous system such as traumatic brain [19] and spinal cord injury positively affect bone regeneration (for a detailed review please see Reference [20]). Finally, complex regional pain syndrome following trauma or surgery was reported to affect brain and bone concurrently [21].
The ‘afferent’ effect of bone on brain function is more difficult to elucidate and far less understood. None the less, rare genetic skeletal disorders are associated with changes of brain activity, potentially caused by interconnecting molecular mechanisms. Furthermore, peripheral bone injury was discovered to negatively modulate or even exacerbate traumatic brain injury [22]. In combination with growing molecular knowledge on bone-derived mediators, these clinical observations provided a first understanding of the skeletal capacity to modulate brain development and function.
2.1. Efferent ‘Brain-Bone’
Neuropsychological dysfunction, caused by shift work, post-traumatic stress and depression as well as the major neurodegenerative pathologies, are associated with bone loss and elevated fracture risk (Table 1) [23]. Considering the impact of brain dysfunction on bone however, direct interactions have to be distinguished from secondary effects following cognitive impairment and long hospitalization which commonly lead to reduced physical activity and altered mechanical loading [24]. Malnourishment, lifestyle behavior and psychotropic medication represent additional factors causing bone loss, the latter particularly implied for epilepsy [11] and schizophrenia [12].
Table 1.
Crosstalk of brain and bone: clinical observations.
| Origin | Condition | Effect on Bone | Clinical Studies/Reviews | |
|---|---|---|---|---|
| brain | neuro-psychological causes | chronic stress and shift work |
|
[13,14,25,41,42,44,45,157,158] reviewed by [159] |
| major depressive disorder (MDD) |
|
[49,50,51,52,53,54,55,56,160] | ||
| stroke |
|
[16,62,63,64,65,66,67,68,69,161,162] | ||
| dementia/Alzheimer’s disease (AD) |
|
[8,73,74,75,76,77,78,86] reviewed by [163] |
||
| Parkinson’s disease (PD) |
|
[23,87,88] | ||
| trauma | traumatic brain injury (TBI) |
|
[19,116,121,128,129,130,131,132,133,134,139,140] [164,165] |
|
| spinal cord injury (SCI) |
|
[20,145,146,147,148,149] [164] reviewed by [166] |
||
| Effect on Brain | ||||
| bone |
chronic
disorders |
osteoporosis |
|
see each condition/disorder for reference; reviewed by [15] |
| genetic | cleidocranial dysplasia (CCD) |
|
reviewed by [90] [91,92] |
|
| hereditary multiple exostoses (HME) |
|
[101,102,103] | ||
| sclerosteosis and van Buchem disease |
|
[99,100] [167] |
||
| trauma | fracture (Fx) |
|
[23] [168,169] reviewed by [22] |
|
| Effect on Brain and Bone | ||||
| brain and bone | genetic | Coffin-Lowry syndrome (CLS) |
|
[94] |
| trauma | complex regional pain syndrome (CRPS) |
|
reviewed by [21] [104,105,106,107] |
Growing evidence provides a strong correlation between shift work and osteoporosis [13,25,26], as working nights are accompanied by numerous endocrinological changes [27] such as reduced levels of melatonin [28,29,30] and elevated levels of stress-induced cortisol [31]. These alterations were associated with an increase in body mass index [31], elevated risk for cardiovascular diseases [32], diabetes [33,34] and low BMD. As bone turnover markers were identified to mirror diurnal oscillations [35], bone remodeling is considered to follow central and peripheral circadian control [36,37]. Therefore, chronic inadequate sleep [38] and a disturbance in the expression of circadian clock genes have been observed to alter the skeletal phenotype [37,39,40].
Post-traumatic stress disorder represents another chronic psychological stress condition associated with bone loss, which poses an elevated risk for osteoporosis in civilian [14] as well as military patients [41]. Although malnutrition could not be excluded, the negative effect on bone mass was proposed to be mediated by elevated serum levels of proinflammatory cytokines such as tumor necrosis factor α (TNFα), interleukin 1 (IL1) and interleukin 6 (IL6) [42] known to stimulate bone resorption [43], as well as through hormones released in response to chronic psychological stress [44]. Stress signaling is predominantly mediated through the hypothalamic-pituitary-adrenal (HPA) axis, whereby pituitary-released adrenocorticotrophic hormone stimulates glucocorticoid synthesis in the adrenal cortex. Along with their important homeostatic, metabolic and immunologic functions, glucocorticoids were shown to directly inhibit osteoblastogenesis [45], which results in reduced bone mass and higher fracture risk [46]. Experimental evidence reported an additional negative effect of glucocorticoids on endochondral ossification in the growth plate, constraining longitudinal and appositional bone growth in adolescent mice [47]. Chronic psychosocial stress was also shown to modulate the immune response through β-adrenoreceptor signaling, resulting in impaired fracture healing [48].
Major depressive disorder (MDD) refers to a psychiatric condition also associated with low BMD [49] and higher fracture risk [50,51,52]. Apart from reduced physical activity and psychotropic medication, bone loss of patients suffering from MDD was proposed to result from inflammatory, metabolic and HPA axis dysregulations [53] with high levels of cortisol and catecholamines [54,55] as well as lower levels of steroids [56,57]. MDD is thought to impair bone and brain in a bidirectional manner, as low BMD and elevated fracture risk potentially result in pain conditions, further deteriorating depression [15]. MDD itself was additionally identified to negatively affect fracture healing through a direct inhibition of osteoblast differentiation [57].
Patients suffering from major neurodegenerative diseases including stroke, Alzheimer’s and Parkinson’s disease are commonly diagnosed with osteoporosis, resulting in high morbidity and mortality [58,59,60]. First, ischemic or hemorrhagic stroke leads to cell death and breakdown of the blood-brain barrier (BBB) [61]. Poststroke fracture is a common complication [62,63], which poses a substantial disadvantage for stroke recovery [63] as a result of immobilization, elevated bone resorption, hypercalcemia and hypovitaminosis D [64]. Interestingly, within the first few days following an acute stroke event, patients already display elevated serum concentrations of bone turnover markers including osteoprotegerin, sclerostin [65], dickkopf-related protein 1 [66] and osteopontin [67,68], suggesting direct correlation of stroke and bone loss. However, hypoxic conditions following stroke were shown to activate angiogenesis and osteogenetic precursor cells, resulting in heterotopic ossification in various parts of the body [69]. While vascular endothelial growth factor (VEGF) signaling is essential for adequate callus formation, hypoxic induction of VEGF further promotes brain edema [70,71]. Thus, experimental fracture, induced shortly before stroke, increased neuroinflammation and further exacerbated ischemic cerebral injury causing substantial secondary damage in a murine stroke-model [72].
Second, Alzheimer’s disease (AD), a chronic neurodegenerative disease, is also closely associated with osteoporosis and an increased fracture risk [73,74,75]. Patients with less brain atrophy show better bone quality [76], indicating central mechanisms of AD contributing to bone loss [7]. Further, patients suffering from AD or mild cognitive impairments display higher levels of osteopontin [77], which correlates with cognitive decline [78] and reduced BMD [79], while AD progression is linked to serum levels of the bone turnover markers osteopontin, osteocalcin and sclerostin [8]. In AD, an accumulation of extracellular amyloid-β (Aβ) plaques and intracellular tau inclusions causing cell degeneration has been observed [80]. In turn, Aβ was identified to increase osteoclast activation and bone resorption [81]. Thus, targeting of Aβ plaques may evolve as a promising therapeutic approach to prevent cognitive decline and bone loss in patients with AD [82].
Third, in line with stroke and AD, patients with Parkinson’s disease (PD) additionally display reduced BMD [18]. While the pathogenesis of PD includes oxidative stress, disturbances of iron metabolism [83] and aggregation of α-synuclein protein [84,85], the associated bone loss already occurs during early stages of disease development. As a result, the majority of PD patients are prone not only to neurological impairment and postural imbalance but also an increased fracture risk [18,86]. Along with correlating vitamin D deficiency, reduced body weight [87] and female gender [88], recent evidence propose a direct effect of PD on bone through degeneration of dopaminergic neurons, resulting in accelerated osteoclastogenesis and suppressed bone formation [89].
2.2. Afferent ‘Bone-Brain’
Although most clinical observations primarily highlight the impact of neurologic disorders on bone integrity, a limited number of genetic bone pathologies are accompanied by structural and cognitive brain impairment, pointing towards an ‘afferent’ bone-brain effect. In this regard, cleidocranial dysplasia (CCD) represents an autosomal dominant skeletal disorder caused by the haploinsufficiency of RUNX2 (also called CBFA1), which is a key transcription factor of osteoblast differentiation. CCD is characterized by skeletal anomalies including brachycephalic skull, collarbones partly or completely missing, midfacial hypoplasia and delayed tooth eruption [90]. Some patients with CCD additionally suffer from a developmental delay of the brain or late-onset progressive cognitive decline [91]. This might potentially be explained by dysfunctional osteoblasts and an insufficient secretion of the osteoblast-derived hormone osteocalcin [92], which was shown to exert neuroprotective effects [6].
Similar to CCD, Coffin-Lowry syndrome (CLS) refers to a genetic skeletal disorder associated with brain malfunction. CLS represents an X-linked disease, caused by loss-of-function mutations in the gene RPS6KA3 encoding for the growth-factor-regulated protein kinase RSK2, which phosphorylates activating transcription factor 4 (ATF4, also called CREB-2) [93]. Although clinical manifestation is highly heterogeneous, CLS patients show profound growth retardation with facial, hand and skeletal malformations as well as serious psychomotor impairments [94]. While the inactivation of RSK2 was associated with severe impaired spatial learning and long-term spatial memory deficit [95], long-term potentiation requires the RSK2 substrate ATF4 [96]. The skeletal malformations are also presumed to be caused by the lack of ATF4, which was shown to regulate osteoblast differentiation and function [94,97] as well as to stimulate osteocalcin expression [98]. Therefore, brain and bone dysfunction of patients suffering from CLS may be explained by altered ATF4 activity [1].
Other skeletal disorders, such as the SOST gene mutations sclerosteosis and van Buchem disease, are associated with raised intracranial pressure and cranial nerve entrapment [99,100], while hereditary multiple exostoses (HME) [101] correlates with symptoms of autism [102] and frontotemporal dementia [103]. Although multiple genetic diseases with concurrent skeletal and mental deficits (selection see Supplementary Table S1) show individually altered brain and bone dysfunction, further evidence of bidirectional molecular interaction is warranted.
2.3. Trauma Affecting Brain and Bone
Clinical studies involving physically injured patients revealed a strong crosslink of brain and bone. In response to general trauma or surgical injury, complex regional pain syndrome (CRPS) was observed to effect the nervous system and bone concurrently [21]. Although not directly affecting brain function, CRPS is characterized by autonomic, sensory and motoric abnormalities with clinical features of neurogenic inflammation, maladaptive neuroplasticity and nociceptive sensitization accompanied by sensory impairments, potentially leading to anxiety and depression [104,105,106]. In bone, CRPS results in loss of BMD and increased periarticular bone turnover with osteoprotegerin proposed as a potential biomarker [107].
Different to general trauma, the external physical insult to the head, causing an alteration of brain function among other brain pathologies, is termed traumatic brain injury (TBI) [108]. Depending on force severity, TBI results in temporary to permanent neurologic dysfunctions as well as a disruption of the circadian rhythm, behavioral and cognitive impairments, with generally increased mortality and morbidity [109,110,111]. During primary TBI, the meninges are commonly damaged causing cerebral hematoma, edema, ischemia and necrosis, leading to a disruption of the BBB [110,112]. Subsequent metabolic disturbance, apoptosis, oxidative stress and neuroinflammation are defined as secondary injury, potentially lasting for weeks [113] with wide-ranging systemic effects on the immune system and other organ function including bone [114]. Clinical TBI studies reported an ‘efferent’ effect on the intact bone, showing patients suffering from isolated TBI to exhibit an elevated fracture risk and reduced BMD [19,115,116]. Although immobilization represents a contributing factor, experimental studies confirmed the negative effect of TBI on bone quality and mineral density without changes in movement [117,118]. This alteration in bone metabolism is stated to be caused by the elevated BBB permeability, peripheral inflammatory response as well as endocrine and sympathetic outflow modulation of the secondary injury [114,119]. Recent evidence implies that the inflammatory stress on bone and its marrow following TBI activates nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells (NF-κB), which in turn induces osteoclastic differentiation resulting in elevated bone resorption [120]. Along with the negative impact on bone metabolism, patients suffering from TBI were furthermore identified to frequently sustain heterotopic ossification (HO) [121,122]. Such musculoskeletal ectopic deposition of lamellar bone in non-osseous tissue is commonly acquired after neurological, soft or bone tissue trauma [119], especially following combined TBI and fracture or high severity injury [123]. Although it has been agreed that neurological HO, initiated by simultaneous central and peripheral nervous injury, represents endochondral ossification, the underlying molecular and cellular mechanisms still remain to be elucidated [122,124,125]. Recently established TBI models sustaining HO potentially provide the foundation for investigations of the pathogenesis while unraveling promising therapeutic targets [126,127].
In contrast to isolated brain injury, patients suffering from TBI with concomitant fracture however were identified to exhibit accelerated bone healing and enhanced callus formation [128,129,130,131,132,133,134]. Similar to the physiologic bone remodeling, bone healing represents a complex process, consisting of the pro- and anti-inflammatory phase, followed by the soft callus, hard callus and the remodeling phases [135,136]. Although continues attempts were made to unravel the underlying molecular bases of this positive ‘efferent’ TBI effect on bone regeneration [137], the exact mechanisms still remain to be elucidated. Clinical studies monitored the systemic regulation of trauma patients suffering from fracture and TBI, to identify potential osteogenic humoral factors [138]. Therefore, proliferation was significantly increased when treating osteoblasts with serum [139] or cerebrospinal fluid of TBI patients [140], pointing towards centrally released osteogenic factors entering the circulation following TBI [138]. For further investigation, experimental studies reproduced the phenomenon [141], reporting an increased bone volume, elevated mineral density and higher rates of gap bridging in mice with TBI and concomitant fracture [142]. As today, different theories have been postulated, considering a complex modulation of the inflammatory response, participating hormones, neuropeptides and neurotransmitters [114,119,137,143]. Most recent evidence proposed a non-humoral pathway with dominance of neuronal mechanisms and neuroinflammation [144]. Similar to TBI, isolated spinal cord injury (SCI) was associated with reduced BMD and osteoporosis [145], predominantly observed within the trabecular metaphysical-epiphyseal areas of the distal femur and proximal tibia [146,147,148,149]. Further, patients suffering from SCI also frequently show HO as well as accelerated and enhanced callus formation [20], which was reproduced in vitro utilizing serum of SCI patients [150,151].
Despite the positive effect of trauma on bone regeneration, the ‘afferent’ bone-brain interaction of peripheral injury such as fracture was discovered to negatively modulate and potentially further deteriorate TBI [22]. While a neurological impact of fracture healing is considered to be constrained by the BBB, trauma patients suffering from TBI and concomitant skeletal injury show higher functional deficits and mortality rates [151]. Clinical multitrauma studies identified systemic inflammatory changes [152,153,154] with the capacity to modulate the neuroinflammatory response following TBI [22]. Such alterations were strongly supported by experimental evidence, reporting concomitant fracture to exacerbate TBI, neuroinflammation [155] and further deteriorate cerebral edema, motor deficits and neurological recovery [156].
3. Molecular Bases of Brain-Bone Crosstalk
Clinical observations continuously unravel pathophysiological processes and interactions, which reveal potential therapeutic benefit and therefore commonly represent the bases for specific mechanistic research. As a result, the combination of clinical and experimental studies gave rise to a continuously improving understanding of the brain-bone crosstalk. Several mutations in the genes encoding the below referenced mediators were discovered in humans to affect development and metabolism of bone and brain, respectively, to various degrees (Supplementary Table S2). In this section, we provide an insight to molecules which directly mediate the signal transmission of these two organs.
3.1. Brain- and Nerve-Derived Mediators Affecting Bone Cell Function
3.1.1. Central Regulation
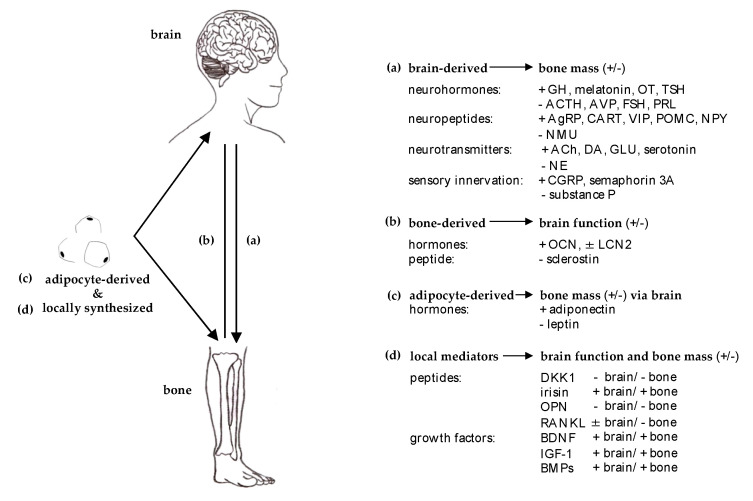
The central nervous system (CNS) is classically known for its major role in coordinating the activity of all parts of the body through neuroendocrine signaling which is primarily funneled by the hypothalamus. Most of these regulatory mediators are either expressed by the hypothalamic nuclei and transported to the posterior pituitary gland or secreted to stimulate hormone release in the anterior pituitary gland. Notably, those hormones, including follicle-stimulating hormone, thyroid-stimulating hormone, prolactin, adrenocorticotrophic hormone, growth hormone, arginine vasopressin, oxytocin and pineal gland-derived melatonin were all discovered to represent potent regulators of skeletal integrity (Figure 1, Table 2) [4,170].
Figure 1.
Molecular bases of brain and bone crosstalk. Summary of the predominant mediator effect of (a) brain-derived on bone, (b) bone-derived on brain, (c) adipocyte-derived on bone via central modulation and (d) locally synthesized mediators affecting brain and bone concurrently. The mediator effect on brain function and bone mass previously reported was summarized as positive (+) and negative (−). Abbreviations: ACh = acetylcholine, ACTH = adrenocorticotrophic hormone, AgRP = agouti-related peptide, AVP = arginine-vasopressin, BDNF = brain-derived neurotrophic factor, BMPs = bone morphogenic proteins, CART = cocaine amphetamine regulated transcript, CGRP = calcitonin gene related peptide, DA = dopamine, FSH = follicular stimulating hormone, DKK1 = dickkopf-related protein 1, GH = growth hormone, GLU = glutamate, IGF-1 = insulin-like growth factor 1, LCN2 = lipocalin 2, NE = norepinephrine, NMU = neuromedin U, NPY = neuropeptide Y, OCN = osteocalcin, OPN = osteopontin, OT = oxytocin, PRL = prolactin, POMC = proopiomelanocortin, RANKL = receptor activator of nuclear factor-κB ligand, TSH = thyroid-stimulating hormone, VIP = vasoactive intestinal peptide.
Table 2.
Crosstalk of brain and bone: experimental observations.
| Origin | Condition | Effect on Bone | Model | Pre-Clinical Studies/Reviews | |
|---|---|---|---|---|---|
| brain | neuro-psychological causes | chronic stress and shift work |
|
in vitro mouse mouse mouse/rat mouse |
[43] [46,47] [48] [37,289,408] [409] |
| depression |
|
rat | [57] | ||
| stroke |
|
mouse | [71] | ||
| dementia/Alzheimer’s disease (AD) |
|
in vitro in vitro |
[81,410] [411,412] reviewed by [163] |
||
| Parkinson’s disease (PD) |
|
mouse | [89] | ||
| trauma | traumatic brain injury (TBI) |
|
mouse/rat rat mouse mouse mouse mouse rat mouse rabbit rat rat rat rat rat |
[117,118] [126] [141,142] [144] [413] [414] [415] [416] [417] [418] [419] [420] [421] [422] |
|
| spinal cord injury (SCI) |
|
in vitro | [423] | ||
| Effect on Brain | |||||
| bone |
chronic
disorders |
osteoporosis |
|
mouse | [424] |
| genetic | cleidocranial dysplasia (CCD) |
|
mouse | [425] | |
| hereditary multiple exostoses (HME) |
|
mouse mouse |
[426] [427] |
||
| trauma | fracture (Fx) |
|
mouse | [155,156,428] | |
| Effect on Brain and Bone | |||||
| brain and bone | genetic | Coffin-Lowry syndrome (CLS) |
|
mouse mouse |
[95] reviewed by [94] [97] |
Growing evidence supports an estrogen-independent, direct effect of follicular stimulating hormone (FSH) on extragonadal tissue, particularly bone and fat [171,172,173,174]. Although this matter remains intensively discussed [175,176,177,178,179], osteoclasts and their precursor cells have been shown to express FSH receptors, allowing FSH to directly stimulate osteoclast formation, function and survival [173,174]. Bone resorption is additionally promoted indirectly following the upregulation of pro-resorptive cytokines in proportion to FSH receptor expression [179]. Especially during late perimenopause, when estrogen levels are still unaltered although ovarian failure is impending, a sharp increase of serum FSH levels was associated with an enhanced rate of bone loss and visceral adiposity onset, proposing FSH as a potential key player in osteoporosis and obesity in women across the menopausal transition [171,178]. Therefore, FSH-neutralizing antibodies were tested in pre-clinical experiments, which lead to increased bone mass and reduced body fat [171,180].
