Abstract
Site-specific recombinases are widely used tools for analysis of genetics, development, and cell biology, and many schemes have been devised to alter gene expression by recombinase-mediated DNA rearrangements. Because the FRT and lox target sites for the commonly used FLP and Cre recombinases are asymmetrical, and must pair in the same direction to recombine, construct design must take into account orientation of the target sites. Both direct and inverted configurations have been used. However, the outcome of recombination between target sites on sister chromatids is frequently overlooked. This is especially consequential with inverted target sites, where exchange between oppositely oriented target sites on sisters will produce dicentric and acentric chromosomes. By using constructs that have inverted target sites in Drosophila melanogaster and in mice, we show here that dicentric chromosomes are produced in the presence of recombinase, and that the frequency of this event is quite high. The negative effects on cell viability and behavior can be significant, and should be considered when using such constructs.
Keywords: Cre, dicentric, Drosophila, FLP, FRT, lox, mouse, recombinase, sister chromatid, site-specific recombination
SITE-specific recombinases have become essential tools for biological experimentation. The FLP-FRT and the Cre-lox systems have been used to great effect in a number of different species. Genes or gene segments may be arranged in a variety of configurations with FRT or lox target sites to activate or inactivate genes at different developmental stages in animal and plant systems. By using such methods, cells and their descendants may be marked to study the fates of mutant cells or to trace cell lineage. Additionally, recombination between widely separated target sites on the same chromosome, or between homologous or nonhomologous chromosomes, has been used to engineer a variety of chromosome rearrangements (Golic and Lindquist 1989; Golic 1991; Lakso et al. 1992; Qin et al. 1994; Ramírez-Solis et al. 1995; Smith et al. 1995; Golic and Golic 1996; Gilbertson 2003; Zong et al. 2005; Wang et al. 2010; Lee 2013; Gierut et al. 2014; Hubbard 2014; Weissman and Pan 2015; Pontes-Quero et al. 2017; Germani et al. 2018).
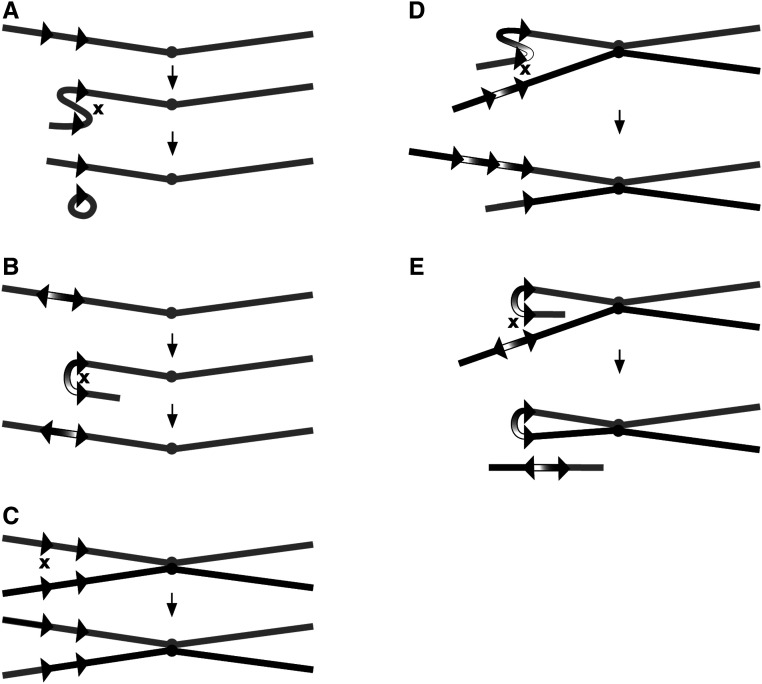
The FRT and lox ttarget sites are both asymmetrical, and FLP- or Cre-mediated recombination generates orientation-specific outcomes. Intramolecular recombination between sites in the same (direct) orientation excises the material between the sites, along with one target site (Figure 1A). Intramolecular recombination between sites that are inverted results in an inversion of the material between them (Figure 1B). Intermolecular recombination may also occur, and the consequence of recombination between sister chromatids is often overlooked. Recombination between target sites at identical locations on sister chromatids is typically without effect (Figure 1C). Recombination between target sites at different locations, though, does produce genetic changes that may be significant. When two target sites are in the same orientation, recombination between staggered sites generates complementary duplications and deletions (Figure 1D). Recombination between inverted target sites on sister chromatids will generate a dicentric and an acentric chromosome (Figure 1E). If the inverted target sites are close to one another, this can be quite frequent. These outcomes are reversed if the target sites are on opposite sides of the centromere, but since the frequency of recombination is strongly dependent on proximity (Golic and Golic 1996), this is of much less concern.