In line with FSH, thyroid stimulating hormone (TSH) was shown to directly affect bone remodeling through binding to the TSH receptor expressed in both osteoblast and osteoclast precursors, independent of thyroid T3 and T4 [181]. TSH was identified to negatively regulate osteoclastogenesis trough binding to TSH receptor directly but also indirectly by suppressing the synthesis of pro-osteoclastic signals [181,182] such as the cytokine TNFα, a critical mediator of the antiresorptive effects of TSH [183]. However, TSH was not only observed to reduce bone loss but also to restore bone mass, bone volume, microarchitecture and strength [184]. In osteoblasts, TSH induces the synthesis of noncanonical Wnt5a, resulting in osteoblastogenesis and stimulation of osteoprotegerin (OPG) synthesis, which in turn reduces bone resorption through an inhibition of receptor activator of nuclear factor κB ligand (RANKL) signaling [185]. However, TSH receptor activation was demonstrated to suppress osteoblast differentiation and the expression of collagen type 1 by impairing the Wnt pathway and decreasing VEGF concentrations [181]. Although these studies define TSH as a single and independent control molecule in bone formation as well as resorption, further investigation are required to unravel the underlying molecular pathophysiology and its therapeutic potential.
Lactotrophic cell-derived prolactin (PRL) is predominantly known for its pivotal role in lactation, mammary development and reproduction, additionally participating in bone homeostasis [186]. Interestingly, G protein-coupled PRL receptors are only expressed on osteoblasts but not osteoclasts [187], with a variable response based on PRL concentration levels. Although PRL is essential for bone growth and homeostasis [187], patients with pathological high PRL concentrations show increased bone resorption and suppressed bone formation activity, which eventually leads to osteoporosis [188]. On a mechanistic level, several studies observed PRL to reduce osteocalcin and alkaline phosphate activity [189], to decrease OPG expression [190,191] and to inhibit osteoblastic proliferation as well as bone mineralization [192]. Besides these direct effects, hyperprolactinemia is also proposed to negatively act on bone in an indirect manner, involving hypogonadism, hypercalcemia and an enhanced secretion of parathyroid hormone-related peptide (PTHrP) [188]. As PRL treatment however showed a positive effect on bone formation in infant rats [193] and a decreased RANKL/OPG ratio in human fetal osteoblasts [192], the effect of PRL on bone is proposed to be dependent on the developmental stage [194] and warrants further mechanistic understanding.
Adrenocorticotrophic hormone (ACTH), the key mediator of corticosteroid-release from the adrenal gland, represents an important co-regulator of immune responses, vascular tone, central metabolism and bone turnover [194,195]. Chronically elevated levels of glucocorticoids are a well-established cause for osteoporosis and presumbly osteonecrosis due to their inhibitory impact on bone-forming osteoblasts [47,196]. Zaidi et al. propose that ACTH in interaction with VEGF supports the prevention of glucocorticoid-induced osteonecrosis [170,194,195]. Additional studies described the expression of ACTH by monocytes/macrophages [197], which acts cortisol-independent on melanocortin 2 receptors (MC2R) expressed by osteoblastic cells [195]. Therefore, local and pituitary-derived ACTH features the capacity to regulate bone cells directly [170] in a concentration-dependent manner [195].
While most anterior pituitary gland-derived hormones show a divergent, rather negative effect on bone remodeling, growth hormone (GH) also known as somatotropin, is a peptide hormone crucial for human development, stimulating cell reproduction and regeneration as well as the regulation of longitudinal bone growth [198]. GH was shown to induce a net gain of bone mass [198], with in vivo studies demonstrating the capacity of GH to stimulate the proliferation of osteoblastic-cells [199]. Although the majority of experimental evidence implies GH to exert primarily insulin-like growth factor 1 (IGF-1)-dependent skeletal effects, the group of Zaidi et al. currently challenge this view by proposing the direct effect of GH on bone cells [170,194].
In contrast to all neurohormones previously discussed, arginine-vasopressin (AVP) commonly known as antidiuretic hormone, is a peptide hormone released from the posterior pituitary gland, which has also been reported to regulate bone metabolism [4]. In bone, AVP receptors (Avpr1α/2) are expressed by osteoblasts and osteoclasts, allowing AVP-binding to reduce osteoblastogenesis as well as to increase osteoclastogenesis [200]. As patients with chronic hyponatremia show severe osteoporosis and high fracture risk, their elevated AVP levels were suggested to mobilize sodium from skeletal cells which increases bone resorption [170,194]. Additionally, AVP was shown to promote the production of corticotropin-releasing hormone, which in turn facilitates the release of ACTH [201].
Oxytocin (OT), also released from the posterior pituitary gland, is known for its role in lactation, parturition [157,202], social behavior, as well as in energy and bone homeostasis [203,204]. Interestingly, OT and AVP were observed to interact with each other’s G protein-coupled receptors in order to control bone metabolism [203]. As opposed to AVP, OT shows a rather anabolic effect on bone [204], promoting osteoblastogenesis while moderately inhibiting osteoclast activity [205]. OT receptor expression was identified in osteoblasts [206] and osteoclasts [207] while bone marrow cells were shown to produce OT themselves, implying an autocrine/paracrine regulation of bone formation [204]. Notably, OT synthesis and OT receptor expression were positively regulated by estrogen signaling [208], explaining the observed sex differences in experimental and clinical studies [171].
Different to all pituitary-derived hormones, melatonin is primarily released from the pineal gland into the cerebrospinal fluid [158,209] and physiologically involved in the synchronization of circadian and seasonal rhythms including the sleep-wake cycle with further regulation of the blood pressure and temperature homeostasis [3]. In bone, both osteoblasts and osteoclasts were observed to express melatonin receptors [210]. As bone remodeling is commonly known to follow circadian rhythmic, melatonin was early discovered to support osteoblastic differentiation [211] and type 1 collagen synthesis [212]. As a result melatonin was identified to inhibit adipogenesis and promote osteogenesis [213], which was further confirmed by the observation of melatonin to induce the expression of bone morphogenetic proteins, alkaline phosphatases, osteocalcin, OPG and the suppression of RANKL [159]. While in vivo studies led to contradictory results [3], clinical observation showed a gain of BMD following melatonin supplementation in postmenopausal women with osteopenia [214], fueling continuous discussions on melatonin’s therapeutic potential [159,215,216].
Complementary to all these neurohormones involved in the central regulation of bone remodeling, further neuropeptides and -transmitters, the endocannabinoid system as well as the central clock (Figure 1) play an important role in bone homeostasis. While the control of energy homeostasis is generally divided among anabolic-active AgRP/NPY and catabolic-active POMC/CART neurons, their impact on bone metabolism appears to be more complex [4]. The multifunctional and orexigenic neuropeptide Y (NPY) is predominantly expressed in the arcuate nucleus (ARC) [217], regulating energy metabolism through induced food intake and increased fat storage [218]. In the peripheral nervous system (PNS), NPY is mainly expressed by neurons of the sympathetic nervous system were it plays a major role in bone metabolism through its receptors Y1 and Y2 [219,220]. When binding to Y1 expressed by osteoblasts [221], NPY inhibits bone formation, proliferation of mesenchymal stem cells and osteoprogenitors [222] and promotes energy storage in white adipose tissue [223], while binding to Y2 reduces bone mass and osteoblast activity through central modulation [3,220,221]. During chronic stress however, NPY was reported to feature protective effects on bone [224], which is potentially explained by a centrally-mediated decrease in sympathetic tone [221].
The neuropeptide agouti-related peptide (AgRP) is co-expressed with NPY. AgRP represents one of the most potent and long-lasting appetite stimulators, in addition to its modulatory impact on metabolism and energy expenditure [225,226]. Interestingly, it has been proposed that altered AgRP neuronal activities affect bone homeostasis independent of metabolic shifts or leptin signaling [227,228]. In this regard, enhanced AgRP neuronal activity was reported to lower sympathetic tone, which favors increased bone mass due to elevated osteoblast activity [229,230].
Cocaine amphetamine regulated transcript (CART), an anorexic neuropeptide precursor protein also involved in the regulation of food intake and energy expenditure [3,231,232,233,234], is released in the ventral tegmental area of the brain according to the serum levels of leptin [234]. While low hypothalamic CART expression was associated with increased bone resorption through higher levels of RANKL, elevated CART expression resulted in increased bone mass phenotype [217,233]. Although the pituitary gland and pancreatic islets additionally release CART into the system [234], no direct effect on osteoblast gene expression was observed [233].
Proopiomelanocortin (POMC) is a large precursor protein for multiple peptide hormones including ACTH, melanocyte-stimulating hormones (MSHs) and beta-endorphin [235]. The melanocortin peptides ACTH and α-, β- and γ-MSH bind with varying affinity to five known melanocortin receptors (MCRs) belonging to the group of G protein-coupled receptors [236]. While all POMC peptides feature immunomodulatory capacity attenuating inflammatory processes [236], melanocortins were particularly identified to show osteo- and chondro-protective effects [237,238,239,240]. Therefore, α-MSH is proposed to potentially delay the process of inflammatory and degenerative joint diseases [240]. Additionally, single nucleotide polymorphisms (SNPs) in the promoter region of the POMC gene were associated with low BMD [241]. Female mice lacking estrogen receptors in POMC neurons display an increase in cortical bone mass and mechanical strength [242], which implies a negative effect of estrogen on bone mass through POMC neurons.
Neuromedin U (NMU), a neuropeptide predominantly expressed in the pituitary and gastrointestinal tract [243], is involved in the regulation of smooth muscle contraction, blood pressure, feeding, energy homeostasis, nociception and stress response [244]. A central, leptin-dependent, regulation of bone remodeling by NMU was demonstrated in NMU-deficient mice which showed higher bone mass due to increased bone formation [245]. In rats, NMU was reported to regulate the corticotropin releasing hormone as a stress response in the pituitary gland [243,246]. In this regard, elevated concentration of both ACTH and corticosterone were observed following NMU injections [247]. Furthermore, NMU stimulates the release of vasopressin and enhances the secretion of steroids from rat adrenal cortex [247]. Taken together, these observations point out potential treatment indications of NMU receptor agonists, which could target bone loss diseases or stress-related disorders without inducing unwanted body weight gain [248,249].
The vasoactive intestinal peptide (VIP) is synthesized in various tissues including the pancreas, gastrointestinal tract as well as the hypothalamus and the autonomic nervous system. VIP is commonly associated with the sympathetic (SNS) as well as the parasympathetic nervous system (PSNS) [250], as ACh and VIP are often co-released from parasympathetic nerve fibers [221] in the periosteum and epiphysis of bone [251,252,253]. G protein-coupled VIP receptors are expressed in both osteoblasts [254] and osteoclasts [255]. VIP was reported to directly suppress receptor activator of nuclear factor κB (RANK) in osteoclasts and RANKL in osteoblasts, while it increases the expression of OPG [256,257] causing an anti-resorptive effect.
The central regulation of bone homeostasis is furthermore controlled by important neurotransmitters of the CNS, including the monoamine serotonin and dopamine as well as the excitatory neurotransmitter glutamate (Figure 1). In bone, different subtypes of G protein-coupled serotonin receptors are expressed by osteoblasts and osteoclasts [258]. Gut-derived serotonin was reported to decrease osteoblast proliferation in a low-density lipoprotein receptor-related protein 5 (Lrp5)-dependent manner [259], as well as to promote maturation of osteoclasts resulting in elevated bone resorption [260]. In fact, it has not been clarified whether Lrp5 signaling via the serotonin or Wnt pathway is more crucial [261]. Overall, the impact of serotonin on bone metabolism is proposed to be origin-dependent, whereby peripherally produced serotonin inhibits and central serotonin enhances bone formation [262]. These findings are supported by clinical data showing an acceleration of postmenopausal bone loss and reduction in bone mass accrual following the use of selective serotonin reuptake inhibitors (SSRIs) [263,264,265].
Dopamine (DA), a member of the catecholamine family, is widely expressed in the CNS and some peripheral areas, which binds to five different dopamine receptors (DR1-DR5). Signaling of DR1 [266], DR2 [267], DR3 and DR5 [268] was evidenced to enhance osteoblastic proliferation and bone mineralization as well as to suppress osteoclastogenesis [269]. The inhibition of these receptors by atypical antipsychotics leads to bone loss [270,271]. Dopamine represents another important molecule of bone homeostasis which gains further importance in diseases with dopamine decline such as AD, PD, depression or schizophrenia, while it demonstrates that the clinical use of neuroleptics potentially affects bone mass [271].
The excitatory neurotransmitter glutamate (GLU) activates ionotropic and metabotropic receptors. All mature bone cells feature glutamate receptors, with N-methyl-D-aspartate (NMDA) receptor being the most commonly expressed by osteoblasts [272] and osteoclasts [273,274]. Glutamate inhibits osteoclast activity [275] while promoting osteoblast differentiation and function [276]. In addition, it was shown that not only bone is innervated densely by glutamate-containing nerve fibers [277] but osteoblasts themselves secret glutamate through exocytosis [278]. Intracellularly, the enzyme glutamine synthase converts active glutamate into inactive glutamine, thus regulating the concentration of glutamate. Interestingly, the basal expression of glutamine synthase itself was shown to be regulated positively by glucocorticoids and negatively by Wnt signaling or Vitamin D [279], establishing glutamine synthase as a key player in the regulation of osteoblastogenesis.
The central regulation of bone remodeling is further controlled by the circadian clock [3] as well as the endocannabinoid system [280]. As the majority of homeostatic and metabolic functions are under circadian control, bone represents no exception, with central and peripheral circadian rhythms controlling bone remodeling. The central pacemaker is located in the hypothalamic suprachiasmatic nucleus, continuously synchronized with the daily light-dark cycle. The heterodimer of pacemaker transcription factors Bmal1 and Clock regulate the gene expression of downstream targets such as periods (Per1–3) and cryptochromes (Cry1, Cry2) [281]. A cyclic expression oscillation of these genes was discovered to regulate glucocorticoids by elevating the hypothalamic secretion of ACTH [282] as well as the sympathetic outflow [283,284], both associated with a negative effect on bone mass. Although continuously synchronized with the central pacemaker, the existence of peripheral clocks was revealed following the observation of time dependent gene expression within different cell types [36,285]. In bone, circadian expression oscillation was primarily reported for osteoblast activity [286,287,288] but also identified in osteoclasts and osteocytes [289,290] as well as mesenchymal stem cells [291]. A further mechanistic understanding of the circadian system and bone remodeling relationship is thus expected to identify novel therapeutic strategies to treat major bone diseases such as osteoporosis.
Cannabinoid signaling is transduced by two cannabinoid receptors (CB1 and CB2), with CB1 predominantly expressed in presynaptic neurons of the CNS and PNS [292,293] and CB2 in peripheral tissues [294]. CB2 receptors expressed in osteoblasts and osteoclasts [295,296,297] were identified to promote bone formation as well as to represses RANKL, thereby inhibiting bone resorption [298]. In clinical studies, genetic variants of the CNR2 gene, encoding for CB2 receptors, were associated with low BMD and osteoporosis [299]. In contrast, centrally expressed CB1 receptors are considered to decrease the sympathetic outflow by modulating adrenergic signaling [221]. In this regard, CB1 receptors are proposed to transmit retrograde signals which inhibit the release of the sympathetic nervous system transmitter norepinephrine and thereby stimulate bone formation [300,301]. As a result, CB1/CB2 agonists and antagonists are under investigation for their therapeutic capacity in bone regeneration [302]. Additionally, the phytocannabinoid cannabidiol was identified to stimulate collagen crosslinks and stabilize callus formation by stimulating lysyl-hydroxylase activity in osteoblasts while respecting the BBB, which promotes cannabidiol as a treatment option for osteoporosis [302] and impaired fracture healing [303].
3.1.2. SNS and PSNS
The autonomic nervous system integrates input from the internal and external microenvironment to ensure essential body function and homeostasis, also in adaption to stressors [221]. The autonomic nervous system is separated into the sympathetic (SNS) and parasympathetic nervous system (PSNS), which are both substantially involved in the regulation of bone homeostasis [3,4,5,221].
The SNS, commonly known to prepare for alert situations [221], signals through the neurotransmitter norepinephrine (NE) which activates G protein-coupled α- and β-adrenergic receptors at effector organs. Noradrenergic fibers were identified within the periosteum, branching into the bone marrow and mineralized bone alongside blood vessels [221]. Although the expression of subtypes from both α- and β-adrenergic receptors were detected in both osteoblasts and osteoclast [221], osteoblastic β-adrenergic receptors are considered as the major transducers of sympathetic signaling in bone remodeling [304]. The activation of β2-adrenergic receptors was identified to inhibit osteoblast function and further shown to induce the release of osteoblast-derived RANKL, thus promoting osteoclast formation [221,233].
As the SNS is considered a negative regulator of bone mass, the PSNS is proposed to counterbalance this impact. In contrast to the SNS, the PSNS emerges from cranial nerves as well as the sacral spinal cord, innervating the rostral and caudal part of the body, respectively. The PSNS promotes restive and digestive responses with a rather positive effect on bone remodeling, signaling through acetylcholine (ACh) at muscarinic and nicotinic cholinergic receptors [221]. Although parasympathetic nerve fibers were discovered within the bone microenvironment [305], specific density and pattern of the cholinergic bone innervation still remains to be determined [221]. The expression of both cholinergic receptor types was identified in osteoclasts and osteoblasts, with osteoclasts being the predominant target of cholinergic signaling inducing an overall inhibitory effect [221]. Notably, ACh inhibition [306] and central muscarinic receptor deletion studies [307] revealed a significant ACh regulation on CNS-mediated bone remodeling. Exclusively the deletion of muscarinic ACh receptor 3 (M3R), which is expressed in different areas of the brain, resulted in significant lower bone formation with elevated bone resorption, identical to the phenotype observed following adrenergic agonists treatment [308]. Therefore, cholinergic signaling is proposed to promote bone mass accrual through the central decrease of sympathetic activity [307].
Historically, the skeletal innervation of the autonomic nervous system was first identified histologically, while its crucial function for bone remodeling started with the discovery of leptin [221]. Leptin represents an adipocyte-derived hormone, encoded by the Ob (Lep) gene. In bone, leptin was discovered to modulate bone metabolism through hypothalamic signaling, leading to an increase in cortical bone and a decrease in trabecular bone [308,309]. Along with its direct impact on the hypothalamus, leptin was observed to bind to hypothalamic receptors, which regulate bone metabolism through the SNS [310,311]. The impact of leptin on bone mass is also related to the intake of energy and IGF-1 pathway [312]. Partially opposing the influence of leptin, the second adipocyte-derived hormone adiponectin was observed to signal in neurons of the locus coeruleus, which decreases the sympathetic tone and therefore leads to an increase in bone mass.
3.1.3. Sensory Innervation
Along with the SNS and PSNS, the sensory nervous system (SeNS) represents the third arm of the autonomic nervous system involved in the regulation of skeletal homeostasis [4]. A network of sensory nerve fibers was identified in compartments of trabecular bone, with rich innervation throughout the periosteum [313,314]. The impact of sensory innervation on bone emerged from denervation studies using capsaicin to disrupt sensory neurons [3,5]. The loss of sensory nerves caused elevated bone resorption with no influence on bone formation, overall resulting in reduced BMD [315,316].
Sensory nerves express calcitonin gene-related peptide and substance P as their main neurotransmitters [221] which substantially regulate bone homeostasis (Figure 1). Calcitonin gene-related peptide (CGRP) represents a neuropeptide occurring as two major isoforms (α and β), both encoded by different genes while sharing similar biological activities [317]. CGRP, expressed in both the CNS and PNS, primarily functions as a potent vasodilator. In bone, CGRP-positive nerve fibers were reported in the periosteum, epiphyseal trabecular bone and within the bone marrow itself [250]. CGRP receptor expression was demonstrated in osteoblasts and bone marrow stromal cells, mediating the stimulatory effect of CGRP on osteoblast proliferation and differentiation [254,318,319]. Additionally, CGRP was identified to inhibit osteoclast differentiation and activity in vitro [320]. In bone regeneration, CGRP plasma levels were observed to be elevated in patients with long-bone fracture [321], similar to rabbits suffering from fracture with concomitant traumatic brain injury [322].
Substance P (SP) represents another sensory neuropeptide, which is released from sensory nerve endings. SP is synthesized from the tachykinin 1 (TAC1) gene and signals through neurokinin 1 receptors [323]. Physiologically, SP is known for its initiating role in neurogenic inflammation, which can be further potentiated by CGRP [323]. Following CNS injury, SP was reported to be substantially elevated [324] and associated with the promotion of brain edema, injury of neurons, crucial BBB disruption and subsequent poor functional outcome [325]. In bone, both osteoblasts and osteoclasts were shown to express neurokinin 1 receptors [318,326]. Although current evidence of SP on bone formation remains inconsistent [327], SP is proposed to stimulate osteoblast proliferation and differentiation in a dose-dependent manner while simultaneously promoting the formation and activity of osteoclasts [328]. In vivo, SP-deficiency was observed to cause a minor reduction in bone resorption but a major impairment of bone formation and mineralization [328]. In bone repair, SP is considered an important regulator of angiogenesis which promotes fracture healing [329], while the antagonization of its receptor neurokinin 1 results in reduced callus formation and biomechanical strength [330,331].
Different to the neuropeptides CGRP and SP, semaphorins represent a group of soluble and membrane-associated proteins, originally identified to regulate axonal guidance and growth [332]. Different semaphorins are considered to be involved in bone homeostasis, such as semaphorin 3A (sema3A), sema4D, sema6D and sema7A [333,334]. Osteoblast-derived sema3A, particularly important for sensory neuronal development, was discovered to feature significant osteoprotective properties by promoting osteoblast function and suppressing osteoclastic activity [335]. While studying conditional sem3A-deficient mice, only the depletion of sema3A in sensory neurons resulted in a lower BMD phenotype but not the inactivation of osteoblastic sema3A [336]. These results contradict direct regulatory effects of sema3A on osteoblasts, indicating an indirect modulation of bone remodeling through sensory nerve development [1,2,4,334,335,337].
In summary, these observations highlight the importance of the nervous system in bone homeostasis and regeneration. While SNS activity results in a suppression of bone formation, PSNS and SeNS activity are proposed to counterbalance this effect by promoting bone formation and reducing bone resorption [1,2,3]. The neuronal regulation of bone mass by SeNS-derived peptides exhibit exceptional features with notable future therapeutic potential.
3.2. Bone-Derived Mediators Acting on the Central Nervous System
Recent studies revealed a bidirectional dependence of brain and bone through bone cell-derived modulators that directly affect behavioral and cognitive function. The main bone-derived mediator affecting the brain represents osteocalcin (OCN), which is encoded by the BGLAP gene and exclusively synthesized by osteoblasts. Undercarboxylated, bioactive OCN, initially considered as an inhibitor of bone mineralization [337], participates in systemic body regulation and homeostasis [338] primarily regulating insulin secretion, muscle adaptation as well as testosterone production in the testis [6]. In addition, OCN was recently discovered to transverse the BBB to enter the CNS, where it promotes spatial learning and memory while preventing anxiety-like behavior or even depression [6]. Cognitive function and circulating levels of OCN are proposed to inversely correlate with age while maternal osteocalcin regulates embryonic brain development by enhancing monoamine neurotransmitters and their synthesis [339]. Although the treatment capacity of OCN in neurodegenerative disorders remains to be elucidated [340], models of movement disorders such as PD demonstrated a neuroprotective effect of OCN [341], while OCN was reported to improve insulin sensitivity and muscle strength [342]. Interestingly, the hormonal role of osteocalcin was currently challenged by two individually performed experimental studies [343,344] that generated novel murine osteocalcin-knockout strains which lacked the previously postulated endocrine dysregulation. Therefore, both OCN-deficient mice strains with a Bglap/Bglap2 double-knockout allele displayed regular bone quantity, glucose metabolism, fertility [343] and muscle mass [344]. Bone microstructure analyses of the two studies revealed an increase in cortical bone carbonate-to-phosphate ratio and collagen maturity [343] but also the disruption of the biological apatite crystallographic orientation which resulted in reduced bone strength [344]. The impact of OCN-deficiency on cognition and brain development within the new models remains to be elucidated.
Another bone-derived mediator, lipocalin 2 (LCN2), also known as neutrophil gelatinase-associated lipocalin, is a glycoprotein which regulates energy metabolism by mediating insulin secretion and improving glucose tolerance as well as insulin sensitivity [345]. Similar to osteocalcin, the predominantly osteoblast-derived LCN2 was recently discovered to cross the BBB in order to activate the anorexigenic pathway by binding to the melanocortin 4 receptor (MC4R) in the hypothalamus [346]. Therefore, the suppression of appetite by LCN2 is referred to as an endocrine function of bone [346].
Along with the osteoblast-derived OCN and LCN2, the osteocyte-specific sclerostin was additionally identified to affect brain function. Sclerostin, a glycoprotein encoded by the SOST gene, antagonizes Wnt signaling by binding to the Lrp4/5/6 [347], which interrupts the Wnt/β-catenin pathway resulting in elevated bone resorption and reduced bone formation [348]. Therefore, the inhibition of sclerostin was proposed as a therapeutic approach in the treatment of bone loss [349], which led to the development and approval in the US and Europe of the monoclonal sclerostin-antibody Romosozumab for severe osteoporosis [350,351,352,353]. In the brain, Wnt/β-catenin signaling is essential for neurogenesis, neuronal survival, synaptic plasticity, BBB integrity [82] and further associated with the pathophysiology of AD [354]. As SOST/sclerostin is expressed by several other tissues in physiological and pathogenic conditions [355], additional studies are required to elucidate whether pharmacologic inhibition of sclerostin may also affect Wnt/β-catenin signaling in the brain [8].
Similar to sclerostin, dickkopf-related protein 1 (DKK1) represents another antagonist of Wnt signaling. In bone, DKK1 binds to Lrp6 also antagonizing the Wnt/β-catenin pathway [356], whereby over-expression of DKK1 results in osteopenia [357]. During early embryogenesis, DKK1 is released from the endoderm in order to control cell death and differentiation [358]. As a result, over-expression of DKK1 leads to synaptic loss and neuronal apoptosis, making it a common marker for neuronal death in neurodegenerative diseases [359]. In the aging brain, Wnt signaling was identified to generally diminish [360], while DKK1 remained up-regulated [361]. Such an elevation of DKK1 was clinically and experimentally reported for individuals suffering from AD, further supporting the hypothesis that Wnt signaling dysfunction contributes to the pathology of AD [362]. Along with its positive effect on bone, restoring the Wnt/β-catenin signaling is regarded as a promising future therapeutic approach to treat neurodegenerative diseases [354,359,362,363,364,365].
In addition to the molecular mediators secreted by bone, bone marrow-derived cells were identified to enter the systemic circulation and migrate into the injured brain [8]. In preclinical studies, bone marrow-derived microglia-like cells were shown to modulate amyloid pathology by restricting Aβ plaque formation and by supporting Aβ plaque clearance, which improved cognitive impairment [366,367,368]. Therefore, recent attempts employed hematopoietic stem cells mobilized from bone marrow into the peripheral blood for autologous microglia-like cell preparation [369], using specific antibodies to convert bone marrow cells into trafficking microglia-like cells [367]. Additionally, the systemic transplantation of bone marrow-derived macrophages reduced neuroinflammation with a limited reversion of Aβ deposition [370]. In summary, growing evidence of experimental AD studies demonstrates the capacity of bone marrow-derived microglia and macrophages to enter the CNS and to suppress the progression of brain degeneration, which provides a promising therapeutic tool for AD patients [8,371].
3.3. Mediators Affecting Both Brain and Bone Function
Both brain and bone share common regulatory factors which are locally synthesized in both organ systems. These mediators include irisin, osteopontin (OPN) and RANKL as well as the growth factors brain-derived neurotrophic factor (BDNF), IGF-1 and bone morphogenic proteins (BMPs). Irisin, a cleaved product of the precursor type 1 membrane protein, is described as an exercise-induced myokine [372,373] with the capacity to change visceral adipose tissue into brown adipose tissue, highlighting its potential role in preventing obesity or even metabolic syndrome [374,375]. In the brain, irisin was discovered to feature neuroprotective effects following ischemic stroke [376] through the activation of phagocytotic cells [377], while rescuing synaptic plasticity and memory defects in models of AD [375,377,378]. In bone, irisin showed osteoanabolic effects by enhancing osteoblastic activity and reducing the number of osteoclasts [372]. Furthermore, irisin was proposed to upregulate RUNX2 and Wnt/β-catenin signaling, potentially transmitted by the suppression of the Wnt antagonist sclerostin [373]. As a result, irisin represents a notable pharmacologic target currently under discussion in osteoporosis-associated neurodegenerative diseases and metabolic research [7,374].
OPN represents a glycoprotein encoded by the SPP1 gene, which is expressed in a variety of tissues including brain and bone. OPN is one of the non-collagenous proteins present in bone matrix, supporting bone demineralization by anchoring osteoclasts to bone mineral matrix which enhances the process of bone resorption [379,380]. Therefore, patients with high serum OPN levels are associated with low BMD [381]. Besides bone tissue, OPN is a multifunctional molecule with high expression in inflammatory diseases, where it serves as a proinflammatory cytokine [382]. In the brain, OPN is proposed to protect neurons and to regulate repair processes in various brain disorders such as ischemia, stroke, TBI and neurodegenerative diseases [77,383,384,385].
RANKL is encoded by the TNFSF11 gene, also expressed in various tissues including brain and bone. In the CNS, RANKL/RANK are particularly present in the lateral septal nucleus of the hypothalamus, controlling the central regulation of fever and gender specific body temperature in females [386], whereas in bone, RANKL represents the key mediator for activation, differentiation, fusion and survival of osteoclasts [387]. For therapeutic treatment of bone loss diseases, the RANKL-neutralizing monoclonal antibody Denosumab [387,388] was developed, which is fully approved in the US and Europe. Recently, Denosumab was reported to improve chronic social defeat stress in mice [389,390], which is proposed as novel treatment indication in patients suffering from depression.
The growth factor BDNF is secreted in the CNS and PNS. In the brain, BDNF supports neurodevelopment, synaptic plasticity, neuronal differentiation and survival while its reduced levels are associated with neuronal dysfunction and degeneration in disorders such as depression, AD and PD [391]. Interestingly, the central inactivation of BDNF expression leads to gender-dependent increased bone mass [392], obesity and leptin resistance [393].
IGF-1, a polypeptide hormone structurally similar to insulin, is primarily synthesized in the liver in response to growth hormone signaling [394]. In the brain, IGF-1 was discovered to perform pleiotropic actions during development, alterations of neuronal excitability and enhancement of nerve cell metabolism, posing anti-apoptotic properties [395]. In bone, the growth hormone/IGF-1 axis stimulates growth directly by activating chondrocyte proliferation and osteoblast differentiation [396,397], while the reduction of IGF-1 serum levels and insulin-deficiency of type 1 diabetes were associated with osteoporosis [397].
Expressed in most body tissues, BMPs represent the largest subgroup of the transforming growth factor beta (TGFβ) family, regulating various developmental processes [398]. In the brain, BMPs are involved in neurogenesis with differential expression levels in adults [399,400], indicating their participation in age-related neuronal dysfunction [401]. Therefore, BMPs are discussed for therapeutic protection of the white matter [402]. In bone, BMPs enhance endochondral ossification and skeletal regeneration [400]. Interestingly, statins, commonly known for their application in cardiovascular diseases, were shown to promote osteogenesis via the BMP pathway by inhibiting osteoclastogenesis and apoptosis of osteoblasts [403,404,405], which highlights their osteoanabolic and anti-resorptive treatment capacity [406,407].
4. Conclusions
Several clinical observations provide key evidence for a bidirectional communication between brain and bone tissue, which is strongly supported by experimental studies that unraveled the underlying mechanistic pathways and identified molecular mediators involved in this crosstalk. The majority of brain-bone crosstalk is ’efferent,’ as the nervous system tightly modulates bone metabolism and regeneration. Thus, bone is regulated by multiple transmitters of both the CNS and PNS. Although evidence of the bone’s regulatory capacity is continuously growing, this ‘afferent’ loop has long been underestimated. Skeletal tissue emerged as a crucial modulator of cerebral development, function and pathologies. Investigating the diverse physiological and pathological interactions of brain and bone has thus revealed a variety of promising pharmacologic targets, supporting anabolic treatment strategies in bone and neuroprotective effects in brain pathologies. Future therapeutic approaches however need to consider the intense and dynamic brain-bone crosstalk as well as genetic and neuropsychological comorbidities of affected patients, potentially requiring additional monitoring or even individualized treatment regimes.
Acknowledgments
We thank Tobias Thiele for his graphical support.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/21/14/4946/s1.
Author Contributions
Conceptualization, E.O., P.-R.K., J.K.; writing—original draft preparation, E.O., P.-R.K., J.K.; writing—review and editing, E.O., P.-R.K., D.J., J.A., K.-H.F., S.T., J.K.; visualization, E.O., P.-R.K.; supervision, J.K.; funding acquisition, S.T., J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded in part by the German Research Foundation (DFG KE 2179/2-1 and TS 303/2-1), the Else-Kröner-Fresenius Stiftung (EKFS 2017_A22) and the Berlin Institute of Health. We acknowledge support from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité—Universitätsmedizin Berlin.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Chamouni A., Schreiweis C., Oury F. Bone, brain & beyond. Rev. Endocr. Metab. Disord. 2015;16:99–113. doi: 10.1007/s11154-015-9312-5. [DOI] [PubMed] [Google Scholar]
- 2.Rousseaud A., Moriceau S., Ramos-Brossier M., Oury F. Bone-brain crosstalk and potential associated diseases. Horm. Mol. Biol. Clin. Investig. 2016;28:69–83. doi: 10.1515/hmbci-2016-0030. [DOI] [PubMed] [Google Scholar]
- 3.Dimitri P., Rosen C. The Central Nervous System and Bone Metabolism: An Evolving Story. Calcif. Tissue Int. 2017;100:476–485. doi: 10.1007/s00223-016-0179-6. [DOI] [PubMed] [Google Scholar]
- 4.Idelevich A., Baron R. Brain to bone: What is the contribution of the brain to skeletal homeostasis? Bone. 2018;115:31–42. doi: 10.1016/j.bone.2018.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maryanovich M., Takeishi S., Frenette P.S. Neural regulation of bone and bone marrow. Cold Spring Harb. Perspect. Med. 2018;8 doi: 10.1101/cshperspect.a031344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Obri A., Khrimian L., Karsenty G., Oury F. Osteocalcin in the brain: From embryonic development to age-related decline in cognition. Nat. Rev. Endocrinol. 2018;14:174–182. doi: 10.1038/nrendo.2017.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frame G., Bretland K.A., Dengler-Crish C.M. Mechanistic complexities of bone loss in Alzheimer’s disease: A review. Connect. Tissue Res. 2020;61:4–18. doi: 10.1080/03008207.2019.1624734. [DOI] [PubMed] [Google Scholar]
- 8.Yuan J., Meloni B.P., Shi T., Bonser A., Papadimitriou J.M., Mastaglia F.L., Zhang C., Zheng M., Gao J. The potential influence of bone-derived modulators on the progression of Alzheimer’s disease. J. Alzheimer’s Dis. 2019;69:59–70. doi: 10.3233/JAD-181249. [DOI] [PubMed] [Google Scholar]
- 9.Tatangelo G., Watts J., Lim K., Connaughton C., Abimanyi-Ochom J., Borgström F., Nicholson G.C., Shore-Lorenti C., Stuart A.L., Iuliano-Burns S., et al. The Cost of Osteoporosis, Osteopenia, and Associated Fractures in Australia in 2017. J. Bone Miner. Res. 2019;34:616–625. doi: 10.1002/jbmr.3640. [DOI] [PubMed] [Google Scholar]
- 10.Siris E.S., Adler R., Bilezikian J., Bolognese M., Dawson-Hughes B., Favus M.J., Harris S.T., Jan De Beur S.M., Khosla S., Lane N.E., et al. The clinical diagnosis of osteoporosis: A position statement from the National Bone Health Alliance Working Group. Osteoporos. Int. 2014;25:1439–1443. doi: 10.1007/s00198-014-2655-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petty S.J., Wilding H., Wark J.D. Osteoporosis Associated with Epilepsy and the Use of Anti-Epileptics—A Review. Curr. Osteoporos. Rep. 2016;14:54–65. doi: 10.1007/s11914-016-0302-7. [DOI] [PubMed] [Google Scholar]
- 12.Kishimoto T., De Hert M., Carlson H.E., Manu P., Correll C.U. Osteoporosis and fracture risk in people with schizophrenia. Curr. Opin. Psychiatry. 2012;25:415–429. doi: 10.1097/YCO.0b013e328355e1ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bukowska-Damska A., Skowronska-Jozwiak E., Peplonska B. Night shift work and osteoporosis: Evidence and hypothesis. Chronobiol. Int. 2019;36:171–180. doi: 10.1080/07420528.2018.1528553. [DOI] [PubMed] [Google Scholar]
- 14.Huang W.S., Hsu J.W., Huang K.L., Bai Y.M., Su T.P., Li C.T., Lin W.C., Chen T.J., Tsai S.J., Liou Y.J., et al. Post-traumatic stress disorder and risk of osteoporosis: A nationwide longitudinal study. Stress Heal. 2018;34:440–445. doi: 10.1002/smi.2806. [DOI] [PubMed] [Google Scholar]
- 15.Aloumanis K., Mavroudis K. The “depressive” face of osteoporosis and the “osteoporotic” face of depression. Hormones. 2013;12:350–362. doi: 10.1007/BF03401301. [DOI] [PubMed] [Google Scholar]
- 16.Carda S., Cisari C., Invernizzi M., Bevilacqua M. Osteoporosis after stroke: A review of the causes and potential treatments. Cerebrovasc. Dis. 2009;28:191–200. doi: 10.1159/000226578. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y.H., Lo R.Y. Alzheimer’s disease and osteoporosis. Tzu Chi Med. J. 2017;29:138–142. doi: 10.4103/tcmj.tcmj_54_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Invernizzi M., Carda S., Viscontini G.S., Cisari C. Osteoporosis in Parkinson’s disease. Park. Relat. Disord. 2009;15:339–346. doi: 10.1016/j.parkreldis.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Smith E., Comiskey C., Carroll A. Prevalence of and risk factors for osteoporosis in adults with acquired brain injury. Ir. J. Med. Sci. 2016;185:473–481. doi: 10.1007/s11845-016-1399-5. [DOI] [PubMed] [Google Scholar]
- 20.Wang L., Yao X., Xiao L., Tang X., Ding H., Zhang H., Yuan J. The effects of spinal cord injury on bone healing in patients with femoral fractures. J. Spinal Cord Med. 2014;37:414–419. doi: 10.1179/2045772313Y.0000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borchers A.T., Gershwin M.E. Complex regional pain syndrome: A comprehensive and critical review. Autoimmun. Rev. 2014;13:242–265. doi: 10.1016/j.autrev.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 22.McDonald S.J., Sun M., Agoston D.V., Shultz S.R. The effect of concomitant peripheral injury on traumatic brain injury pathobiology and outcome. J. Neuroinflammation. 2016;13 doi: 10.1186/s12974-016-0555-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennison E.M., Compston J.E., Flahive J., Siris E.S., Gehlbach S.H., Adachi J.D., Boonen S., Chapurlat R., Díez-Pérez A., Anderson F.A., et al. Effect of co-morbidities on fracture risk: Findings from the Global Longitudinal Study of Osteoporosis in Women (GLOW) Bone. 2012;50:1288–1293. doi: 10.1016/j.bone.2012.02.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellman R., Spatz J., Cloutier A., Palme R., Christiansen B.A., Bouxsein M.L. Partial reductions in mechanical loading yield proportional changes in bone density, bone architecture, and muscle mass. J. Bone Miner. Res. 2013;28:875–885. doi: 10.1002/jbmr.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feskanich D., Hankinson S.E., Schernhammer E.S. Nightshift work and fracture risk: The Nurses’ Health Study. Osteoporos. Int. 2009;20:537–542. doi: 10.1007/s00198-008-0729-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quevedo I., Zuniga A.M. Low bone mineral density in rotating-shift workers. J. Clin. Densitom. 2010;13:467–469. doi: 10.1016/j.jocd.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Weibel L., Brandenberger G. Disturbances in Hormonal Profiles of Night Workers during Their Usual Sleep and Work Times. J. Biol. Rhythms. 1998;13:202–208. doi: 10.1177/074873098129000048. [DOI] [PubMed] [Google Scholar]
- 28.Dumont M., Lanctt V., Cadieux-Viau R., Paquet J. Melatonin production and light exposure of rotating night workers. Chronobiol. Int. 2012;29:203–210. doi: 10.3109/07420528.2011.647177. [DOI] [PubMed] [Google Scholar]
- 29.Ulhôa M.A., Marqueze E.C., Burgos L.G.A., Moreno C.R.C. Shift work and endocrine disorders. Int. J. Endocrinol. 2015;2015 doi: 10.1155/2015/826249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schernhammer E.S., Rosner B., Willet W.C., Laden F., Colditz G.A., Hankinson S.E. Epidemiology of urinary melatonin in women and its relation to other hormones and night work. Cancer Epidemiol. Biomarkers Prev. 2004;13:936–943. [PubMed] [Google Scholar]
- 31.Manenschijn L., Van Kruysbergen R.G.P.M., De Jong F.H., Koper J.W., Van Rossum E.F.C. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J. Clin. Endocrinol. Metab. 2011;96:1862–1865. doi: 10.1210/jc.2011-1551. [DOI] [PubMed] [Google Scholar]
- 32.Pimenta A.M., Kac G., Campos e Souza R.R., Maria de Barros Almeida Ferreira L., Maria de Fátima Silqueira S. Night-shift work and cardiovascular risk among employees of a public university. Rev. da Assoc. Médica Bras. (English Ed.) 2012;58:168–177. doi: 10.1016/S0104-4230(12)70177-X. [DOI] [PubMed] [Google Scholar]