Figure 1.
Intramolecular and intermolecular site-specific recombination. Schematic representations of recombination between recombinase targets on the same chromatid or on sister chromatids. (A) Intramolecular recombination between targets in direct orientation results in excision of the material between the targets as an extrachromosomal circle. (B) Intramolecular recombination between inverted repeats inverts the orientation of the DNA between the target sites. (C) Intermolecular recombination between targets at the same location on sister chromatids results in equal exchange. (D) Exchange between targets in direct orientation at different sites on sisters results in duplication and deletion of intervening DNA. (E) Recombination between inverted target sites on sisters produces a dicentric chromosome and an acentric chromosome. Chromatids, are indicated as lines, target sites as arrowheads, and centromeres as filled circles.
In mitosis, a dicentric chromosome can break, leading to breakage-fusion-bridge cycles, chromosomal and segmental aneuploidy, and cell death. In cells that do not die, continuing problems with chromosome segregation and aneuploidy can cause aberrant behavior. Falco et al. (1982) were the first to recognize the problem created by inversely oriented recombinase target sites within a chromosome. They saw that chromosomal integration of the 2µ plasmid of Saccharomyces cerevisiae, which carries inverted FRTs and encodes the FLP recombinase, caused chromosome instability, which they attributed to dicentric/acentric formation. Subsequently, this event was directly demonstrated in Drosophila melanogaster (Golic 1994), and inferentially shown in mice with Cre-lox (Lewandoski and Martin 1997). In the latter case, when inverted lox sites were placed on the mouse Y chromosome, and males with this Y were mated to females with a β-actin-Cre gene, almost all XY zygotes developed as XO females, owing to loss of the lox-bearing chromosome during embryonic development.
Since those examples, there have been a number of studies that have utilized FLP- or Cre-mediated recombination between inverted target sites to cause dicentric chromosome formation (Ahmad and Golic 1998, 1999; Grégoire and Kmita 2008; Otsuji et al. 2008; Titen and Golic 2008, 2010; Tada et al. 2009; Jones et al. 2010; Zhu et al. 2010, 2012; Kurzhals et al. 2011; Sato et al. 2017; Thomas et al. 2017). Other studies have identified cell death as a consequence of recombination between inverted target sites without directly pinpointing the cause (Eckrich et al. 2019).
In spite of these many examples of inverted target sites causing dicentric chromosome formation in the presence of the recombinase, their use for gene switching and lineage tracing continues, apparently without recognition that dicentric chromosomes are formed. Flex and FlipFlox constructs, used for gene switching in mice, rely upon Cre-mediated inversion (Schnütgen et al. 2003; Xin 2005). The polylox system for barcoding cells and tracing cell lineage in mice relies on both direct and inverted lox repeats (Pei et al. 2017). The Confetti method for cell marking, and other methods based on Brainbow 2.0 or 2.1, also utilize inverted target sites (Livet et al. 2007; Snippert et al. 2010; Richier and Salecker 2014; de Roo et al. 2019). In flies, Flybow, FlpStop, Flip-Flop and other implementations have used inverted target sites to alter gene expression during development (Hadjieconomou et al. 2011; Chin et al. 2014; Fisher et al. 2017; Nagarkar-Jaiswal et al. 2017; Williams et al. 2019). In yeast, the SCRAMBLE system for rearranging chromosomes uses engineered symmetrical lox sites that may recombine in either direction (Dymond et al. 2011; Annaluru et al. 2014; Wu et al. 2018).
Studies that use these constructs will be impacted by the death of cells with broken chromosomes, or by the persistence of cells with aneuploidy and continuing genome instability, possibly leading to misinterpretation or invalid conclusions. Our purpose here is to raise awareness of the fact that dicentric chromosomes are formed when inverted recombinase target sites are used in eukaryotes, and that this event can be very frequent. This outcome should be considered when planning experiments with such constructs.
Materials and Methods
Dicentric chromosomes in Drosophila melanogaster
Flies were maintained on standard medium at 25°. The 70FLP gene has been previously described (Golic et al. 1997). FlipFlop stocks were obtained from the Bloomington, IN, Drosophila Stock Center. Female y w 70FLP3F; Sb/TM6, Ubx flies were crossed to male y w; Mi{FlipFlop} Nedd8 [MI13776-FF.PT-EGFP]/CyO (Bloomington 76596) or male y w; Mi{FlipFlop} Cdep[MI12769-FF.PT-EGFP] (Bloomington 76595). Parental flies were removed from the vials after 7 days and vials were placed into a circulating water bath for 1 hr at 38° to induce FLP. Larval brains were dissected either 4–5 hr after heat shock, or 23 hr after heat shock. Neuroblast chromosomes were prepared as described (Fanti and Pimpinelli 2004) and then stained with 4′,6-diamidino-2-phenylindole (DAPI). Multiple brains were scored, and each clear mitotic figure was scored as showing acentric/dicentric chromosomes or unaffected normal chromosomes. Nuclei in which all chromosomes were not visible or could not be distinguished were not scored.
Generation of inverted loxP transgenic mice
The HPRTCre constitutive and germline Cre expression lines were obtained from Jackson Laboratories. The mouse line bearing inverted loxP sites targeted to the middle of chromosome 15, at the Ext1 locus, is described in Jones et al. (2010). The mouse line bearing inverted loxP sites targeted to the distal tip of chromosome 7, to the Fgf4 locus, is described in Moon et al. (2000), except that the loxP site in the 5′ UTR is inverted with respect the other loxP site. The mouse line with inverted loxP sites and the tetracycline-repressible tdTomato transgene targeted to the tip of chromosome 19 was generated using homology arms with sequence similarity to the region distal to the last exon of the coding region of Grk5. Briefly, this construct contains a minimal CMV promoter immediately upstream of seven copies of the tetracycline operon operator sequence immediately upstream of the coding sequence of a tandem dimer of the tomato fluorescent protein and a polyadenylation sequence. Downstream of tomato are the two inverted loxP sites followed by a CAG promoter driving expression of the fusion protein consisting of TetR fused to the strong transcriptional silencing Kruppel-associated box domain (Tet-KRAB).
Mouse metaphase chromosome analysis
For preparation of mitotic figures, mouse embryonic fibroblasts (MEF) and embryonic stem (ES) cell lines cells were grown to 50–70% confluence in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with Na pyruvate, nonessential amino acids (NEAA), and 10% fetal bovine serum (FBS) for MEFs, or DMEM supplemented with Na pyruvate and NEAA and 15% FBS and leukemia inhibitory factor (LIF) for ES cells. MEFs were synchronized by double block with 50 ng/ml methotrexate and 1 mM thymidine, and then arrested in metaphase with 1 μg/ml colcemid. ES cells were arrested at metaphase with colcemid at 100 ng/ml. (Detailed protocol available upon request.)
CreTAT treatment
Rapidly dividing cells were grown to 50–70% confluence. Cells were then washed with PBS and incubated in serum-free medium supplemented with 4 μM CreTAT protein for 1 hr at 37°.
Data availability statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are represented fully within the article.
Results
As a practical demonstration of dicentric chromosome formation, we obtained flies carrying an insertion of the Flip-Flop construct, either on chromosome 2 or chromosome 3. The Flip-Flop cassette was developed as a method for switching between GFP and mCherry labeling of gene traps in Drosophila (Nagarkar-Jaiswal et al. 2017). Inverted FRTs were placed so that inversion of the flanked segment switches between the two markers.