- 33.Strohmaier S., Devore E.E., Zhang Y., Schernhammer E.S. A Review of Data of Findings on Night Shift Work and the Development of DM and CVD Events: A Synthesis of the Proposed Molecular Mechanisms. Curr. Diab. Rep. 2018;18 doi: 10.1007/s11892-018-1102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan A., Schernhammer E.S., Sun Q., Hu F.B. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao P., Ohtsuka-Isoya M., Shinoda H. Circadian rhythms in serum bone markers and their relation to the effect of etidronate in rats. Chronobiol. Int. 2003;20:325–336. doi: 10.1081/CBI-120019343. [DOI] [PubMed] [Google Scholar]
- 36.Schibler U., Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111:919–922. doi: 10.1016/S0092-8674(02)01225-4. [DOI] [PubMed] [Google Scholar]
- 37.Maronde E., Schilling A.F., Seitz S., Schinke T., Schmutz I., van der Horst G., Amling M., Albrecht U. The clock genes Period 2 and Cryptochrome 2 differentially balance bone formation. PLoS ONE. 2010;5:1–8. doi: 10.1371/journal.pone.0011527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Everson C.A., Folley A.E., Toth J.M. Chronically inadequate sleep results in abnormal bone formation and abnormal bone marrow in rats. Exp. Biol. Med. 2012;237:1101–1109. doi: 10.1258/ebm.2012.012043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swanson C.M., Kohrt W.M., Buxton O.M., Everson C.A., Wright K.P., Orwoll E.S., Shea S.A. The importance of the circadian system & sleep for bone health. Metabolism. 2018;84:28–43. doi: 10.1016/j.metabol.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Swanson C.M., Shea S.A., Wolfe P., Cain S.W., Munch M., Vujović N., Czeisler C.A., Buxton O.M., Orwoll E.S. Bone turnover markers after sleep restriction and circadian disruption: A mechanism for sleep-related bone loss in humans. J. Clin. Endocrinol. Metab. 2017;102:3722–3730. doi: 10.1210/jc.2017-01147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.El-Gabalawy R., Blaney C., Tsai J., Sumner J.A., Pietrzak R.H. Physical health conditions associated with full and subthreshold PTSD in U.S. military veterans: Results from the National Health and Resilience in Veterans Study. J. Affect. Disord. 2018;227:849–853. doi: 10.1016/j.jad.2017.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Toft H., Bramness J.G., Lien L., Abebe D.S., Wampold B.E., Tilden T., Hestad K., Neupane S.P. PTSD patients show increasing cytokine levels during treatment despite reduced psychological distress. Neuropsychiatr. Dis. Treat. 2018;14:2367–2378. doi: 10.2147/NDT.S173659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfeilschifter J., Chenu C., Bird A., Mundy G.R., Roodman D.G. Interleukin-1 and tumor necrosis factor stimulate the formation of human osteoclastlike cells in vitro. J. Bone Miner. Res. 1989;4:113–118. doi: 10.1002/jbmr.5650040116. [DOI] [PubMed] [Google Scholar]
- 44.Daskalakis N.P., Lehrner A., Yehuda R. Endocrine Aspects of Post-traumatic Stress Disorder and Implications for Diagnosis and Treatment. Endocrinol. Metab. Clin. N. Am. 2013;42:503–513. doi: 10.1016/j.ecl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Hasan K.M.M., Rahman M.S., Arif K.M.T., Sobhani M.E. Psychological stress and aging: Role of glucocorticoids (GCs) Age. 2012;34:1421–1433. doi: 10.1007/s11357-011-9319-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weinstein R.S., Jilka R.L., Michael Parfitt A., Manolagas S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts end osteocytes by glucocorticoids potential mechanisms of their deleterious effects on bone. J. Clin. Investig. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foertsch S., Haffner-Luntzer M., Kroner J., Gross F., Kaiser K., Erber M., Reber S.O., Ignatius A. Chronic psychosocial stress disturbs long-bone growth in adolescent mice. DMM Dis. Model. Mech. 2017;10:1399–1409. doi: 10.1242/dmm.030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haffner-Luntzer M., Foertsch S., Fischer V., Prystaz K., Tschaffon M., Mödinger Y., Bahney C.S., Marcucio R.S., Miclau T., Ignatius A., et al. Chronic psychosocial stress compromises the immune response and endochondral ossification during bone fracture healing via β-AR signaling. Proc. Natl. Acad. Sci. USA. 2019;116:8615–8622. doi: 10.1073/pnas.1819218116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schweiger J.U., Schweiger U., Hüppe M., Kahl K.G., Greggersen W., Fassbinder E. Bone density and depressive disorder: A meta-analysis. Brain Behav. 2016;6:1–10. doi: 10.1002/brb3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cizza G. Major depressive disorder is a risk factor for low bone mass, central obesity, and other medical conditions. Dialogues Clin. Neurosci. 2011;13:73–87. doi: 10.31887/DCNS.2011.13.1/gcizza. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cizza G., Primma S., Coyle M., Gourgiotis L., Csako G. Depression and osteoporosis: A research synthesis with meta-analysis. Horm. Metab. Res. 2010;42:467–482. doi: 10.1055/s-0030-1252020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yirmiya R., Bab I. Major Depression Is a Risk Factor for Low Bone Mineral Density: A Meta-Analysis. Biol. Psychiatry. 2009;66:423–432. doi: 10.1016/j.biopsych.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Keller J., Gomez R., Williams G., Lembke A., Lazzeroni L., Murphy G.M., Schatzberg A.F. HPA axis in major depression: Cortisol, clinical symptomatology and genetic variation predict cognition. Mol. Psychiatry. 2017;22:527–536. doi: 10.1038/mp.2016.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yirmiya R., Goshen I., Bajayo A., Kreisel T., Feldman S., Tam J., Trembovler V., Csernus V., Shohami E., Bab I. Depression induces bone loss through stimulation of the sympathetic nervous system. Proc. Natl. Acad. Sci. USA. 2006;103:16876–16881. doi: 10.1073/pnas.0604234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mezuk B., Eaton W.W., Golden S.H. Depression and osteoporosis: Epidemiology and potential mediating pathways. Osteoporos. Int. 2008;19:1–12. doi: 10.1007/s00198-007-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schweiger U., Weber B., Deuschle M., Heuser I. Lumbar bone mineral density in patients with major depression: Evidence of increased bone loss at follow-up. Am. J. Psychiatry. 2000;157:118–120. doi: 10.1176/ajp.157.1.118. [DOI] [PubMed] [Google Scholar]
- 57.Nie C., Wang Z., Liu X. The effect of depression on fracture healing and osteoblast differentiation in rats. Neuropsychiatr. Dis. Treat. 2018;14:1705–1713. doi: 10.2147/NDT.S168653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roth G.A., Feigin V.L., Nguyen G., Cercy K., Johnson C.O., Alam T., Parmar P.G., Abajobir A.A., Abate K.H., Abd-Allah F., et al. Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N. Engl. J. Med. 2018;379:2429–2437. doi: 10.1056/NEJMoa1804492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mughal N., Inderjeeth A.J., Inderjeeth C.A. Osteoporosis in patients with dementia is associated with high morbidity and mortality: Findings from a single orthogeriatric unit. Aust. J. Gen. Pract. 2019;48:53–58. doi: 10.31128/AJGP-04-18-4544. [DOI] [PubMed] [Google Scholar]
- 60.Posada I.J., Benito-León J., Louis E.D., Trincado R., Villarejo A., Medrano M.J., Bermejo-Pareja F. Mortality from Parkinson’s disease: A population-based prospective study (NEDICES) Mov. Disord. 2011;26:2522–2529. doi: 10.1002/mds.23921. [DOI] [PubMed] [Google Scholar]
- 61.Abdullahi W., Tripathi D., Ronaldson P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018;315:C343–C356. doi: 10.1152/ajpcell.00095.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramnemark A., Nyberg L., Borssén B., Olsson T., Gustafson Y. Fractures after stroke. Osteoporos. Int. 1998;8:92–95. doi: 10.1007/s001980050053. [DOI] [PubMed] [Google Scholar]
- 63.Wei M., Lyu H., Huo K., Su H. Impact of bone fracture on ischemic stroke recovery. Int. J. Mol. Sci. 2018;19:1533. doi: 10.3390/ijms19051533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato Y., Kuno H., Kaji M., Etoh K., Oizumi K. Influence of immobilization upon calcium metabolism in the week following hemiplegic stroke. J. Neurol. Sci. 2000;175:135–139. doi: 10.1016/S0022-510X(00)00298-7. [DOI] [PubMed] [Google Scholar]
- 65.He X.W., Wang E., Bao Y.Y., Wang F., Zhu M., Hu X.F., Jin X.P. High serum levels of sclerostin and Dickkopf-1 are associated with acute ischaemic stroke. Atherosclerosis. 2016;253:22–28. doi: 10.1016/j.atherosclerosis.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 66.Zhu Z., Guo D., Zhong C., Wang A., Xie X., Xu T., Chen C.S., Peng Y., Peng H., Li Q., et al. Serum Dkk-1 (Dickkopf-1) Is a Potential Biomarker in the Prediction of Clinical Outcomes among Patients with Acute Ischemic Stroke. Arterioscler. Thromb. Vasc. Biol. 2019;39:285–293. doi: 10.1161/ATVBAHA.118.311960. [DOI] [PubMed] [Google Scholar]
- 67.Mathold K., Wanby P., Brudin L., Von S.P., Carlsson M. Alterations in bone turnover markers in patients with noncardio-embolic ischemic stroke. PLoS ONE. 2018;13:1–14. doi: 10.1371/journal.pone.0207348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beaupre G.S., Lew H.L. Bone-density changes after stroke. Am. J. Phys. Med. Rehabil. 2006;85:464–472. doi: 10.1097/01.phm.0000214275.69286.7a. [DOI] [PubMed] [Google Scholar]
- 69.Dey D., Wheatley B.M., Cholok D., Agarwal S., Yu P.B., Levi B., Davis T.A. The traumatic bone: Trauma-induced heterotopic ossification. Transl. Res. 2017;186:95–111. doi: 10.1016/j.trsl.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gerber H.P., Vu T.H., Ryan A.M., Kowalski J., Werb Z., Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat. Med. 1999;5:623–628. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 71.Street J., Bao M., DeGuzman L., Bunting S., Peale F.V., Ferrara N., Steinmetz H., Hoeffel J., Cleland J.L., Daugherty A., et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc. Natl. Acad. Sci. USA. 2002;99:9656–9661. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L., Kang S., Zou D., Zhan L., Li Z., Zhu W., Su H. Bone fracture pre-ischemic stroke exacerbates ischemic cerebral injury in mice. PLoS ONE. 2016;11:e0153835. doi: 10.1371/journal.pone.0153835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baker N.L., Cook M.N., Arrighi H.M., Bullock R. Hip fracture risk and subsequent mortality among Alzheimer’s disease patients in the United Kingdom, 1988–2007. Age Ageing. 2011;40:49–54. doi: 10.1093/ageing/afq146. [DOI] [PubMed] [Google Scholar]
- 74.Zhao Y., Shen L., Ji H.F. Alzheimer’s disease and risk of hip fracture: A meta-analysis study. Sci. World J. 2012;2012 doi: 10.1100/2012/872173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang H.K., Hung C.M., Lin S.H., Tai Y.C., Lu K., Liliang P.C., Lin C.W., Lee Y.C., Fang P.H., Chang L.C., et al. Increased risk of hip fractures in patients with dementia: A nationwide population-based study. BMC Neurol. 2014;14 doi: 10.1186/s12883-014-0175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loskutova N., Honea R.A., Vidoni E.D., Brooks W.M., Burns J.M. Bone density and brain atrophy in early Alzheimer’s disease. J. Alzheimer’s Dis. 2009;18:777–785. doi: 10.3233/JAD-2009-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun Y., Yin X.S., Guo H., Han R.K., He R.D., Chi L.J. Elevated osteopontin levels in mild cognitive impairment and Alzheimer’s disease. Mediators Inflamm. 2013;2013 doi: 10.1155/2013/615745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Comi C., Carecchio M., Chiocchetti A., Nicola S., Galimberti D., Fenoglio C., Cappellano G., Monaco F., Scarpini E., Dianzani U. Osteopontin is increased in the cerebrospinal fluid of patients with Alzheimer’s disease and its levels correlate with cognitive decline. J. Alzheimer’s Dis. 2010;19:1143–1148. doi: 10.3233/JAD-2010-1309. [DOI] [PubMed] [Google Scholar]
- 79.Fodor D., Bondor C., Albu A., Simon S.P., Craciun A., Muntean L. The value of osteopontin in the assessment of bone mineral density status in postmenopausal women. J. Investig. Med. 2013;61:15–21. doi: 10.2310/JIM.0b013e3182761264. [DOI] [PubMed] [Google Scholar]
- 80.He Z., Guo J.L., McBride J.D., Narasimhan S., Kim H., Changolkar L., Zhang B., Gathagan R.J., Yue C., Dengler C., et al. Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med. 2018;24:29–38. doi: 10.1038/nm.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li S., Yang B., Teguh D., Zhou L., Xu J., Rong L. Amyloid β peptide enhances RANKL-induced osteoclast activation through NF-κB, ERK, and calcium oscillation signaling. Int. J. Mol. Sci. 2016;17:1683. doi: 10.3390/ijms17101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jia L., Piña-Crespo J., Li Y. Restoring Wnt/β-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Mol. Brain. 2019;12:1–11. doi: 10.1186/s13041-019-0525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berg D., Gerlach M., Youdim M.B.H., Double K.L., Zecca L., Riederer P., Becker G. Brain iron pathways and their relevance to Parkinson’s disease. J. Neurochem. 2001;79:225–236. doi: 10.1046/j.1471-4159.2001.00608.x. [DOI] [PubMed] [Google Scholar]
- 84.Fink A.L. The aggregation and fibrillation of α-synuclein. Acc. Chem. Res. 2006;39:628–634. doi: 10.1021/ar050073t. [DOI] [PubMed] [Google Scholar]
- 85.Burré J., Sharma M., Südhof T.C. Definition of a molecular pathway mediating α-synuclein neurotoxicity. J. Neurosci. 2015;35:5221–5232. doi: 10.1523/JNEUROSCI.4650-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kapral M.K., Fang J., Alibhai S.M.H., Cram P., Cheung A.M., Casaubon L.K., Prager M., Stamplecoski M., Rashkovan B., Austin P.C. Risk of fractures after stroke: Results from the Ontario Stroke Registry. Neurology. 2017;88:57–64. doi: 10.1212/WNL.0000000000003457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Den Bos F., Speelman A.D., Van Nimwegen M., Van Der Schouw Y.T., Backx F.J.G., Bloem B.R., Munneke M., Verhaar H.J.J. Bone mineral density and vitamin D status in Parkinson’s disease patients. J. Neurol. 2013;260:754–760. doi: 10.1007/s00415-012-6697-x. [DOI] [PubMed] [Google Scholar]
- 88.Torsney K.M., Noyce A.J., Doherty K.M., Bestwick J.P., Dobson R., Lees A.J. Bone health in Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry. 2014;85:1159–1166. doi: 10.1136/jnnp-2013-307307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Handa K., Kiyohara S., Yamakawa T., Ishikawa K., Hosonuma M., Sakai N., Karakawa A., Chatani M., Tsuji M., Inagaki K., et al. Bone loss caused by dopaminergic degeneration and levodopa treatment in Parkinson’s disease model mice. Sci. Rep. 2019;9:1–16. doi: 10.1038/s41598-019-50336-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mundlos S. Cleidocranial dysplasia: Clinical and molecular genetics. J. Med. Genet. 1999;36:177–182. doi: 10.1136/jmg.36.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Izumi K., Yahagi N., Fujii Y., Higuchi M., Kosaki R., Naito Y., Nishimura G., Hosokai N., Takahashi T., Kosaki K. Cleidocranial dysplasia plus vascular anomalies with 6p21.2 microdeletion spanning RUNX2 and VEGF (4) Am. J. Med. Genet. 2006;140 A:398–401. doi: 10.1002/ajmg.a.31061. [DOI] [PubMed] [Google Scholar]
- 92.Takenouchi T., Sato W., Torii C., Kosaki K. Progressive cognitive decline in an adult patient with cleidocranial dysplasia. Eur. J. Med. Genet. 2014;57:319–321. doi: 10.1016/j.ejmg.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 93.Trivier E., De Cesare D., Jacquot S., Pannetier S., Zackai E., Young I., Mandel J.L., Sassone-Corsi P., Hanauer A. Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature. 1996;384:567–570. doi: 10.1038/384567a0. [DOI] [PubMed] [Google Scholar]
- 94.Marques Pereira P., Schneider A., Pannetier S., Heron D., Hanauer A. Coffin-Lowry syndrome. Eur. J. Hum. Genet. 2010;18:627–633. doi: 10.1038/ejhg.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Poirier R., Jacquot S., Vaillend C., Soutthiphong A.A., Libbey M., Davis S., Laroche S., Hanauer A., Welzl H., Lipp H.P., et al. Deletion of the Coffin-Lowry syndrome gene Rsk2 in mice is associated with impaired spatial learning and reduced control of exploratory behavior. Behav. Genet. 2007;37:31–50. doi: 10.1007/s10519-006-9116-1. [DOI] [PubMed] [Google Scholar]
- 96.Kandel E.R., Dudai Y., Mayford M.R. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 97.Yang X., Matsuda K., Bialek P., Jacquot S., Masuoka H.C., Schinke T., Li L., Brancorsini S., Sassone-Corsi P., Townes T.M., et al. ATF4 is a substrate of RSK2 and an essential regulator of osteoblast biology: Implication for Coffin-Lowry syndrome. Cell. 2004;117:387–398. doi: 10.1016/S0092-8674(04)00344-7. [DOI] [PubMed] [Google Scholar]
- 98.Xiao G., Jiang D., Ge C., Zhao Z., Lai Y., Boules H., Phimphilai M., Yang X., Karsenty G., Franceschi R.T. Cooperative interactions between activating transcription factor 4 and Runx2/Cbfa1 stimulate osteoblast-specific osteocalcin gene expression. J. Biol. Chem. 2005;280:30689–30696. doi: 10.1074/jbc.M500750200. [DOI] [PubMed] [Google Scholar]
- 99.Balemans W. Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST) Hum. Mol. Genet. 2001;10:537–543. doi: 10.1093/hmg/10.5.537. [DOI] [PubMed] [Google Scholar]
- 100.Balemans W., Patel N., Ebeling M., Van Hul E., Wuyts W., Lacza C., Dioszegi M., Dikkers F.G., Hildering P., Willems P.J., et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J. Med. Genet. 2002;39:91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alvarez C.M., De Vera M.A., Heslip T.R., Casey B. Evaluation of the anatomic burden of patients with hereditary multiple exostoses. Clin. Orthop. Relat. Res. 2007:73–79. doi: 10.1097/BLO.0b013e3181334b51. [DOI] [PubMed] [Google Scholar]
- 102.Li H., Yamagata T., Mori M., Momoi M.Y. Association of autism in two patients with hereditary multiple exostoses caused by novel deletion mutations of EXT1. J. Hum. Genet. 2002;47:262–265. doi: 10.1007/s100380200036. [DOI] [PubMed] [Google Scholar]
- 103.Narvid J., Gorno-Tempini M.L., Slavotinek A., DeArmond S.J., Cha Y.H., Miller B.L., Rankin K. Of brain and bone: The unusual case of Dr. A. Neurocase. 2009;15:190–205. doi: 10.1080/13554790802632967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marinus J., Moseley L., Birklein F., Baron R., Maihöfner C., Kingery W.S., Hilten J.J. Van Syndrome–current state of the art. Lancet Neurol. 2011;10:637–648. doi: 10.1016/S1474-4422(11)70106-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lohnberg J.A., Altmaier E.M. A review of psychosocial factors in complex regional pain syndrome. J. Clin. Psychol. Med. Settings. 2013;20:247–254. doi: 10.1007/s10880-012-9322-3. [DOI] [PubMed] [Google Scholar]
- 106.Bazika-Gerasch B., Maier C., Kumowski N., Fiege C., Kaisler M., Vollert J., Dietrich J.W. Compared to limb pain of other origin, ultrasonographic osteodensitometry reveals loss of bone density in complex regional pain syndrome. Pain. 2019;160:1261–1269. doi: 10.1097/j.pain.0000000000001520. [DOI] [PubMed] [Google Scholar]
- 107.Krämer H.H., Hofbauer L.C., Szalay G., Breimhorst M., Eberle T., Zieschang K., Rauner M., Schlereth T., Schreckenberger M., Birklein F. Osteoprotegerin: A new biomarker for impaired bone metabolism in complex regional pain syndrome? Pain. 2014;155:889–895. doi: 10.1016/j.pain.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 108.Menon D.K., Schwab K., Wright D.W., Maas A.I. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010;91:1637–1640. doi: 10.1016/j.apmr.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 109.Lu J., Marmarou A., Choi S., Maas A., Murray G., Steyerberg E.W. Mortality from traumatic brain injury. Acta Neurochir. Suppl. 2005:281–285. doi: 10.1007/3-211-32318-X_58. [DOI] [PubMed] [Google Scholar]
- 110.Blennow K., Hardy J., Zetterberg H. The Neuropathology and Neurobiology of Traumatic Brain Injury. Neuron. 2012;76:886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 111.Barshikar S., Bell K.R. Sleep Disturbance After TBI. Curr. Neurol. Neurosci. Rep. 2017;17 doi: 10.1007/s11910-017-0792-4. [DOI] [PubMed] [Google Scholar]