To test whether dicentric chromosomes would form after expression of the FLP recombinase, we generated progeny that contained the Flip-Flop construct and a heat shock inducible FLP gene (70FLP). We heat shocked larvae for 1 hr at 38° and then examined neuroblast mitotic spreads from third instar larvae (Figure 2). With a Flip-Flop insertion on 2L, 63% of metaphases showed dicentric/acentric chromosome 2 figures at 4–5 hr after heat shock. On 3R, 46% of chromosome 3 figures showed the same. At 23 hr after heat shock, cells showing dicentric/acentric figures were approximately half as frequent as the earlier time point, owing, no doubt, to the arrest and death of cells suffering from aneuploidy and broken chromosomes during the intervening period (Table 1; Titen and Golic (2008)). These results demonstrate that FLP recombinase does not benignly switch markers within the Flip-Flop cassette, but also causes dicentric chromosome formation. Our previous work shows that this is frequently, but not inevitably, followed by apoptosis (Golic 1994; Titen and Golic 2008; Kurzhals et al. 2011).
Figure 2.
Dicentric chromosomes in Drosophila larval neuroblasts. (A) The normal D. melanogaster karyotype. (B) Dicentric and acentric chromosomes formed by unequal sister chromatid exchange using a FlipFlop construct inserted at 37B on chromosome 2. Normal chromosomes are indicated in blue; dicentric (Dc) and acentric (Ac) products indicated in red. (C) Dicentric/acentric chromosomes produced using a FlipFlop construct at 82E on chromosome 3.
Table 1. Dicentric chromosome frequencies with FlipFlop insertions.
| Location | Hours after heat shock (hr) | Number of brains | Dicentric/acentric karyotype | Normal karyotype | Total metaphases |
|---|---|---|---|---|---|
| 2L, 37B | 4–5 | 5 | 67 (46%) | 80 | 147 |
| 23 | 2 | 9 (20%) | 37 | 46 | |
| 3R, 82E | 4–5 | 7 | 132 (63%) | 76 | 208 |
| 23 | 3 | 21 (30%) | 49 | 70 |
In the mouse, there is also ample evidence that the use of inverted lox sites produces dicentric chromosomes. As a direct demonstration of this, we generated ES cells carrying a targeted insertion of a construct with inverted loxP sites telomere adjacent on chromosome 7 within the coding region of fgf4, and MEFs with inverted loxP sites near the centromere of chromosome 15 within the coding region of ext1. The ES cells were transfected with Cre recombinase expression vector and the MEFs were treated with the CreTAT protein on plates. In either case, we would expect to see splitting of the targeted chromosome into acentric and dicentric portions (Figure 3). Following incubation in the presence of the Cre recombinase we observed dicentric and acentric chromosome formation (Figure 4, A–D and Table 2). Upon extended culture we observed further chromosome rearrangements, polyploidy, and evidence of breakage-fusion-bridge cycles (Figure 4, E–H). We also directly visualized dicentric chromosome formation in live embryos carrying the inverted loxP sites on the tip of chromosome 7 and an HPRTCRE transgene—a constitutive Cre allele whose expression results in maternal deposition of the Cre protein (Figure 5).
Figure 3.
Dicentric chromosomes produced in mouse cells. Following exposure to Cre, asymmetric recombination between inverted loxP sites targeted to a locus near (A) the end of chromosome 7, or (B) near the centromere of chromosome 15, would result in a dicentric chromosome and an acentric chromosome fragment. As indicated, the recombinant chromosome products are expected to be significantly different in size.
Figure 4.
Evidence of Cre-mediated dicentric and acentric chromosome formation in mouse cells. Pictured here are mitotic figures of cultured cells transfected with a Cre expression vector or purified Cre protein. The targeted chromosomes were visualized with DAPI (A, C, E, and G), or DAPI plus whole chromosome paints (B, D, F, and H) specific for either chromosome 15 (A, B, E, and F) or 7 (C, D, G, and H). Exposure to Cre for a short duration (∼24 hr) results in large acentric fragments (A and B) or short acentric fragments and a large dicentric chromosome (C and D). Longer exposure to Cre results in evidence of further chromosome damage, such as smaller than expected chromosomes (E and F) or extraordinarily long chromosomes (G and H). Arrows indicate acentric fragments and arrowheads indicate dicentric chromosomes.
Table 2. Dicentric chromosome production in mouse cells.
| Insertion site | Normal karyotype | Dicentric chromosome | Acentric chromosome | >2n | N optical fields |
|---|---|---|---|---|---|
| chr. 7 | 40 | 15 | 73 | 16 | 100 |
| chr. 15 | 40 | 19 | 23 | 19 | 29 |
Cells with inverted loxP targets, inserted on chromosome 7 or chromosome 15, were exposed to Cre protein and scored for dicentric or acentric figures, or polyploidy (>2n), in metaphase. Chromosome-specific paints were used to recognize chromosome 7 or 15
Figure 5.
Dicentric bridge formation in early embryos. Mouse embryos carrying two inverted loxP sites on chromosome 7 with maternal Cre and Histone-GFP were used to make time-lapse movies of the early cleavage divisions. (A and B) are images of two successive timepoints during anaphase showing persistence of a dicentric chromosome during mitotic anaphase (arrow).
As a second test of dicentric/acentric formation in live embryos, we generated mice carrying a construct with the Tet Repressor (TetR) gene distal to inverted loxP sites inserted in the subtelomeric region of chromosome 19. Proximal to the loxP sites is a tetracycline-repressible reporter gene encoding the red fluorescent protein tdTomato (Figure 6). When dicentric/acentric chromosomes are formed by Cre activity, and the acentric portion carrying the TetR is lost, tdTomato can be expressed. We examined 2–32 cell early mouse embryos carrying this construct and HPRTCre. We observed tdTomato activation in a subset of cells, indicating that TetR had been lost (Figure 6).
Figure 6.
Marking cells after loss of acentric chromosome. (A) Schematic representation of the transgene targeted to the tip of chromosome 19. The transgene encodes a minimal CMV promoter (PCMV), the tetracycline operator sequence (tetO7), and the tdTomato coding sequence, centromere proximal to inverted loxP sites; a strong CAG promoter (PCAG) controlling expression of the TetR-KRAB repressor gene lies distal to the loxP sites. Loss of the PCAG-tetR-KRAB portion relieves repression of tdTomato expression, resulting in cells that fluoresce red. Early embryos carrying this construct, and maternally deposited Cre protein, were examined for tdTomato fluorescence. Nuclei are visualized with histone-GFP (green). Cells that have lost the TetR gene express tdTomato (red): two cells in (B) and ∼6 cells in (C).
Discussion
The occurrence of dicentric/acentric chromosomes will almost certainly confound the interpretation of experiments that use inverted target sites. We have shown, here and previously, that generation of dicentric/acentric chromosomes can be very frequent—in some cases nearly 100% of cells that express FLP and carry inverted FRTs can experience this event (Titen and Golic 2010; Kurzhals et al. 2017). These cells frequently, but not inevitably, undergo apoptosis (Ahmad and Golic 1999; Titen and Golic 2008; Kurzhals et al. 2011). This outcome can also be quite frequent when using Cre-lox in mice (Grégoire and Kmita 2008).
Experimental outcomes may be impacted, and possibly misinterpreted, by dicentric/acentric chromosome formation. As one possible example, recombination with the Confetti construct was used to mark and examine cell clones in the mouse intestine. Death of some clones, and their replacement by other clones, was observed (Snippert et al. 2010). It is difficult to exclude the possibility that this outcome was influenced by dicentric/acentric formation and subsequent death of cells with broken chromosomes or aneuploidy. More recently, a Brainbow construct was injected into single cell zebrafish embryos, along with a Cre expression vector, and the multicolor output was used to trace cell fate in the developing brain. The authors observed death of clones of cells during brain development (Brockway et al. 2019). While the particular Brainbow construct used carries only directly repeated lox sites, the method of transformation typically generates tandem arrays of the injected DNA, and in many, if not most, cases, some of the inserted copies are in opposite orientation to the others (Stuart et al. 1988; 1990; Culp et al. 1991). In this case, the formation of dicentric/acentric chromosomes is likely. Although it is possible that clonal death is a normal part of development of the central nervous system, in this experiment it may instead be a consequence of the formation of dicentric and acentric chromosomes. In this context, it is worth noting that cells which experience dicentric/acentric formation can divide several times to produce a clone before they die. Such cells can even differentiate into adult tissues, though they are less successful than wildtype cells (Golic 1994; Otsuji et al. 2008; Titen and Golic 2008; Tada et al. 2009; Zhu et al. 2010; Kurzhals et al. 2011).