- 112.Werner C., Engelhard K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 113.Strbian D., Durukan A., Pitkonen M., Marinkovic I., Tatlisumak E., Pedrono E., Abo-Ramadan U., Tatlisumak T. The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neuroscience. 2008;153:175–181. doi: 10.1016/j.neuroscience.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 114.McDonald S.J., Sharkey J.M., Sun M., Kaukas L.M., Shultz S.R., Turner R.J., Leonard A.V., Brady R.D., Corrigan F. Beyond the Brain: Peripheral Interactions after Traumatic Brain Injury. J. Neurotrauma. 2020;37:770–781. doi: 10.1089/neu.2019.6885. [DOI] [PubMed] [Google Scholar]
- 115.Trentz O.A., Handschin A.E., Bestmann L., Hoerstrup S.P., Trentz O.L., Platz A. Influence of brain injury on early posttraumatic bone metabolism. Crit. Care Med. 2005;33:399–406. doi: 10.1097/01.CCM.0000152221.87477.21. [DOI] [PubMed] [Google Scholar]
- 116.Banham-Hall N., Kothwal K., Pipkin J., Bentley J., Dickens G.L. Prevalence of low bone mineral density in inpatients with traumatic brain injury receiving neurobehavioural rehabilitation: A postoperative, observational study. Physiother. 2013;99:328–334. doi: 10.1016/j.physio.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 117.Yu H., Watt H., Mohan S. The negative impact of traumatic brain injury (TBI) on bone in a mouse model. Brain Inj. 2014;28:244–251. doi: 10.3109/02699052.2013.859735. [DOI] [PubMed] [Google Scholar]
- 118.Brady R.D., Shultz S.R., Sun M., Romano T., van der Poel C., Wright D.K., Wark J.D., O’Brien T.J., Grills B.L., McDonald S.J. Experimental Traumatic Brain Injury Induces Bone Loss in Rats. J. Neurotrauma. 2016;33:2154–2160. doi: 10.1089/neu.2014.3836. [DOI] [PubMed] [Google Scholar]
- 119.Bajwa N.M., Kesavan C., Mohan S. Long-term consequences of Traumatic brain injury in bone metabolism. Front. Neurol. 2018;9 doi: 10.3389/fneur.2018.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singleton Q., Vaibhav K., Braun M., Patel C., Khayrullin A., Mendhe B., Lee B., Kolhe R., Kaiser H., Awad M., et al. Bone Marrow Derived Extracellular Vesicles Activate Osteoclast Differentiation in Traumatic Brain Injury Induced Bone Loss. Cells. 2019;8:63. doi: 10.3390/cells8010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simonsen L.L., Sonne-Holm S., Krasheninnikoff M., Engberg A.W. Symptomatic heterotopic ossification after very severe traumatic brain injury in 114 patients: Incidence and risk factors. Injury. 2007;38:1146–1150. doi: 10.1016/j.injury.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 122.Almangour W., Schnitzler A., Salga M., Debaud C., Denormandie P., Genêt F. Recurrence of heterotopic ossification after removal in patients with traumatic brain injury: A systematic review. Ann. Phys. Rehabil. Med. 2016;59:263–269. doi: 10.1016/j.rehab.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 123.Huang H., Cheng W.X., Hu Y.P., Chen J.H., Zheng Z.T., Zhang P. Relationship between heterotopic ossification and traumatic brain injury: Why severe traumatic brain injury increases the risk of heterotopic ossification. J. Orthop. Transl. 2018;12:16–25. doi: 10.1016/j.jot.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Brady R.D., Shultz S.R., McDonald S.J., O’Brien T.J. Neurological heterotopic ossification: Current understanding and future directions. Bone. 2018;109:35–42. doi: 10.1016/j.bone.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 125.Davis E.L., Davis A.R., Gugala Z., Olmsted-Davis E.A. Is heterotopic ossification getting nervous?: The role of the peripheral nervous system in heterotopic ossification. Bone. 2018;109:22–27. doi: 10.1016/j.bone.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Anthonissen J., Steffen C.T., Alessandri B., Baranowski A., Rommens P.M., Victor J., Hofmann A. Traumatic brain injury enhances the formation of heterotopic ossification around the hip: An animal model study. Arch. Orthop. Trauma Surg. 2019 doi: 10.1007/s00402-019-03326-0. [DOI] [PubMed] [Google Scholar]
- 127.Brady R.D., Zhao M.Z., Wong K.R., Casilla-Espinosa P.M., Yamakawa G.R., Wortman R.C., Sun M., Grills B.L., Mychasiuk R., O’Brien T.J., et al. A novel rat model of heterotopic ossification after polytrauma with traumatic brain injury. Bone. 2020;133 doi: 10.1016/j.bone.2020.115263. [DOI] [PubMed] [Google Scholar]
- 128.Newman R.J., Stone M.H., Mukherjee S.K. Accelerated fracture union in association with severe head injury. Injury. 1987;18:241–246. doi: 10.1016/0020-1383(87)90006-4. [DOI] [PubMed] [Google Scholar]
- 129.Perkins R., Skirving A.P. Callus formation and the rate of healing of femoral fractures in patients with head injuries. J. Bone Jt. Surg. Ser. B. 1987;69:521–524. doi: 10.1302/0301-620X.69B4.3611150. [DOI] [PubMed] [Google Scholar]
- 130.Spencer R.F. The effect of head injury on fracture healing. A quantitative assessment. J. Bone Jt. Surg. Ser. B. 1987;69:525–528. doi: 10.1302/0301-620X.69B4.3611151. [DOI] [PubMed] [Google Scholar]
- 131.Giannoudis P.V., Mushtaq S., Harwood P., Kambhampati S., Dimoutsos M., Stavrou Z., Pape H.C. Accelerated bone healing and excessive callus formation in patients with femoral fracture and head injury. Injury. 2006;37 doi: 10.1016/j.injury.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 132.Cadosch D., Gautschi O.P., Thyer M., Song S., Skirving A.P., Filgueira L., Zellweger R. Humoral factors enhance fracture-healing and callus formation in patients with traumatic brain injury. J. Bone Jt. Surg. Ser. A. 2009;91:282–288. doi: 10.2106/JBJS.G.01613. [DOI] [PubMed] [Google Scholar]
- 133.Huang W., Li Z., Li Z., Yang R. Does traumatic brain injury result in accelerated mandibular fracture healing? J. Oral Maxillofac. Surg. 2012;70:2135–2142. doi: 10.1016/j.joms.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 134.Yang T.Y., Wang T.C., Tsai Y.H., Huang K.C. The effects of an injury to the brain on bone healing and callus formation in young adults with fractures of the femoral shaft. J. Bone Jt. Surg. Ser. B. 2012;94 B:227–230. doi: 10.1302/0301-620X.94B2.28193. [DOI] [PubMed] [Google Scholar]
- 135.Claes L., Recknagel S., Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat. Rev. Rheumatol. 2012;8:133–143. doi: 10.1038/nrrheum.2012.1. [DOI] [PubMed] [Google Scholar]
- 136.Schell H., Duda G.N., Peters A., Tsitsilonis S., Johnson K.A., Schmidt-Bleek K. The haematoma and its role in bone healing. J. Exp. Orthop. 2017;4 doi: 10.1186/s40634-017-0079-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hofman M., Koopmans G., Kobbe P., Poeze M., Andruszkow H., Brink P.R.G., Pape H.C. Improved fracture healing in patients with concomitant traumatic brain injury: Proven or not? Mediators Inflamm. 2015;2015 doi: 10.1155/2015/204842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Toffoli A.M., Gautschi O.P., Frey S.P., Filgueira L., Zellweger R. From brain to bone: Evidence for the release of osteogenic humoral factors after traumatic brain injury. Brain Inj. 2008;22:511–518. doi: 10.1080/02699050802158235. [DOI] [PubMed] [Google Scholar]
- 139.Bidner S.M., Rubins I.M., Desjardins J.V., Zukor D.J., Goltzman D. Evidence for a humoral mechanism for enhanced osteogenesis after head injury. J. Bone Jt. Surg. Ser. A. 1990;72:1144–1149. doi: 10.2106/00004623-199072080-00004. [DOI] [PubMed] [Google Scholar]
- 140.Gautschi O.P., Toffoli A.M., Joesbury K.A., Skirving A.P., Filgueira L., Zellweger R. Osteoinductive effect of cerebrospinal fluid from brain-injured patients. J. Neurotrauma. 2007;24:154–162. doi: 10.1089/neu.2006.0166. [DOI] [PubMed] [Google Scholar]
- 141.Tsitsilonis S., Seemann R., Misch M., Wichlas F., Haas N.P., Schmidt-Bleek K., Kleber C., Schaser K.D. The effect of traumatic brain injury on bone healing: An experimental study in a novel in vivo animal model. Injury. 2015;46:661–665. doi: 10.1016/j.injury.2015.01.044. [DOI] [PubMed] [Google Scholar]
- 142.Locher R.J., Lünnemann T., Garbe A., Schaser K.D., Schmidt-Bleek K., Duda G., Tsitsilonis S. Traumatic brain injury and bone healing: Radiographic and biomechanical analyses of bone formation and stability in a combined murine trauma model. J. Musculoskelet. Neuronal Interact. 2015;15:309–315. [PMC free article] [PubMed] [Google Scholar]
- 143.Morley J., Marsh S., Drakoulakis E., Pape H.C., Giannoudis P.V. Does traumatic brain injury result in accelerated fracture healing? Injury. 2005;36:363–368. doi: 10.1016/j.injury.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 144.Morioka K., Marmor Y., Sacramento J.A., Lin A., Shao T., Miclau K.R., Clark D.R., Beattie M.S., Marcucio R.S., Miclau T., et al. Differential fracture response to traumatic brain injury suggests dominance of neuroinflammatory response in polytrauma. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-48126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang S.D., Dai L.Y., Jiang L.S. Osteoporosis after spinal cord injury. Osteoporos. Int. 2006;17:180–192. doi: 10.1007/s00198-005-2028-8. [DOI] [PubMed] [Google Scholar]
- 146.Dauty M., Perrouin Verbe B., Maugars Y., Dubois C., Mathe J.F. Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone. 2000;27:305–309. doi: 10.1016/S8756-3282(00)00326-4. [DOI] [PubMed] [Google Scholar]
- 147.BIERING-SØRENSEN F., BOHR H.H., SCHAADT O.P. Longitudinal study of bone mineral content in the lumbar spine, the forearm and the lower extremities after spinal cord injury. Eur. J. Clin. Investig. 1990;20:330–335. doi: 10.1111/j.1365-2362.1990.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 148.Frey-Rindova P., De Bruin E.D., Stüssi E., Dambacher M.A., Dietz V. Bone mineral density in upper and lower extremities during 12 months after spinal cord injury measured by peripheral quantitative computed tomography. Spinal Cord. 2000;38:26–32. doi: 10.1038/sj.sc.3100905. [DOI] [PubMed] [Google Scholar]
- 149.de Bruin E.D., Dietz V., Dambacher M.A., Stüssi E. Longitudinal changes in bone in men with spinal cord injury. Clin. Rehabil. 2000;14:145–152. doi: 10.1191/026921500670532165. [DOI] [PubMed] [Google Scholar]
- 150.Sullivan M.P., Torres S.J., Mehta S., Ahn J. Heterotopic ossification after central nervous system trauma. Bone Joint Res. 2013;2:51–57. doi: 10.1302/2046-3758.23.2000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Albrecht J.S., Al Kibria G., Gruber-Baldini A., Magaziner J. Risk of Mortality in Individuals with Hip Fracture and Traumatic Brain Injury. J. Am. Geriatr. Soc. 2019;67:124–127. doi: 10.1111/jgs.15661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hensler T., Sauerland S., Bouillon B., Raum M., Rixen D., Helling H.J., Andermahr J., Neugebauer E.A.M. Association between injury pattern of patients with multiple injuries and circulating levels of soluble tumor necrosis factor receptors, interleukin-6 and interleukin-10, and polymorphonuclear neutrophil elastase. J. Trauma. 2002;52:962–970. doi: 10.1097/00005373-200205000-00023. [DOI] [PubMed] [Google Scholar]
- 153.Kumar A., Loane D.J. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain. Behav. Immun. 2012;26:1191–1201. doi: 10.1016/j.bbi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 154.Kumar R.G., Diamond M.L., Boles J.A., Berger R.P., Tisherman S.A., Kochanek P.M., Wagner A.K. Acute CSF interleukin-6 trajectories after TBI: Associations with neuroinflammation, polytrauma, and outcome. Brain. Behav. Immun. 2015;45:253–262. doi: 10.1016/j.bbi.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 155.Shultz S.R., Sun M., Wright D.K., Brady R.D., Liu S., Beynon S., Schmidt S.F., Kaye A.H., Hamilton J.A., O’Brien T.J., et al. Tibial fracture exacerbates traumatic brain injury outcomes and neuroinflammation in a novel mouse model of multitrauma. J. Cereb. Blood Flow Metab. 2015;35:1339–1347. doi: 10.1038/jcbfm.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang L., Guo Y., Wen D., Yang L., Chen Y., Zhang G., Fan Z. Bone Fracture Enhances Trauma Brain Injury. Scand. J. Immunol. 2016;83:26–32. doi: 10.1111/sji.12393. [DOI] [PubMed] [Google Scholar]
- 157.Elabd C., Cousin W., Upadhyayula P., Chen R.Y., Chooljian M.S., Li J., Kung S., Jiang K.P., Conboy I.M. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat. Commun. 2014;5 doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tan D.-X., Manchester L.C., Sanchez-Barcelo E., Mediavilla M.D., Reiter R.J. Significance of High Levels of Endogenous Melatonin in Mammalian Cerebrospinal Fluid and in the Central Nervous System. Curr. Neuropharmacol. 2010;8:162–167. doi: 10.2174/157015910792246182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li T., Jiang S., Lu C., Yang W., Yang Z., Hu W., Xin Z., Yang Y. Melatonin: Another avenue for treating osteoporosis? J. Pineal Res. 2019;66:1–12. doi: 10.1111/jpi.12548. [DOI] [PubMed] [Google Scholar]
- 160.Schweiger U., Deuschle M., Körner A., Lammers C.H., Schmider J., Gotthardt U., Holsboer F., Heuser I. Low lumbar bone mineral density in patients with major depression. Am. J. Psychiatry. 1994;151:1691–1693. doi: 10.1176/ajp.151.11.1691. [DOI] [PubMed] [Google Scholar]
- 161.Ramnemark A., Nilsson M., Borssén B., Gustafson Y. Stroke, a major and increasing risk factor for femoral neck fracture. Stroke. 2000;31:1572–1577. doi: 10.1161/01.STR.31.7.1572. [DOI] [PubMed] [Google Scholar]
- 162.Pouwels S., Lalmohamed A., Leufkens B., De Boer A., Cooper C., Van Staa T., De Vries F. Risk of hip/femur fracture after stroke: A population-based case-control study. Stroke. 2009;40:3281–3285. doi: 10.1161/STROKEAHA.109.554055. [DOI] [PubMed] [Google Scholar]
- 163.Liu L., Wan W., Xia S., Kalionis B., Li Y. Dysfunctional Wnt/β-catenin signaling contributes to blood-brain barrier breakdown in Alzheimer’s disease. Neurochem. Int. 2014;75:19–25. doi: 10.1016/j.neuint.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 164.Genêt F., Jourdan C., Schnitzler A., Lautridou C., Guillemot D., Judet T., Poiraudeau S., Denormandie P. Troublesome Heterotopic ossification after central nervous system damage: A survey of 570 surgeries. PLoS ONE. 2011;6:2–8. doi: 10.1371/journal.pone.0016632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Perumal R., SP S., Shankar V., Gaddam S.R., Jayaramaraju D., Rajasekaran S. Does accelerated bone healing associated with traumatic brain injury (TBI) occur in open fractures. Int. J. Orthop. Sci. 2018;4:677–681. doi: 10.22271/ortho.2018.v4.i2j.99. [DOI] [Google Scholar]
- 166.Cipriano C.A., Pill S.G., Keenan M.A. Heterotopic ossification following traumatic brain injury and spinal cord injury. J. Am. Acad. Orthop. Surg. 2009;17:689–697. doi: 10.5435/00124635-200911000-00003. [DOI] [PubMed] [Google Scholar]
- 167.van Bezooijen R.L., Papapoulos S.E., Hamdy N.A., ten Dijke P., Löwik C.W. Control of bone formation by osteocytes? lessons from the rare skeletal disorders sclerosteosis and van Buchem disease. BoneKEy-Osteovision. 2005;2:33–38. doi: 10.1138/20050189. [DOI] [Google Scholar]
- 168.Tsai C.H., Chuang C.S., Hung C.H., Lin C.L., Sung F.C., Tang C.H., Hsu H.C., Chung C.J. Fracture as an independent risk factor of dementia: A nationwide population-based cohort study. Medicine. 2014;93:1–7. doi: 10.1097/MD.0000000000000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jodoin M., Rouleau D.M., Charlebois-Plante C., Benoit B., Leduc S., Laflamme G.Y., Gosselin N., Larson-Dupuis C., De Beaumont L. Incidence rate of mild traumatic brain injury among patients who have suffered from an isolated limb fracture: Upper limb fracture patients are more at risk. Injury. 2016;47:1835–1840. doi: 10.1016/j.injury.2016.05.036. [DOI] [PubMed] [Google Scholar]
- 170.Zaidi M., Yuen T., Sun L., Rosen C.J. Regulation of skeletal homeostasis. Endocr. Rev. 2018;39:701–718. doi: 10.1210/er.2018-00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zaidi M., Lizneva D., Kim S.M., Sun L., Iqbal J., New M.I., Rosen C.J., Yuen T. FSH, Bone Mass, Body Fat, and Biological Aging. Endocrinology. 2018;159:3503–3514. doi: 10.1210/en.2018-00601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Lizneva D., Rahimova A., Kim S.M., Atabiekov I., Javaid S., Alamoush B., Taneja C., Khan A., Sun L., Azziz R., et al. FSH beyond fertility. Front. Endocrinol. 2019;10:1–10. doi: 10.3389/fendo.2019.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Coss D. Commentary on the Recent FSH Collection: Known Knowns and Known Unknowns. Endocrinology. 2020;161:1–6. doi: 10.1210/endocr/bqz035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Seibel M.J., Dunstan C.R., Zhou H., Allan C.M., Handelsman D.J. Sex Steroids, Not FSH, Influence Bone Mass. Cell. 2006;127:1079. doi: 10.1016/j.cell.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 175.Prior J.C. FSH and bone—Important physiology or not? Trends Mol. Med. 2007;13:1–3. doi: 10.1016/j.molmed.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 176.García-Martín A., Reyes-García R., García-Castro J.M., Rozas-Moreno P., Escobar-Jiménez F., Muñoz-Torres M. Role of serum FSH measurement on bone resorption in postmenopausal women. Endocrine. 2012;41:302–308. doi: 10.1007/s12020-011-9541-7. [DOI] [PubMed] [Google Scholar]
- 177.Sun L., Peng Y., Sharrow A.C., Iqbal J., Zhang Z., Papachristou D.J., Zaidi S., Zhu L.L., Yaroslavskiy B.B., Zhou H., et al. FSH Directly Regulates Bone Mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 178.Iqbal J., Blair H.C., Zallone A., Sun L., Zaidi M. Further evidence that FSH causes bone loss independently of low estrogen. Endocrine. 2012;41:171–175. doi: 10.1007/s12020-012-9626-y. [DOI] [PubMed] [Google Scholar]
- 179.Zaidi S., Zhu L.L., Mali R., Iqbal J., Yang G., Zaidi M., Sun L. Regulation of FSH receptor promoter activation in the osteoclast. Biochem. Biophys. Res. Commun. 2007;361:910–915. doi: 10.1016/j.bbrc.2007.07.081. [DOI] [PubMed] [Google Scholar]
- 180.Ji Y., Liu P., Yuen T., Haider S., He J., Romero R., Chen H., Bloch M., Kim S.M., Lizneva D., et al. Epitope-specific monoclonal antibodies to FSHβ increase bone mass. Proc. Natl. Acad. Sci. USA. 2018;115:2192–2197. doi: 10.1073/pnas.1718144115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Abe E., Marians R.C., Yu W., Wu X.B., Ando T., Li Y., Iqbal J., Eldeiry L., Rajendren G., Blair H.C., et al. TSH is a negative regulator of skeletal remodeling. Cell. 2003;115:151–162. doi: 10.1016/S0092-8674(03)00771-2. [DOI] [PubMed] [Google Scholar]
- 182.Ma R., Morshed S., Latif R., Zaidi M., Davies T.F. The influence of thyroid-stimulating hormone and thyroid-stimulating hormone receptor antibodies on osteoclastogenesis. Thyroid. 2011;21:897–906. doi: 10.1089/thy.2010.0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Hase H., Ando T., Eldeiry L., Brebene A., Peng Y., Liu L., Amano H., Davies T.F., Sun L., Zaidi M., et al. TNFα mediates the skeletal effects of thyroid-stimulating hormone. Proc. Natl. Acad. Sci. USA. 2006;103:12849–12854. doi: 10.1073/pnas.0600427103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sampath T.K., Simic P., Sendak R., Draca N., Bowe A.E., O’Brien S., Schiavi S.C., McPherson J.M., Vukicevic S. Thyroid-stimulating hormone restores bone volume, microarchitecture, and strength in aged ovariectomized rats. J. Bone Miner. Res. 2007;22:849–859. doi: 10.1359/jbmr.070302. [DOI] [PubMed] [Google Scholar]
- 185.Baliram R., Latif R., Berkowitz J., Frid S., Colaianni G., Sun L., Zaidi M., Davies T.F. Thyroid-stimulating hormone induces a Wnt-dependent, feed-forward loop for osteoblastogenesis in embryonic stem cell cultures. Proc. Natl. Acad. Sci. USA. 2011;108:16277–16282. doi: 10.1073/pnas.1110286108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bernard V., Young J., Binart N. Prolactin—A pleiotropic factor in health and disease. Nat. Rev. Endocrinol. 2019;15 doi: 10.1038/s41574-019-0194-6. [DOI] [PubMed] [Google Scholar]
- 187.Clément-Lacroix P., Ormandy C., Lepescheux L., Ammann P., Damotte D., Goffin V., Bouchard B., Amling M., Gaillard-Kelly M., Binart N., et al. Osteoblasts are a new target for prolactin: Analysis of bone formation in prolactin receptor knockout mice. Endocrinology. 1999;140:96–105. doi: 10.1210/endo.140.1.6436. [DOI] [PubMed] [Google Scholar]
- 188.Chiloiro S., Giampietro A., Bianchi A., De Marinis L. Prolactinoma and Bone. Curr. Opin. Endocr. Metab. Res. 2018;3:21–24. doi: 10.1016/j.coemr.2018.02.006. [DOI] [Google Scholar]
- 189.Coss D., Yang L., Kuo C.B., Xu X., Luben R.A., Walker A.M. Effects of prolactin on osteoblast alkaline phosphatase and bone formation in the developing rat. Am. J. Physiol. Endocrinol. Metab. 2000;279:1216–1225. doi: 10.1152/ajpendo.2000.279.6.E1216. [DOI] [PubMed] [Google Scholar]