Our results, and those from other groups, make it exceedingly clear that the use of inverted target sites for a site-specific recombinase will frequently lead to the production of dicentric and acentric chromosomes, with significant effects on the behaviors and fates of cells in which they occur. There are schemes that use directly repeated target sites for gene switches and for lineage tracing (Nagy 2000; Kwan 2002; Zong et al. 2005; Gierut et al. 2014; Hubbard 2014; Richier and Salecker 2014; Weissman and Pan 2015; Muñoz-Jiménez et al. 2017; Pontes-Quero et al. 2017; Germani et al. 2018). These do not suffer the drawback of generating dicentric and acentric chromosomes and can be applied to many experimental situations.
Acknowledgments
This work was supported by grants R01 GM065604 (K.G.G.) and R01 MH093595 (M.C.) from the National Institutes of Health (NIH), and by a grant from the Halt Cancer at X Foundation (S.W.A.T.). M.T.B.J. was partially supported by Training Grant T32GM007464 from the NIH. We thank Anne Boulet for the generous gift of the Fgf4-targeted mouse line.
Footnotes
Communicating editor: B. Calvi
Literature Cited
- Ahmad K., and Golic K. G., 1998. The transmission of fragmented chromosomes in Drosophila melanogaster. Genetics 148: 775–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad K., and Golic K. G., 1999. Telomere loss in somatic cells of Drosophila causes cell cycle arrest and apoptosis. Genetics 151: 1041–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annaluru N., Muller H., Mitchell L. A., Ramalingam S., Stracquadanio G. et al. , 2014. Total synthesis of a functional designer eukaryotic chromosome. Science 344: 55–58 (erratum: Science 344: 816). 10.1126/science.1249252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockway N. L., Cook Z. T., O’Gallagher M. J., Tobias Z. J. C., Gedi M. et al. , 2019. Multicolor lineage tracing using in vivo time-lapse imaging reveals coordinated death of clonally related cells in the developing vertebrate brain. Dev. Biol. 453: 130–140. 10.1016/j.ydbio.2019.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin A.-L., Lin C.-Y., Fu T.-F., Dickson B. J., and Chiang A.-S., 2014. Diversity and wiring variability of visual local neurons in the Drosophila medulla M6 stratum. J. Comp. Neurol. 522: 3795–3816. 10.1002/cne.23622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culp P., Nüsslein-Volhard C., and Hopkins N., 1991. High-frequency germ-line transmission of plasmid DNA sequences injected into fertilized zebrafish eggs. Proc. Natl. Acad. Sci. USA 88: 7953–7957. 10.1073/pnas.88.18.7953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roo J. J., Vloemans S. A., Vrolijk H., de Haas E. F., and Staal F. J., 2019. Development of an in vivo model to study clonal lineage relationships in hematopoietic cells using Brainbow2.1/Confetti mice. Future Sci. OA 5: FSO427 10.2144/fsoa-2019-0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dymond J. S., Richardson S. M., Coombes C. E., Babatz T., Muller H. et al. , 2011. Synthetic chromosome arms function in yeast and generate phenotypic diversity by design. Nature 477: 471–476. 10.1038/nature10403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckrich S., Hecker D., Sorg K., Blum K., Fischer K. et al. , 2019. Cochlea-specific deletion of Cav1.3 calcium channels arrests inner hair cell differentiation and unravels pitfalls of conditional mouse models. Front. Cell. Neurosci. 13: 225 10.3389/fncel.2019.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falco S. C., Li Y., Broach J. R., and Botstein D., 1982. Genetic properties of chromosomally integrated 2 mu plasmid DNA in yeast. Cell 29: 573–584. 10.1016/0092-8674(82)90173-8 [DOI] [PubMed] [Google Scholar]