- 190.Seriwatanachai D., Thongchote K., Charoenphandhu N., Pandaranandaka J., Tudpor K., Teerapornpuntakit J., Suthiphongchai T., Krishnamra N. Prolactin directly enhances bone turnover by raising osteoblast-expressed receptor activator of nuclear factor κB ligand/osteoprotegerin ratio. Bone. 2008;42:535–546. doi: 10.1016/j.bone.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 191.Seriwatanachai D., Charoenphandhu N., Suthiphongchai T., Krishnamra N. Prolactin decreases the expression ratio of receptor activator of nuclear factor κB ligand/osteoprotegerin in human fetal osteoblast cells. Cell Biol. Int. 2008;32:1126–1135. doi: 10.1016/j.cellbi.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 192.Seriwatanachai D., Krishnamra N., Van Leeuwen J.P.T.M. Evidence for direct effects of prolactin on human osteoblasts: Inhibition of cell growth and mineralization. J. Cell. Biochem. 2009;107:677–685. doi: 10.1002/jcb.22161. [DOI] [PubMed] [Google Scholar]
- 193.Krishnamra N., Seemoung J. Effects of acute and long-term administration of prolactin on bone 45Ca uptake, calcium deposit, and calcium resorption in weaned, young, and mature rats. Can. J. Physiol. Pharmacol. 1996;74:1157–1165. doi: 10.1139/y96-123. [DOI] [PubMed] [Google Scholar]
- 194.Zaidi M., Sun L., Liu P., Davies T.F., New M., Zallone A., Yuen T. Pituitary-bone connection in skeletal regulation. Horm. Mol. Biol. Clin. Investig. 2016;28:85–94. doi: 10.1515/hmbci-2016-0015. [DOI] [PubMed] [Google Scholar]
- 195.Isales C.M., Zaidi M., Blair H.C. ACTH is a novel regulator of bone mass. Ann. N. Y. Acad. Sci. 2010;1192:110–116. doi: 10.1111/j.1749-6632.2009.05231.x. [DOI] [PubMed] [Google Scholar]
- 196.Weinstein R.S. Glucocorticoid-induced osteonecrosis. Endocrine. 2012;41:183–190. doi: 10.1007/s12020-011-9580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pállinger É., Csaba G. A hormone map of human immune cells showing the presence of adrenocorticotropic hormone, triiodothyronine and endorphin in immunophenotyped white blood cells. Immunology. 2008;123:584–589. doi: 10.1111/j.1365-2567.2007.02731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ohlsson C., Bengtsson B.-Å., Isaksson O.G.P., Andreassen T.T., Slootweg M.C. Growth Hormone and Bone. Endocr. Rev. 1998;19:55–79. doi: 10.1210/edrv.19.1.0324. [DOI] [PubMed] [Google Scholar]
- 199.Giustina A., Mazziotti G., Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tamma R., Sun L., Cuscito C., Lu P., Corcelli M., Li J., Colaianni G., Moonga S.S., Di Benedetto A., Grano M., et al. Regulation of bone remodeling by vasopressin explains the bone loss in hyponatremia. Proc. Natl. Acad. Sci. USA. 2013;110:18644–18649. doi: 10.1073/pnas.1318257110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mavani G.P., DeVita M.V., Michelis M.F. A review of the nonpressor and nonantidiuretic actions of the hormone vasopressin. Front. Med. 2015;2:19. doi: 10.3389/fmed.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Arrowsmith S., Wray S. Oxytocin: Its mechanism of action and receptor signalling in the myometrium. J. Neuroendocrinol. 2014;26:356–369. doi: 10.1111/jne.12154. [DOI] [PubMed] [Google Scholar]
- 203.Sun L., Tamma R., Yuen T., Colaianni G., Ji Y., Cuscito C., Bailey J., Dhawan S., Lu P., Calvano C.D., et al. Functions of vasopressin and oxytocin in bone mass regulation. Proc. Natl. Acad. Sci. USA. 2016;113:164–169. doi: 10.1073/pnas.1523762113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Colaianni G., Sun L., Zaidi M., Zallone A. Oxytocin and bone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R970–R977. doi: 10.1152/ajpregu.00040.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tamma R., Colaianni G., Zhu L.L., DiBenedetto A., Greco G., Montemurro G., Patano N., Strippoli M., Vergari R., Mancini L., et al. Oxytocin is an anabolic bone hormone. Proc. Natl. Acad. Sci. USA. 2009;106:7149–7154. doi: 10.1073/pnas.0901890106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Copland J.A., Ives K.L., Simmons D.J., Soloff M.S. Functional oxytocin receptors discovered in human osteoblasts. Endocrinology. 1999;140:4371–4374. doi: 10.1210/endo.140.9.7130. [DOI] [PubMed] [Google Scholar]
- 207.Colucci S., Colaianni G., Mori G., Grano M., Zallone A. Human osteoclasts express oxytocin receptor. Biochem. Biophys. Res. Commun. 2002;297:442–445. doi: 10.1016/S0006-291X(02)02009-0. [DOI] [PubMed] [Google Scholar]
- 208.Colaianni G., Sun L., Di Benedetto A., Tamma R., Zhu L.L., Cao J., Grano M., Yuen T., Colucci S., Cuscito C., et al. Bone marrow oxytocin mediates the anabolic action of estrogen on the skeleton. J. Biol. Chem. 2012;287:29159–29167. doi: 10.1074/jbc.M112.365049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Leston J., Harthé C., Brun J., Mottolese C., Mertens P., Sindou M., Claustrat B. Melatonin is released in the third ventricle in humans. A study in movement disorders. Neurosci. Lett. 2010;469:294–297. doi: 10.1016/j.neulet.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 210.Slominski R.M., Reiter R.J., Schlabritz-Loutsevitch N., Ostrom R.S., Slominski A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Roth J.A., Kim B.G., Lin W.L., Cho M. Il Melatonin promotes osteoblast differentiation and bone formation. J. Biol. Chem. 1999;274:22041–22047. doi: 10.1074/jbc.274.31.22041. [DOI] [PubMed] [Google Scholar]
- 212.Nakade O., Koyama H., Ariji H., Yajima A., Kaku T. Melatonin stimulates proliferation and type I collagen synthesis in human bone cells in vitro. J. Pineal Res. 1999;27:106–110. doi: 10.1111/j.1600-079X.1999.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 213.Zhang L., Su P., Xu C., Chen C., Liang A., Du K., Peng Y., Huang D. Melatonin inhibits adipogenesis and enhances osteogenesis of human mesenchymal stem cells by suppressing PPARγ expression and enhancing Runx2 expression. J. Pineal Res. 2010;49:364–372. doi: 10.1111/j.1600-079X.2010.00803.x. [DOI] [PubMed] [Google Scholar]
- 214.Amstrup A.K., Sikjaer T., Heickendorff L., Mosekilde L., Rejnmark L. Melatonin improves bone mineral density at the femoral neck in postmenopausal women with osteopenia: A randomized controlled trial. J. Pineal Res. 2015;59:221–229. doi: 10.1111/jpi.12252. [DOI] [PubMed] [Google Scholar]
- 215.Sanchez-Barcelo E.J., Mediavilla M.D., Tan D.X., Reiter R.J. Clinical Uses of Melatonin: Evaluation of Human Trials. Curr. Med. Chem. 2010;17:2070–2095. doi: 10.2174/092986710791233689. [DOI] [PubMed] [Google Scholar]
- 216.Koch B.C.P., Nagtegaal J.E., Hagen E.C., Van Der Westerlaken M.M.L., Boringa J.B.S., Kerkhof G.A., Ter Wee P.M. The effects of melatonin on sleep-wake rhythm of daytime haemodialysis patients: A randomized, placebo-controlled, cross-over study (EMSCAP study) Br. J. Clin. Pharmacol. 2009;67:68–75. doi: 10.1111/j.1365-2125.2008.03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Driessler F., Baldock P.A. Hypothalamic regulation of bone. J. Mol. Endocrinol. 2010;45:175–181. doi: 10.1677/JME-10-0015. [DOI] [PubMed] [Google Scholar]
- 218.Gehlert D.R. Role of hypothalamic neuropeptide Y in feeding and obesity. Neuropeptides. 1999;33:329–338. doi: 10.1054/npep.1999.0057. [DOI] [PubMed] [Google Scholar]
- 219.Teixeira L., Sousa D.M., Nunes A.F., Sousa M.M., Herzog H., Lamghari M. NPY revealed as a critical modulator of osteoblast function in vitro: New insights into the role of Y1 and Y2 receptors. J. Cell. Biochem. 2009;107:908–916. doi: 10.1002/jcb.22194. [DOI] [PubMed] [Google Scholar]
- 220.Baldock P.A., Allison S.J., Lundberg P., Lee N.J., Slack K., Lin E.J.D., Enriquez R.F., McDonald M.M., Zhang L., During M.J., et al. Novel role of Y1 receptors in the coordinated regulation of bone and energy homeostasis. J. Biol. Chem. 2007;282:19092–19102. doi: 10.1074/jbc.M700644200. [DOI] [PubMed] [Google Scholar]
- 221.Elefteriou F. Impact of the autonomic nervous system on the skeleton. Physiol. Rev. 2018;98:1083–1112. doi: 10.1152/physrev.00014.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Horsnell H., Baldock P.A. Osteoblastic Actions of the Neuropeptide Y System to Regulate Bone and Energy Homeostasis. Curr. Osteoporos. Rep. 2016;14:26–31. doi: 10.1007/s11914-016-0300-9. [DOI] [PubMed] [Google Scholar]
- 223.Zhang W., Cline M.A., Gilbert E.R. Hypothalamus-adipose tissue crosstalk: Neuropeptide y and the regulation of energy metabolism. Nutr. Metab. 2014;11:1–12. doi: 10.1186/1743-7075-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Baldock P., Lin S., Zhang L., Karl T., Shi Y., Driessler F., Zengin A., Hörmer B., Lee N., Wong I., et al. Neuropeptide Y Attenuates Stress-Induced Bone Loss Through Suppression of Noradrenaline Circuits. J. Bone Miner. Res. 2014;29:2238–2249. doi: 10.1002/jbmr.2205. [DOI] [PubMed] [Google Scholar]
- 225.Ilnytska O., Argyropoulos G. The role of the Agouti-related protein in energy balance regulation. Cell. Mol. Life Sci. 2008;65:2721–2731. doi: 10.1007/s00018-008-8104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Deng J., Yuan F., Guo Y., Xiao Y., Niu Y., Deng Y., Han X., Guan Y., Chen S., Guo F. Deletion of ATF4 in AgRP neurons promotes fat loss mainly via increasing energy expenditure. Diabetes. 2017;66:640–650. doi: 10.2337/db16-0954. [DOI] [PubMed] [Google Scholar]
- 227.Kim J.G., Sun B.H., Dietrich M.O., Koch M., Yao G.Q., Diano S., Insogna K., Horvath T.L. AgRP Neurons Regulate Bone Mass. Cell Rep. 2015;13:8–14. doi: 10.1016/j.celrep.2015.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Swart I., Jahng J.W., Overton J.M., Houpt T.A. Hypothalamic NPY, AGRP, and POMC mRNA responses to leptin and refeeding in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:1020–1026. doi: 10.1152/ajpregu.00501.2001. [DOI] [PubMed] [Google Scholar]
- 229.Kuperman Y., Weiss M., Dine J., Staikin K., Golani O., Ramot A., Nahum T., Kühne C., Shemesh Y., Wurst W., et al. CRFR1 in AgRP Neurons Modulates Sympathetic Nervous System Activity to Adapt to Cold Stress and Fasting. Cell Metab. 2016;23:1185–1199. doi: 10.1016/j.cmet.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Shi Z., Madden C.J., Brooks V.L. Arcuate neuropeptide y inhibits sympathetic nerve activity via multiple neuropathways. J. Clin. Investig. 2017;127:2868–2880. doi: 10.1172/JCI92008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Morton G.J., Cummings D.E., Baskin D.G., Barsh G.S., Schwartz M.W. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 232.Maletínská L., Maixnerová J., Matyšková R., Haugvicová R., Pirník Z., Kiss A., Železná B. Synergistic effect of CART (cocaine- and amphetamine-regulated transcript) peptide and cholecystokinin on food intake regulation in lean mice. BMC Neurosci. 2008;9 doi: 10.1186/1471-2202-9-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Elefteriou F., Ahn J.D., Takeda S., Starbuck M., Yang X., Liu X., Kondo H., Richards W.G., Bannon T.W., Noda M., et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- 234.Singh M.K., Elefteriou F., Karsenty G. Cocaine and amphetamine-regulated transcript may regulate bone remodeling as a circulating molecule. Endocrinology. 2008;149:3933–3941. doi: 10.1210/en.2008-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Yamamoto H., Yamane T., Iguchi K., Tanaka K., Iddamalgoda A., Unno K., Hoshino M., Takeda A. Melanin production through novel processing of proopiomelanocortin in the extracellular compartment of the auricular skin of C57BL/6 mice after UV-irradiation. Sci. Rep. 2015;5:1–11. doi: 10.1038/srep14579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Böhm M., Grässel S. Role of proopiomelanocortin-derived peptides and their receptors in the osteoarticular system: From basic to translational research. Endocr. Rev. 2012;33:623–651. doi: 10.1210/er.2011-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Cornish J., Callon K.E., Mountjoy K.G., Bava U., Lin J.M., Myers D.E., Naot D., Reid I.R. A-Melanocyte-Stimulating Hormone Is a Novel Regulator of Bone. Am. J. Physiol. Endocrinol. Metab. 2003;284 doi: 10.1152/ajpendo.00412.2002. [DOI] [PubMed] [Google Scholar]
- 238.Farooqi I.S., Yeo G.S.H., Keogh J.M., Aminian S., Jebb S.A., Butler G., Cheetham T., O’Rahilly S. Dominant and recessive inheritance of morbid obesity associate with melanocortin 4 receptor deficiency. J. Clin. Investig. 2000;106:271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Garg G., Kumar J., McGuigan F.E., Ridderstråle M., Gerdhem P., Luthman H., Åkesson K. Variation in the MC4R gene is associated with bone phenotypes in elderly Swedish women. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0088565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Grässel S., Muschter D. Do neuroendocrine peptides and their receptors qualify as novel therapeutic targets in osteoarthritis? Int. J. Mol. Sci. 2018;19:367. doi: 10.3390/ijms19020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sudo Y., Ezura Y., Kajita M., Yoshida H., Suzuki T., Hosoi T., Inoue S., Shiraki M., Ito H., Emi M. Association of single nucleotide polymorphisms in the promoter region of the pro-opiomelanocortin gene (POMC) with low bone mineral density in adult women. J. Hum. Genet. 2005;50:235–240. doi: 10.1007/s10038-005-0244-x. [DOI] [PubMed] [Google Scholar]
- 242.Farman H.H., Windahl S.H., Westberg L., Isaksson H., Egecioglu E., Schele E., Ryberg H., Jansson J.O., Tuukkanen J., Koskela A., et al. Female mice lacking estrogen receptor-α in hypothalamic proopiomelanocortin (POMC) neurons display enhanced estrogenic response on cortical bone mass. Endocrinology. 2016;157:3242–3252. doi: 10.1210/en.2016-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fujii R., Hosoya M., Fukusumi S., Kawamata Y., Habata Y., Hinuma S., Onda H., Nishimura O., Fujino M. Identification of neuromedin U as the cognate ligand of the orphan G protein-coupled receptor FM-3. J. Biol. Chem. 2000;275:21068–21074. doi: 10.1074/jbc.M001546200. [DOI] [PubMed] [Google Scholar]
- 244.Brighton P.J., Szekeres P.G., Willars G.B. Neuromedin U and its receptors: Structure, function, and physiological roles. Pharmacol. Rev. 2004;56:231–248. doi: 10.1124/pr.56.2.3. [DOI] [PubMed] [Google Scholar]
- 245.Sato S., Hanada R., Kimura A., Abe T., Matsumoto T., Iwasaki M., Inose H., Ida T., Mieda M., Takeuchi Y., et al. Central control of bone remodeling by neuromedin U. Nat. Med. 2007;13:1234–1240. doi: 10.1038/nm1640. [DOI] [PubMed] [Google Scholar]
- 246.Hanada T., Date Y., Shimbara T., Sakihara S., Murakami N., Hayashi Y., Kanai Y., Suda T., Kangawa K., Nakazato M. Central actions of neuromedin U via corticotropin-releasing hormone. Biochem. Biophys. Res. Commun. 2003;311:954–958. doi: 10.1016/j.bbrc.2003.10.098. [DOI] [PubMed] [Google Scholar]
- 247.Wren A.M., Small C.J., Abbott C.R., Jethwa P.H., Kennedy A.R., Murphy K.G., Stanley S.A., Zollner A.N., Ghatei M.A., Bloom S.R. Hypothalamic actions of neuromedin U. Endocrinology. 2002;143:4227–4234. doi: 10.1210/en.2002-220308. [DOI] [PubMed] [Google Scholar]
- 248.Kaisho T., Nagai H., Asakawa T., Suzuki N., Fujita H., Matsumiya K., Nishizawa N., Kanematsu-Yamaki Y., Dote K., Sakamoto J.I., et al. Effects of peripheral administration of a Neuromedin U receptor 2-selective agonist on food intake and body weight in obese mice. Int. J. Obes. 2017;41:1790–1797. doi: 10.1038/ijo.2017.176. [DOI] [PubMed] [Google Scholar]
- 249.Sampson C.M., Kasper J.M., Felsing D.E., Raval S.R., Ye N., Wang P., Patrikeev I., Rytting E., Zhou J., Allen J.A., et al. Small-Molecule Neuromedin U Receptor 2 Agonists Suppress Food Intake and Decrease Visceral Fat in Animal Models. Pharmacol. Res. Perspect. 2018;6:1–11. doi: 10.1002/prp2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jones K.B., Mollano A.V., Morcuende J.A., Cooper R.R., Saltzman C.L. Bone and brain: A review of neural, hormonal, and musculoskeletal connections. Iowa Orthop. J. 2004;24:123–132. [PMC free article] [PubMed] [Google Scholar]
- 251.Hohmann E., Elde R., Rysavy J., Einzig S., Gebhard R. Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science. 1986;232:868–871. doi: 10.1126/science.3518059. [DOI] [PubMed] [Google Scholar]
- 252.Bjurholm A., Kreicbergs A., Terenius L., Goldstein M., Schultzberg M. Neuropeptide Y-, tyrosine hydroxylase- and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J. Auton. Nerv. Syst. 1988;25:119–125. doi: 10.1016/0165-1838(88)90016-1. [DOI] [PubMed] [Google Scholar]
- 253.Hill E.L., Elde R. Distribution of CGRP-, VIP-, DβH-, SP-, and NPY-immunoreactive nerves in the periosteum of the rat. Cell Tissue Res. 1991;264:469–480. doi: 10.1007/BF00319037. [DOI] [PubMed] [Google Scholar]
- 254.Togari A., Arai M., Mizutani S., Mizutani S., Koshihara Y., Nagatsu T. Expression of mRNAs for neuropeptide receptors and β-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci. Lett. 1997;233:125–128. doi: 10.1016/S0304-3940(97)00649-6. [DOI] [PubMed] [Google Scholar]
- 255.Ransjö M., Lie A., Mukohyama H., Lundberg P., Lerner U.H. Microisolated mouse osteoclasts express VIP-1 and PACAP receptors. Biochem. Biophys. Res. Commun. 2000;274:400–404. doi: 10.1006/bbrc.2000.3151. [DOI] [PubMed] [Google Scholar]
- 256.Mukohyama H., Ransjö M., Taniguchi H., Ohyama T., Lerner U.H. The inhibitory effects of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide on osteoclast formation are associated with upregulation of osteoprotegerin and downregulation of RANKL and RANK. Biochem. Biophys. Res. Commun. 2000;271:158–163. doi: 10.1006/bbrc.2000.2599. [DOI] [PubMed] [Google Scholar]
- 257.Juarranz Y., Abad C., Martinez C., Arranz A., Gutierrez-Cañas I., Rosignoli F., Gomariz R.P., Leceta J. Protective effect of vasoactive intestinal peptide on bone destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res. Ther. 2005;7:R1034. doi: 10.1186/ar1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.de Vernejoul M.-C., Collet C., Chabbi-Achengli Y. Serotonin: Good or bad for bone. Bonekey Rep. 2012;1 doi: 10.1038/bonekey.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yadav V.K., Ryu J.H., Suda N., Tanaka K.F., Gingrich J.A., Schütz G., Glorieux F.H., Chiang C.Y., Zajac J.D., Insogna K.L., et al. Lrp5 Controls Bone Formation by Inhibiting Serotonin Synthesis in the Duodenum. Cell. 2008;135:825–837. doi: 10.1016/j.cell.2008.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Park K.R., Kim E.C., Hong J.T., Yun H.M. Dysregulation of 5-hydroxytryptamine 6 receptor accelerates maturation of bone-resorbing osteoclasts and induces bone loss. Theranostics. 2018;8:3087–3098. doi: 10.7150/thno.24426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Cui Y., Niziolek P.J., MacDonald B.T., Zylstra C.R., Alenina N., Robinson D.R., Zhong Z., Matthes S., Jacobsen C.M., Conlon R.A., et al. Lrp5 functions in bone to regulate bone mass. Nat. Med. 2011;17:684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Ducy P., Karsenty G. The two faces of serotonin in bone biology. J. Cell Biol. 2010;191:7–13. doi: 10.1083/jcb.201006123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sheu Y.H., Lanteigne A., Stürmer T., Pate V., Azrael D., Miller M. SSRI use and risk of fractures among perimenopausal women without mental disorders. Inj. Prev. 2015;21:397–403. doi: 10.1136/injuryprev-2014-041483. [DOI] [PubMed] [Google Scholar]
- 264.Feuer A.J., Demmer R.T., Thai A., Vogiatzi M.G. Use of selective serotonin reuptake inhibitors and bone mass in adolescents: An NHANES study. Bone. 2015;78:28–33. doi: 10.1016/j.bone.2015.04.042. [DOI] [PubMed] [Google Scholar]
- 265.Rauma P.H., Honkanen R.J., Williams L.J., Tuppurainen M.T., Kröger H.P., Koivumaa-Honkanen H. Effects of antidepressants on postmenopausal bone loss—A 5-year longitudinal study from the OSTPRE cohort. Bone. 2016;89:25–31. doi: 10.1016/j.bone.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 266.Wang C.X., Ge X.Y., Wang M.Y., Ma T., Zhang Y., Lin Y. Dopamine D1 receptor-mediated activation of the ERK signaling pathway is involved in the osteogenic differentiation of bone mesenchymal stem cells. Stem Cell Res. Ther. 2020;11:12. doi: 10.1186/s13287-019-1529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Cheong P., Ma T., Zheng Y., Ge X., Zhang Y., Lin Y. Dopamine receptor expression on primary osteoblasts and bone marrow mesenchymal stem cells of rats. Int. J. Clin. Exp. Med. 2018;11:1765–1771. [Google Scholar]
- 268.Lee D.J., Tseng H.C., Wong S.W., Wang Z., Deng M., Ko C.C. Dopaminergic effects on in vitro osteogenesis. Bone Res. 2015;3 doi: 10.1038/boneres.2015.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Hanami K., Nakano K., Saito K., Okada Y., Yamaoka K., Kubo S., Kondo M., Tanaka Y. Dopamine D2-like receptor signaling suppresses human osteoclastogenesis. Bone. 2013;56:1–8. doi: 10.1016/j.bone.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 270.Motyl K.J., Beauchemin M., Barlow D., Le P.T., Nagano K., Treyball A., Contractor A., Baron R., Rosen C.J., Houseknecht K.L. A novel role for dopamine signaling in the pathogenesis of bone loss from the atypical antipsychotic drug risperidone in female mice. Bone. 2017;103:168–176. doi: 10.1016/j.bone.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Chen C.Y., Lane H.Y., Lin C.H. Effects of Antipsychotics on Bone Mineral Density in Patients with Schizophrenia: Gender Differences. Clin. Psychopharmacol. Neurosci. 2016;14:238–249. doi: 10.9758/cpn.2016.14.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Gu Y., Genever P.G., Skerry T.M., Publicover S.J. The NMDA type glutamate receptors expressed by primary rat osteoblasts have the same electrophysiological characteristics as neuronal receptors. Calcif. Tissue Int. 2002;70:194–203. doi: 10.1007/s00223-001-2004-z. [DOI] [PubMed] [Google Scholar]
- 273.Chenu C., Serre C.M., Raynal C., Burt-Pichat B., Delmas P.D. Glutamate receptors are expressed by bone cells and are involved in bone resorption. Bone. 1998;22:295–299. doi: 10.1016/S8756-3282(97)00295-0. [DOI] [PubMed] [Google Scholar]
- 274.Merle B., Itzstein C., Delmas P.D., Chenu C. NMDA glutamate receptors are expressed by osteoclast precursors and involved in the regulation of osteoclastogenesis. J. Cell. Biochem. 2003;90:424–436. doi: 10.1002/jcb.10625. [DOI] [PubMed] [Google Scholar]
- 275.Morimoto R., Uehara S., Yatsushiro S., Juge N., Hua Z., Senoh S., Echigo N., Hayashi M., Mizoguchi T., Ninomiya T., et al. Secretion of L-glutamate from osteoclasts through transcytosis. EMBO J. 2006;25:4175–4186. doi: 10.1038/sj.emboj.7601317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Taylor A.F. Osteoblastic glutamate receptor function regulates bone formation and resorption. J. Musculoskelet. Neuronal Interact. 2002;2:285–290. [PubMed] [Google Scholar]
- 277.Serre C.M., Farlay D., Delmas P.D., Chenu C. Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone. 1999;25:623–629. doi: 10.1016/S8756-3282(99)00215-X. [DOI] [PubMed] [Google Scholar]
- 278.Bhangu P.S., Genever P.G., Spencer G.J., Grewal T.S., Skerry T.M. Evidence for targeted vesicular glutamate exocytosis in osteoblasts. Bone. 2001;29:16–23. doi: 10.1016/S8756-3282(01)00482-3. [DOI] [PubMed] [Google Scholar]
- 279.Olkku A., Mahonen A. Wnt and steroid pathways control glutamate signalling by regulating glutamine synthetase activity in osteoblastic cells. Bone. 2008;43:483–493. doi: 10.1016/j.bone.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 280.Idris A.I., Ralston S.H. Role of cannabinoids in the regulation of bone remodeling. Front. Endocrinol. 2012;3:1–8. doi: 10.3389/fendo.2012.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Reppert S.M., Weaver D.R. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- 282.Kalsbeek A., Palm I.F., La Fleur S.E., Scheer F.A.J.L., Perreau-Lenz S., Ruiter M., Kreier F., Cailotto C., Buijs R.M. SCN outputs and the hypothalamic balance of life. J. Biol. Rhythms. 2006;21:458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 283.Bartness T.J., Song C.K., Demas G.E. SCN efferents to peripheral tissues: Implications for biological rhythms. J. Biol. Rhythms. 2001;16:196–204. doi: 10.1177/074873040101600302. [DOI] [PubMed] [Google Scholar]
- 284.Buijs R.M., Escobar C., Swaab D.F. The circadian system and the balance of the autonomic nervous system. Handb. Clin. Neurol. 2013;117:173–191. doi: 10.1016/B978-0-444-53491-0.00015-8. [DOI] [PubMed] [Google Scholar]
- 285.Buijs F.N., León-Mercado L., Guzmán-Ruiz M., Guerrero-Vargas N.N., Romo-Nava F., Buijs R.M. The circadian system: A regulatory feedback network of periphery and brain. Physiology. 2016;31:170–181. doi: 10.1152/physiol.00037.2015. [DOI] [PubMed] [Google Scholar]
- 286.Takarada T., Xu C., Ochi H., Nakazato R., Yamada D., Nakamura S., Kodama A., Shimba S., Mieda M., Fukasawa K., et al. Bone Resorption Is Regulated by Circadian Clock in Osteoblasts. J. Bone Miner. Res. 2017;32:872–881. doi: 10.1002/jbmr.3053. [DOI] [PubMed] [Google Scholar]
- 287.Russell J.E., Walker W.V., Fenster R.J., Simmons D.J. In Vitro Evaluation of Circadian Patterns of Bone Collagen Formation. Proc. Soc. Exp. Biol. Med. 1985;180:375–381. doi: 10.3181/00379727-180-42192. [DOI] [PubMed] [Google Scholar]
- 288.McElderry J.D.P., Zhao G., Khmaladze A., Wilson C.G., Franceschi R.T., Morris M.D. Tracking circadian rhythms of bone mineral deposition in murine calvarial organ cultures. J. Bone Miner. Res. 2013;28:1846–1854. doi: 10.1002/jbmr.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Xu C., Ochi H., Fukuda T., Sato S., Sunamura S., Takarada T., Hinoi E., Okawa A., Takeda S. Circadian Clock Regulates Bone Resorption in Mice. J. Bone Miner. Res. 2016;31:1344–1355. doi: 10.1002/jbmr.2803. [DOI] [PubMed] [Google Scholar]
- 290.Kawai M., Kinoshita S., Shimba S., Ozono K., Michigami T. Sympathetic activation induces skeletal Fgf23 expression in a circadian rhythm-dependent manner. J. Biol. Chem. 2014;289:1457–1466. doi: 10.1074/jbc.M113.500850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Boucher H., Vanneaux V., Domet T., Parouchev A., Larghero J. Circadian clock genes modulate human bone marrow mesenchymal stem cell differentiation, migration and cell cycle. PLoS ONE. 2016;11:1–16. doi: 10.1371/journal.pone.0146674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Herkenham M., Lynn A.B., Little M.D., Johnson M.R., Melvin L.S., De Costa B.R., Rice K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ishac E.J.N., Jiang L., Lake K.D., Varga K., Abood M.E., Kunos G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996;118:2023–2028. doi: 10.1111/j.1476-5381.1996.tb15639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Munro S., Thomas K.L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 295.Howlett A.C. The cannabinoid receptors. Prostaglandins Other Lipid Mediat. 2002;68–69:619–631. doi: 10.1016/S0090-6980(02)00060-6. [DOI] [PubMed] [Google Scholar]
- 296.Pertwee R.G., Ross R.A. Cannabinoid receptors and their ligands. Prostaglandins Leukot. Essent. Fat. Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- 297.Mackie K. Signaling via CNS cannabinoid receptors. Mol. Cell. Endocrinol. 2008;286 doi: 10.1016/j.mce.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Bab I., Zimmer A., Melamed E. Cannabinoids and the skeleton: From marijuana to reversal of bone loss. Ann. Med. 2009;41:560–567. doi: 10.1080/07853890903121025. [DOI] [PubMed] [Google Scholar]
- 299.Karsak M., Malkin I., Tolia M.R., Kubisch C., Nürnberg P., Zimmer A., Livshits G. The cannabinoid receptor type 2 (CNR2) gene is associated with hand bone strength phenotypes in an ethnically homogeneous family sample. Hum. Genet. 2009;126:629–636. doi: 10.1007/s00439-009-0708-8. [DOI] [PubMed] [Google Scholar]
- 300.Tam J., Trembovler V., Di Marzo V., Petrosino S., Leo G., Alexandrovich A., Regev E., Casap N., Shteyer A., Ledent C., et al. The cannabinoid CB1 receptor regulates bone formation by modulating adrenergic signaling. FASEB J. 2008;22:285–294. doi: 10.1096/fj.06-7957com. [DOI] [PubMed] [Google Scholar]
- 301.Deis S., Srivastava R.K., de Azua I.R., Bindila L., Baraghithy S., Lutz B., Bab I., Tam J. Age-related regulation of bone formation by the sympathetic cannabinoid CB1 receptor. Bone. 2018;108:34–42. doi: 10.1016/j.bone.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 302.Apostu D., Lucaciu O., Mester A., Benea H., Oltean-Dan D., Onisor F., Baciut M., Bran S. Cannabinoids and bone regeneration. Drug Metab. Rev. 2019;51:65–75. doi: 10.1080/03602532.2019.1574303. [DOI] [PubMed] [Google Scholar]
- 303.Kogan N.M., Melamed E., Wasserman E., Raphael B., Breuer A., Stok K.S., Sondergaard R., Escudero A.V.V., Baraghithy S., Attar-Namdar M., et al. Cannabidiol, a major non-psychotropic cannabis constituent enhances fracture healing and stimulates lysyl hydroxylase activity in osteoblasts. J. Bone Miner. Res. 2015;30:1905–1913. doi: 10.1002/jbmr.2513. [DOI] [PubMed] [Google Scholar]
- 304.Elefteriou F., Campbell P., Ma Y. Control of bone remodeling by the peripheral sympathetic nervous system. Calcif. Tissue Int. 2014;94:140–151. doi: 10.1007/s00223-013-9752-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Bajayo A., Bar A., Denes A., Bachar M., Kram V., Attar-Namdar M., Zallone A., Kovács K.J., Yirmiya R., Bab I. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc. Natl. Acad. Sci. USA. 2012;109:15455–15460. doi: 10.1073/pnas.1206061109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Eimar H., Tamimi I., Murshed M., Tamimi F. Cholinergic regulation of bone. J. Musculoskelet. Neuronal Interact. 2013;13:124–132. [PubMed] [Google Scholar]
- 307.Shi Y., Oury F., Yadav V.K., Wess J., Liu X.S., Guo X.E., Murshed M., Karsenty G. Signaling through the M3 Muscarinic Receptor Favors Bone Mass Accrual by Decreasing Sympathetic Activity. Cell Metab. 2010;11:231–238. doi: 10.1016/j.cmet.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Kondo H., Togari A. Continuous treatment with a low-dose β-agonist reduces bone mass by increasing bone resorption without suppressing bone formation. Calcif. Tissue Int. 2011;88:23–32. doi: 10.1007/s00223-010-9421-9. [DOI] [PubMed] [Google Scholar]
- 309.Ducy P., Amling M., Takeda S., Priemel M., Schilling A.F., Beil F.T., Shen J., Vinson C., Rueger J.M., Karsenty G. Leptin inhibits bone formation through a hypothalamic relay: A central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/S0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 310.Takeda S., Elefteriou F., Levasseur R., Liu X., Zhao L., Parker K.L., Armstrong D., Ducy P., Karsenty G. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/S0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- 311.Hamrick M.W., Ferrari S.L. Leptin and the sympathetic connection of fat to bone. Osteoporos. Int. 2008;19:905–912. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- 312.Martin A., David V., Malaval L., Lafage-Proust M.H., Vico L., Thomas T. Opposite effects of leptin on bone metabolism: A dose-dependent balance related to energy intake and insulin-like growth factor-I pathway. Endocrinology. 2007;148:3419–3425. doi: 10.1210/en.2006-1541. [DOI] [PubMed] [Google Scholar]
- 313.Mach D.B., Rogers S.D., Sabino M.C., Luger N.M., Schwei M.J., Pomonis J.D., Keyser C.P., Clohisy D.R., Adams D.J., O’leary P., et al. Origins of skeletal pain: Sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/S0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 314.Chartier S.R., Mitchell S.A.T., Majuta L.A., Mantyh P.W. The Changing Sensory and Sympathetic Innervation of the Young, Adult and Aging Mouse Femur. Neuroscience. 2018;387:178–190. doi: 10.1016/j.neuroscience.2018.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Offley S.C., Guo T.Z., Wei T., Clark J.D., Vogel H., Lindsey D.P., Jacobs C.R., Yao W., Lane N.E., Kingery W.S. Capsaicin-sensitive sensory neurons contribute to the maintenance of trabecular bone integrity. J. Bone Miner. Res. 2005;20:257–267. doi: 10.1359/JBMR.041108. [DOI] [PubMed] [Google Scholar]
- 316.Ding Y., Arai M., Kondo H., Togari A. Effects of capsaicin-induced sensory denervation on bone metabolism in adult rats. Bone. 2010;46:1591–1596. doi: 10.1016/j.bone.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 317.Russell F.A., King R., Smillie S.J., Kodji X., Brain S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014;94:1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Wang L., Shi X., Zhao R., Halloran B.P., Clark D.J., Jacobs C.R., Kingery W.S. Calcitonin-gene-related peptide stimulates stromal cell osteogenic differentiation and inhibits RANKL induced NF-κB activation, osteoclastogenesis and bone resorption. Bone. 2010;46:1369–1379. doi: 10.1016/j.bone.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Zhou R., Yuan Z., Liiu J., Liu J. Calcitonin gene-related peptide promotes the expression of osteoblastic genes and activates the WNT signal transduction pathway in bone marrow stromal stem cells. Mol. Med. Rep. 2016;13:4689–4696. doi: 10.3892/mmr.2016.5117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ishizuka K., Hirukawa K., Nakamura H., Togari A. Inhibitory effect of CGRP on osteoclast formation by mouse bone marrow cells treated with isoproterenol. Neurosci. Lett. 2005;379:47–51. doi: 10.1016/j.neulet.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 321.Onuoha G.N., Alpar E.K. Elevation of plasma CGRP and SP levels in orthopedic patients with fracture neck of femur. Neuropeptides. 2000;34:116–120. doi: 10.1054/npep.2000.0803. [DOI] [PubMed] [Google Scholar]
- 322.Xu Y.Q., Qin M.L., Feng S.Y., Huang Y., Jia Z. Expressions and significance of calcitonin gene-related peptide and nerve growth factor in rabbit model of traumatic brain injury complicated with tibial fracture: Preliminary results. Eur. Rev. Med. Pharmacol. Sci. 2019;23:5040–5050. doi: 10.26355/eurrev_201906_18167. [DOI] [PubMed] [Google Scholar]
- 323.Harrison S., Geppetti P. Substance P. Int. J. Biochem. Cell Biol. 2001;33:555–576. doi: 10.1016/S1357-2725(01)00031-0. [DOI] [PubMed] [Google Scholar]
- 324.Cook N.L., Vink R., Donkin J.J., van den Heuvel C. Validation of reference genes for normalization of real-time quantitative RT-PCR data in traumatic brain injury. J. Neurosci. Res. 2009;87:34–41. doi: 10.1002/jnr.21846. [DOI] [PubMed] [Google Scholar]
- 325.Donkin J.J., Nimmo A.J., Cernak I., Blumbergs P.C., Vink R. Substance P is associated with the development of brain edema and functional deficits after traumatic brain injury. J. Cereb. Blood Flow Metab. 2009;29:1388–1398. doi: 10.1038/jcbfm.2009.63. [DOI] [PubMed] [Google Scholar]
- 326.Wang L., Zhao R., Shi X., Wei T., Halloran B.P., Clark D.J., Jacobs C.R., Kingery W.S. Substance P stimulates bone marrow stromal cell osteogenic activity, osteoclast differentiation, and resorption activity in vitro. Bone. 2009;45:309–320. doi: 10.1016/j.bone.2009.04.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Li F.X.Z., Xu F., Lin X., Wu F., Zhong J.Y., Wang Y., Guo B., Zheng M.H., Shan S.K., Yuan L.Q. The Role of Substance P in the Regulation of Bone and Cartilage Metabolic Activity. Front. Endocrinol. 2020;11 doi: 10.3389/fendo.2020.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Niedermair T., Schirner S., Seebröker R., Straub R.H., Grässel S. Substance P modulates bone remodeling properties of murine osteoblasts and osteoclasts. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-27432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ding W.G., Zhang Z.M., Zhang Y.H., Jiang S.D., Jiang L.S., Dai L.Y. Changes of substance P during fracture healing in ovariectomized mice. Regul. Pept. 2010;159:28–34. doi: 10.1016/j.regpep.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 330.Hofman M., Rabenschlag F., Andruszkow H., Andruszkow J., Möckel D., Lammers T., Kolejewska A., Kobbe P., Greven J., Teuben M.P.J., et al. Effect of neurokinin-1-receptor blockage on fracture healing in rats. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-46278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zheng X.F., Zhao E.D., He J.Y., Zhang Y.H., Jiang S.D., Jiang L.S. Inhibition of substance P signaling aggravates the bone loss in ovariectomy-induced osteoporosis. Prog. Biophys. Mol. Biol. 2016;122:112–121. doi: 10.1016/j.pbiomolbio.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 332.Tran T.S., Kolodkin A.L., Bharadwaj R. Semaphorin Regulation of Cellular Morphology. Annu. Rev. Cell Dev. Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- 333.Wee N.K.Y., Kulkarni R.N., Horsnell H., Baldock P.A. The brain in bone and fuel metabolism. Bone. 2016;82:56–63. doi: 10.1016/j.bone.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 334.Huang S., Li Z., Liu Y., Gao D., Zhang X., Hao J., Yang F. Neural regulation of bone remodeling: Identifying novel neural molecules and pathways between brain and bone. J. Cell. Physiol. 2019;234:5466–5477. doi: 10.1002/jcp.26502. [DOI] [PubMed] [Google Scholar]
- 335.Hayashi M., Nakashima T., Taniguchi M., Kodama T., Kumanogoh A., Takayanagi H. Osteoprotection by semaphorin 3A. Nature. 2012;485:69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- 336.Fukuda T., Takeda S., Xu R., Ochi H., Sunamura S., Sato T., Shibata S., Yoshida Y., Gu Z., Kimura A., et al. Sema3A regulates bone-mass accrual through sensory innervations. Nature. 2013;497:490–493. doi: 10.1038/nature12115. [DOI] [PubMed] [Google Scholar]
- 337.Ducy P., Desbois C., Boyce B., Pinero G., Story B., Dunstan C., Smith E., Bonadio J., Goldstein S., Gundberg C., et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 338.Diaz-Franco M.C., Franco-Diaz de Leon R., Villafan-Bernal J.R. Osteocalcin-GPRC6A: An update of its clinical and biological multi-organic interactions (Review) Mol. Med. Rep. 2019;19:15–22. doi: 10.3892/mmr.2018.9627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Oury F., Khrimian L., Denny C.A., Gardin A., Chamouni A., Goeden N., Huang Y.Y., Lee H., Srinivas P., Gao X.B., et al. XMaternal and offspring pools of osteocalcin influence brain development and functions. Cell. 2013;155:228. doi: 10.1016/j.cell.2013.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Bradburn S., Mcphee J.S., Bagley L., Sipila S., Stenroth L., Narici M.V., Pääsuke M., Gapeyeva H., Osborne G., Sassano L., et al. Association between osteocalcin and cognitive performance in healthy older adults. Age Ageing. 2016;45:844–849. doi: 10.1093/ageing/afw137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Shan C., Ghosh A., Guo X.Z., Wang S.M., Hou Y.F., Li S.T., Liu J.M. Roles for osteocalcin in brain signalling: Implications in cognition- and motor-related disorders. Mol. Brain. 2019;12:1–11. doi: 10.1186/s13041-019-0444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Moser S.C., van der Eerden B.C.J. Osteocalcin — A versatile bone-derived hormone. Front. Endocrinol. 2019;10:4–9. doi: 10.3389/fendo.2018.00794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Diegel C.R., Hann S., Ayturk U.M., Hu J.C.W., Lim K.E., Droscha C.J., Madaj Z.B., Foxa G.E., Izaguirre I., Transgenics Core V.V.A., et al. An osteocalcin-deficient mouse strain without endocrine abnormalities. PLoS Genet. 2020;16:e1008361. doi: 10.1371/journal.pgen.1008361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Moriishi T., Ozasa R., Ishimoto T., Nakano T., Hasegawa T., Miyazaki T., Liu W., Fukuyama R., Wang Y., Komori H., et al. Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genet. 2020;16:e1008586. doi: 10.1371/journal.pgen.1008586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Mera P., Ferron M., Mosialou I. Regulation of energy metabolism by bone-derived hormones. Cold Spring Harb. Perspect. Med. 2018;8 doi: 10.1101/cshperspect.a031666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Mosialou I., Shikhel S., Liu J.M., Maurizi A., Luo N., He Z., Huang Y., Zong H., Friedman R.A., Barasch J., et al. MC4R-dependent suppression of appetite by bone-derived lipocalin 2. Nature. 2017;543:385–390. doi: 10.1038/nature21697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Koide M., Kobayashi Y. Regulatory mechanisms of sclerostin expression during bone remodeling. J. Bone Miner. Metab. 2019;37:9–17. doi: 10.1007/s00774-018-0971-7. [DOI] [PubMed] [Google Scholar]
- 348.Baron R., Kneissel M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013;19:179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 349.Shah A.D., Shoback D., Michael Lewiecki E. Sclerostin inhibition: A novel therapeutic approach in the treatment of osteoporosis. Int. J. Womens. Health. 2015;7:565–580. doi: 10.2147/IJWH.S73244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.McClung M.R., Grauer A., Boonen S., Bolognese M.A., Brown J.P., Diez-Perez A., Langdahl B.L., Reginster J.Y., Zanchetta J.R., Wasserman S.M., et al. Romosozumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 2014;370:412–420. doi: 10.1056/NEJMoa1305224. [DOI] [PubMed] [Google Scholar]
- 351.Cosman F., Crittenden D.B., Ferrari S., Khan A., Lane N.E., Lippuner K., Matsumoto T., Milmont C.E., Libanati C., Grauer A. FRAME Study: The Foundation Effect of Building Bone With 1 Year of Romosozumab Leads to Continued Lower Fracture Risk After Transition to Denosumab. J. Bone Miner. Res. 2018;33:1219–1226. doi: 10.1002/jbmr.3427. [DOI] [PubMed] [Google Scholar]
- 352.Graeff C., Campbell G.M., Peña J., Borggrefe J., Padhi D., Kaufman A., Chang S., Libanati C., Glüer C.C. Administration of romosozumab improves vertebral trabecular and cortical bone as assessed with quantitative computed tomography and finite element analysis. Bone. 2015;81:364–369. doi: 10.1016/j.bone.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 353.Saag K.G., Petersen J., Brandi M.L., Karaplis A.C., Lorentzon M., Thomas T., Maddox J., Fan M., Meisner P.D., Grauer A. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N. Engl. J. Med. 2017;377:1417–1427. doi: 10.1056/NEJMoa1708322. [DOI] [PubMed] [Google Scholar]
- 354.Inestrosa N.C., Varela-Nallar L. Wnt signaling in the nervous system and in Alzheimer’s disease. J. Mol. Cell Biol. 2014;6:64–74. doi: 10.1093/jmcb/mjt051. [DOI] [PubMed] [Google Scholar]
- 355.Weivoda M.M., Youssef S.J., Oursler M.J. Sclerostin expression and functions beyond the osteocyte. Bone. 2017;96:45–50. doi: 10.1016/j.bone.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Pinzone J.J., Hall B.M., Thudi N.K., Vonau M., Qiang Y.W., Rosol T.J., Shaughnessy J.D. The role of Dickkopf-1 in bone development, homeostasis, and disease. Blood. 2009;113:517–525. doi: 10.1182/blood-2008-03-145169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Li J., Sarosi I., Cattley R.C., Pretorius J., Asuncion F., Grisanti M., Morony S., Adamu S., Geng Z., Qiu W., et al. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 358.Lieven O., Knobloch J., Rüther U. The regulation of Dkk1 expression during embryonic development. Dev. Biol. 2010;340:256–268. doi: 10.1016/j.ydbio.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 359.Huang Y., Liu L., Liu A. Dickkopf-1: Current knowledge and related diseases. Life Sci. 2018;209:249–254. doi: 10.1016/j.lfs.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 360.Okamoto M., Inoue K., Iwamura H., Terashima K., Soya H., Asashima M., Kuwabara T. Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB J. 2011;25:3570–3582. doi: 10.1096/fj.11-184697. [DOI] [PubMed] [Google Scholar]
- 361.Ren C., Gu X., Li H., Lei S., Wang Z., Wang J., Yin P., Zhang C., Wang F., Liu C. The role of DKK1 in Alzheimer’s disease: A potential intervention point of brain damage prevention? Pharmacol. Res. 2019;144:331–335. doi: 10.1016/j.phrs.2019.04.033. [DOI] [PubMed] [Google Scholar]
- 362.Purro S.A., Dickins E.M., Salinas P.C. The secreted Wnt antagonist dickkopf-1 is required for amyloid β-mediated synaptic loss. J. Neurosci. 2012;32:3492–3498. doi: 10.1523/JNEUROSCI.4562-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Marchetti B. Wnt/β-catenin signaling pathway governs a full program for dopaminergic neuron survival, neurorescue and regeneration in the MPTP mouse model of Parkinson’s disease. Int. J. Mol. Sci. 2018;19:3743. doi: 10.3390/ijms19123743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Corrado A., Sanpaolo E.R., Di Bello S., Cantatore F.P. Osteoblast as a target of anti-osteoporotic treatment. Postgrad. Med. 2017;129:858–865. doi: 10.1080/00325481.2017.1362312. [DOI] [PubMed] [Google Scholar]
- 365.Florio M., Gunasekaran K., Stolina M., Li X., Liu L., Tipton B., Salimi-Moosavi H., Asuncion F.J., Li C., Sun B., et al. A bispecific antibody targeting sclerostin and DKK-1 promotes bone mass accrual and fracture repair. Nat. Commun. 2016;7 doi: 10.1038/ncomms11505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Simard A.R., Soulet D., Gowing G., Julien J.P., Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 367.Han K.H., Arlian B.M., Macauley M.S., Paulson J.C., Lerner R.A. Migration-based selections of antibodies that convert bone marrow into trafficking microglia-like cells that reduce brain amyloid β. Proc. Natl. Acad. Sci. USA. 2018;115:E372–E381. doi: 10.1073/pnas.1719259115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Kawanishi S., Takata K., Itezono S., Nagayama H., Konoya S., Chisaki Y., Toda Y., Nakata S., Yano Y., Kitamura Y., et al. Bone-Marrow-Derived Microglia-Like Cells Ameliorate Brain Amyloid Pathology and Cognitive Impairment in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018;64:563–585. doi: 10.3233/JAD-170994. [DOI] [PubMed] [Google Scholar]
- 369.Kuroda E., Takata K., Nishimura K., Oka H., Sueyoshi M., Aitani M., Kouda A., Satake S., Shima C., Toda Y., et al. Peripheral Blood-Derived Microglia-Like Cells Decrease Amyloid-β Burden and Ameliorate Cognitive Impairment in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020;73:413–429. doi: 10.3233/JAD-190974. [DOI] [PubMed] [Google Scholar]
- 370.Costa-Marques L., Arnold K., Pardon M.C., Leovsky C., Swarbrick S., Fabian C., Stolzing A. Transplantation of bone marrow derived macrophages reduces markers of neuropathology in an APP/PS1 mouse model. Transl. Neurodegener. 2019;8:1–11. doi: 10.1186/s40035-019-0173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Lampron A., Gosselin D., Rivest S. Targeting the hematopoietic system for the treatment of Alzheimer’s disease. Brain. Behav. Immun. 2011;25:71–79. doi: 10.1016/j.bbi.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 372.Colaianni G., Cuscito C., Mongelli T., Pignataro P., Buccoliero C., Liu P., Lu P., Sartini L., Comite M.D., Mori G., et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA. 2015;112:12157–12162. doi: 10.1073/pnas.1516622112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Colaianni G., Mongelli T., Cuscito C., Pignataro P., Lippo L., Spiro G., Notarnicola A., Severi I., Passeri G., Mori G., et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017;7:1–16. doi: 10.1038/s41598-017-02557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Arhire L.I., Mihalache L., Covasa M. Irisin: A Hope in Understanding and Managing Obesity and Metabolic Syndrome. Front. Endocrinol. 2019;10:1–12. doi: 10.3389/fendo.2019.00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Munoz I.Y.M., Del Socorro Camarillo Romero E., De Jesus Garduno Garcia J. Irisin a novel metabolic biomarker: Present knowledge and future directions. Int. J. Endocrinol. 2018;2018 doi: 10.1155/2018/7816806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Asadi Y., Gorjipour F., Behrouzifar S., Vakili A. Irisin Peptide Protects Brain Against Ischemic Injury Through Reducing Apoptosis and Enhancing BDNF in a Rodent Model of Stroke. Neurochem. Res. 2018;43:1549–1560. doi: 10.1007/s11064-018-2569-9. [DOI] [PubMed] [Google Scholar]
- 377.Ting S.-M., Zhao X., Sun G., Obertas L., Ricote M., Aronowski J. Brain Cleanup as a Potential Target for Poststroke Recovery. Stroke. 2020:958–966. doi: 10.1161/STROKEAHA.119.027315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Lourenco M.V., Frozza R.L., de Freitas G.B., Zhang H., Kincheski G.C., Ribeiro F.C., Gonçalves R.A., Clarke J.R., Beckman D., Staniszewski A., et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019;25:165–175. doi: 10.1038/s41591-018-0275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Reinholt F.P., Hultenby K., Oldbergf A., Heinegardt D. Osteopontin-a possible anchor of osteoclasts to bone (bone matrix proteins/cell adhesion/vitronectin receptor) Proc. Nadl. Acad. Sci. USA. 1990;87:4473–4475. doi: 10.1073/pnas.87.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Singh A., Gill G., Kaur H., Amhmed M., Jakhu H. Role of osteopontin in bone remodeling and orthodontic tooth movement: A review. Prog. Orthod. 2018;19 doi: 10.1186/s40510-018-0216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Cho E.H., Cho K.H., Lee H.A., Kim S.W. High serum osteopontin levels are associated with low bone mineral density in postmenopausal women. J. Korean Med. Sci. 2013;28:1496–1499. doi: 10.3346/jkms.2013.28.10.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Scatena M., Liaw L., Giachelli C.M. Osteopontin: A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 383.Brown A. Osteopontin: A key link between immunity, inflammation and the central nervous system. Transl. Neurosci. 2012;3:288–293. doi: 10.2478/s13380-012-0028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Gliem M., Krammes K., Liaw L., van Rooijen N., Hartung H.P., Jander S. Macrophage-derived osteopontin induces reactive astrocyte polarization and promotes re-establishment of the blood brain barrier after ischemic stroke. Glia. 2015;63:2198–2207. doi: 10.1002/glia.22885. [DOI] [PubMed] [Google Scholar]
- 385.Carecchio M., Comi C. The role of osteopontin in neurodegenerative diseases. J. Alzheimer’s Dis. 2011;25:179–185. doi: 10.3233/JAD-2011-102151. [DOI] [PubMed] [Google Scholar]
- 386.Hanada R., Leibbrandt A., Hanada T., Kitaoka S., Furuyashiki T., Fujihara H., Trichereau J., Paolino M., Qadri F., Plehm R., et al. Central control of fever and female body temperature by RANKL/RANK. Nature. 2009;462:505–509. doi: 10.1038/nature08596. [DOI] [PubMed] [Google Scholar]
- 387.Liu W., Zhang X. Receptor activator of nuclear factor-κB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (Review) Mol. Med. Rep. 2015;11:3212–3218. doi: 10.3892/mmr.2015.3152. [DOI] [PubMed] [Google Scholar]
- 388.Lacey D.L., Boyle W.J., Simonet W.S., Kostenuik P.J., Dougall W.C., Sullivan J.K., Martin J.S., Dansey R. Bench to bedside: Elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat. Rev. Drug Discov. 2012;11:401–419. doi: 10.1038/nrd3705. [DOI] [PubMed] [Google Scholar]
- 389.Lipton A., Fizazi K., Stopeck A.T., Henry D.H., Brown J.E., Yardley D.A., Richardson G.E., Siena S., Maroto P., Clemens M., et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: A combined analysis of 3 pivotal, randomised, phase 3 trials. Eur. J. Cancer. 2012;48:3082–3092. doi: 10.1016/j.ejca.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 390.Zhang J., Fujita Y., Chang L., Pu Y., Qu Y., Wang S., Hashimoto K. Beneficial effects of anti-RANKL antibody in depression-like phenotype, inflammatory bone markers, and bone mineral density in male susceptible mice after chronic social defeat stress. Behav. Brain Res. 2020;379 doi: 10.1016/j.bbr.2019.112397. [DOI] [PubMed] [Google Scholar]
- 391.Mattson M.P. Glutamate and neurotrophic factors in neuronal plasticity and disease. Ann. N. Y. Acad. Sci. 2008;1144:97–112. doi: 10.1196/annals.1418.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Kajiya M., Shiba H., Fujita T., Ouhara K., Takeda K., Mizuno N., Kawaguchi H., Kitagawa M., Takata T., Tsuji K., et al. Brain-derived neurotrophic factor stimulates bone/cementum-related protein gene expression in cementoblasts. J. Biol. Chem. 2008;283:16259–16267. doi: 10.1074/jbc.M800668200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Camerino C., Zayzafoon M., Rymaszewski M., Heiny J., Rios M., Hauschka P.V. Central depletion of brain-derived neurotrophic, factorin mice results in high bone mass and metabolic phenotype. Endocrinology. 2012;153:5394–5405. doi: 10.1210/en.2012-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Laviola L., Natalicchio A., Perrini S., Giorgino F. Abnormalities of IGF-I signaling in the pathogenesis of diseases of the bone, brain, and fetoplacental unit in humans. Am. J. Physiol. Endocrinol. Metab. 2008;295 doi: 10.1152/ajpendo.90452.2008. [DOI] [PubMed] [Google Scholar]
- 395.Wrigley S., Arafa D., Tropea D. Insulin-like growth factor 1: At the crossroads of brain development and aging. Front. Cell. Neurosci. 2017;11:1–15. doi: 10.3389/fncel.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Yakar S., Boisclair Y.R., Leroith D., Yakar S., Rosen C.J., Beamer W.G., Ackert-bicknell C.L., Wu Y., Liu J., Ooi G.T., et al. Circulating levels of IGF-1 directly regulate bone growth and density. J. Clin. Investig. 2002;110:771–781. doi: 10.1172/JCI0215463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Rosen C.J. Insulin-like growth factor I and bone mineral density: Experience from animal models and human observational studies. Best Pract. Res. Clin. Endocrinol. Metab. 2004;18:423–435. doi: 10.1016/j.beem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 398.Bragdon B., Moseychuk O., Saldanha S., King D., Julian J., Nohe A. Bone Morphogenetic Proteins: A critical review. Cell. Signal. 2011;23:609–620. doi: 10.1016/j.cellsig.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 399.Chen H.L., Lein P.J., Wang J.Y., Gash D., Hoffer B.J., Chiang Y.H. Expression of bone morphogenetic proteins in the brain during normal aging and in 6-hydroxydopamine-lesioned animals. Brain Res. 2003;994:81–90. doi: 10.1016/j.brainres.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 400.Sato T., Mikawa S., Sato K. BMP2 expression in the adult rat brain. J. Comp. Neurol. 2010;518:4513–4530. doi: 10.1002/cne.22469. [DOI] [PubMed] [Google Scholar]
- 401.Bond A.M., Bhalala O.G., Kessler J.A. The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation. Dev. Neurobiol. 2012;72:1068–1084. doi: 10.1002/dneu.22022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Chang J., Dettman R.W., Dizon M.L.V. Bone morphogenetic protein signaling: A promising target for white matter protection in perinatal brain injury. Neural Regen. Res. 2018;13:1183–1184. doi: 10.4103/1673-5374.235025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Staal A., Frith J.C., French M.H., Swartz J., Güngör T., Harrity T.W., Tamasi J., Rogers M.J., Feyen J.H.M. The ability of statins to inhibit bone resorption is directly related to their inhibitory effect on HMG-CoA reductase activity. J. Bone Miner. Res. 2003;18:88–96. doi: 10.1359/jbmr.2003.18.1.88. [DOI] [PubMed] [Google Scholar]
- 404.Mori M., Nishikawa T., Masuno K., Okamura T., Tanaka A., Shikimori M. Statins: Candidates for promoting bone formation via BMP-2. Oral Med. Pathol. 2010;14:81–87. doi: 10.3353/omp.14.81. [DOI] [Google Scholar]
- 405.Ruan F., Zheng Q., Wang J. Mechanisms of bone anabolism regulated by statins. Biosci. Rep. 2012;32:511–519. doi: 10.1042/BSR20110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Uzzan B., Cohen R., Nicolas P., Cucherat M., Perret G.Y. Effects of statins on bone mineral density: A meta-analysis of clinical studies. Bone. 2007;40:1581–1587. doi: 10.1016/j.bone.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 407.Morse L.R., Coker J., Battaglino R.A. Statins and bone health: A mini review. Actual. osteol. 2018;14:31–35. [PMC free article] [PubMed] [Google Scholar]
- 408.Samsa W.E., Vasanji A., Midura R.J., Kondratov R.V. Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype. Bone. 2016;84:194–203. doi: 10.1016/j.bone.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Liu H., Liu Z., Man C.W., Guo J., Han X., Hu Z., Ng T.B., Zhao Z., Li J., Wang W., et al. The effect of exogenous melatonin on reducing scoliotic curvature and improving bone quality in melatonin-deficient C57BL/6J mice. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-42467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Li S., Liu B., Zhang L., Rong L. Amyloid beta peptide is elevated in osteoporotic bone tissues and enhances osteoclast function. Bone. 2014;61:164–175. doi: 10.1016/j.bone.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 411.Zhu P., Zhang Z., Huang X., Liang S., Khandekar N., Song Z., Lin S. RANKL reduces body weight and food intake via the modulation of hypothalamic NPY/CART expression. Int. J. Med. Sci. 2018;15:969–977. doi: 10.7150/ijms.24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Laksitorini M.D., Yathindranath V., Xiong W., Hombach-Klonisch S., Miller D.W. Modulation of Wnt/β-catenin signaling promotes blood-brain barrier phenotype in cultured brain endothelial cells. Sci. Rep. 2019;9:1–13. doi: 10.1038/s41598-019-56075-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Eger M., Bader M., Bree D., Hadar R., Nemerovski A., Tam J., Levy D., Pick C.G., Gabet Y. Bone Anabolic Response in the Calvaria Following Mild Traumatic Brain Injury is Mediated by the Cannabinoid-1 Receptor. Sci. Rep. 2019;9:1–8. doi: 10.1038/s41598-019-51720-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Seemann R., Graef F., Garbe A., Keller J., Huang F., Duda G., Schmidt-Bleek K., Schaser K.D., Tsitsilonis S. Leptin-deficiency eradicates the positive effect of traumatic brain injury on bone healing: Histological analyses in a combined trauma mouse model. J. Musculoskelet. Neuronal Interact. 2018;18:32–41. [PMC free article] [PubMed] [Google Scholar]
- 415.Song Y., Han G.X., Chen L., Zhai Y.Z., Dong J., Chen W., Li T.S., Zhu H.Y. The role of the hippocampus and the function of calcitonin gene-related peptide in the mechanism of traumatic brain injury accelerating fracture-healing. Eur. Rev. Med. Pharmacol. Sci. 2017;21:1522–1531. [PubMed] [Google Scholar]
- 416.Liu X., Zhou C., Li Y., Ji Y., Xu G., Wang X., Yan J. SDF-1 Promotes Endochondral Bone Repair during Fracture Healing at the Traumatic Brain Injury Condition. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0054077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Yan H., Zhang H.W., Fu P., Liu B.L., Jin W.Z., Duan S.B., Xue J., Liu K., Sun Z.M., Zeng X.W. Leptin’s effect on accelerated fracture healing after traumatic brain injury. Neurol. Res. 2013;35:537–544. doi: 10.1179/1743132813Y.0000000201. [DOI] [PubMed] [Google Scholar]
- 418.Yang S., Ma Y., Liu Y., Que H., Zhu C., Liu S. Arachidonic acid: A bridge between traumatic brain injury and fracture healing. J. Neurotrauma. 2012;29:2696–2705. doi: 10.1089/neu.2012.2442. [DOI] [PubMed] [Google Scholar]
- 419.Song Y., Bi L., Zhang Z., Huang Z., Hou W., Lu X., Sun P., Han Y. Increased levels of calcitonin gene-related peptide in serum accelerate fracture healing following traumatic brain injury. Mol. Med. Rep. 2012;5:432–438. doi: 10.3892/mmr.2011.645. [DOI] [PubMed] [Google Scholar]
- 420.Zhang D., Zhang P., Wang Y., Han N., Tang C., Jiang B. The influence of brain injury or peripheral nerve injury on calcitonin gene-related peptide concentration variation and fractures healing process. Artif. Cells Blood Substitutes Biotechnol. 2009;37:85–91. doi: 10.1080/10731190902743149. [DOI] [PubMed] [Google Scholar]
- 421.Wei Y., Wang L., Clark J.C.M., Dass C.R., Choong P.F.M. Elevated leptin expression in a rat model of fracture and traumatic brain injury. J. Pharm. Pharmacol. 2008;60:1667–1672. doi: 10.1211/jpp.60.12.0013. [DOI] [PubMed] [Google Scholar]
- 422.Boes M., Kain M., Kakar S., Nicholls F., Cullinane D., Gerstenfeld L., Einhorn T.A., Tornetta P. Osteogenic effects of traumatic brain injury on experimental fracture-healing. J. Bone Jt. Surg. Ser. A. 2006;88:738–743. doi: 10.2106/JBJS.D.02648. [DOI] [PubMed] [Google Scholar]
- 423.Khallaf F.G., Kehinde E.O. The Effect of Serum from Acute Traumatic Brain or Spinal Cord Injury Patients on the Growth of Human Bone Marrow-Derived Mesenchymal Stem Cells. J. Trauma Treat. 2016;5 doi: 10.4172/2167-1222.1000299. [DOI] [Google Scholar]
- 424.Dengler-Crish C.M., Ball H.C., Lin L., Novak K.M., Cooper L.N. Evidence of Wnt/β-catenin alterations in brain and bone of a tauopathy mouse model of Alzheimer’s disease. Neurobiol. Aging. 2018;67:148–158. doi: 10.1016/j.neurobiolaging.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 425.Khrimian L., Obri A., Karsenty G. Modulation of cognition and anxiety-like behavior by bone remodeling. Mol. Metab. 2017;6:1610–1615. doi: 10.1016/j.molmet.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Inatani M., Irie F., Plump A.S., Tessier-Lavigne M., Yamaguchi Y. Mammalian Brain Morphogenesis and Midline Axon Guidance Require Heparan Sulfate. Science. 2003;302:1044–1046. doi: 10.1126/science.1090497. [DOI] [PubMed] [Google Scholar]
- 427.Irie F., Badie-Mahdavi H., Yamaguchi Y. Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate. Proc. Natl. Acad. Sci. USA. 2012;109:5052–5056. doi: 10.1073/pnas.1117881109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Suto Y., Nagata K., Ahmed S.M., Jacovides C.L., Browne K.D., Cognetti J., Johnson V.E., Leone R., Kaplan L.J., Smith D.H., et al. Cerebral Edema and Neurological Recovery after Traumatic Brain Injury Are Worsened if Accompanied by a Concomitant Long Bone Fracture. J. Neurotrauma. 2019;36:609–618. doi: 10.1089/neu.2018.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.