- Fanti L., and Pimpinelli S., 2004. Immunostaining of squash preparations of chromosomes of larval brains, pp. 353–361 in Methods in Molecular Biology, edited by Henderson D. S. Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- Fisher Y. E., Yang H. H., Isaacman-Beck J., Xie M., Gohl D. M. et al. , 2017. FlpStop, a tool for conditional gene control in Drosophila. eLife 6: e22279 10.7554/eLife.22279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germani F., Bergantiños C., and Johnston L. A., 2018. Mosaic analysis in Drosophila. Genetics 208: 473–490. 10.1534/genetics.117.300256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierut J. J., Jacks T. E., and Haigis K. M., 2014. Strategies to achieve conditional gene mutation in mice. Cold Spring Harb Protoc 2014: 339–349. 10.1101/pdb.top069807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson L., 2003. Cre–lox recombination: Cre-ative tools for plant biotechnology. Trends Biotechnol. 21: 550–555. 10.1016/j.tibtech.2003.09.011 [DOI] [PubMed] [Google Scholar]
- Golic K. G., 1991. Site-specific recombination between homologous chromosomes in Drosophila. Science 252: 958–961. 10.1126/science.2035025 [DOI] [PubMed] [Google Scholar]
- Golic K. G., 1994. Local transposition of P elements in Drosophila melanogaster and recombination between duplicated elements using a site-specific recombinase. Genetics 137: 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golic K. G., and Golic M. M., 1996. Engineering the Drosophila genome: chromosome rearrangements by design. Genetics 144: 1693–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golic K. G., and Lindquist S., 1989. The FLP recombinase of yeast catalyzes site-specific recombination in the Drosophila genome. Cell 59: 499–509. 10.1016/0092-8674(89)90033-0 [DOI] [PubMed] [Google Scholar]
- Golic M. M., Rong Y. S., Petersen R. B., Lindquist S. L., and Golic K. G., 1997. FLP-mediated DNA mobilization to specific target sites in Drosophila chromosomes. Nucleic Acids Res. 25: 3665–3671. 10.1093/nar/25.18.3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grégoire D., and Kmita M., 2008. Recombination between inverted loxP sites is cytotoxic for proliferating cells and provides a simple tool for conditional cell ablation. Proc. Natl. Acad. Sci. USA 105: 14492–14496. 10.1073/pnas.0807484105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjieconomou D., Rotkopf S., Alexandre C., Bell D. M., Dickson B. J. et al. , 2011. Flybow: genetic multicolor cell labeling for neural circuit analysis in Drosophila melanogaster. Nat. Methods 8: 260–266. 10.1038/nmeth.1567 [DOI] [PubMed] [Google Scholar]
- Hubbard E. J. A., 2014. FLP/FRT and Cre/lox recombination technology in C. elegans. Methods 68: 417–424. 10.1016/j.ymeth.2014.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. B., Piombo V., Searby C., Kurriger G., Yang B. et al. , 2010. A mouse model of osteochondromagenesis from clonal inactivation of Ext1 in chondrocytes. Proc. Natl. Acad. Sci. USA 107: 2054–2059. 10.1073/pnas.0910875107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzhals R. L., Titen S. W. A., Xie H. B., and Golic K. G., 2011. Chk2 and p53 are haploinsufficient with dependent and independent functions to eliminate cells after telomere loss. PLoS Genet. 7: e1002103 10.1371/journal.pgen.1002103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzhals R. L., Fanti L., Ebsen A. C. G., Rong Y. S., Pimpinelli S. et al. , 2017. Chromosome healing is promoted by the telomere cap component hiphop in Drosophila. Genetics 207: 949–959. 10.1534/genetics.117.300317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan K.-M., 2002. Conditional alleles in mice: practical considerations for tissue-specific knockouts. Genesis 32: 49–62. 10.1002/gene.10068 [DOI] [PubMed] [Google Scholar]
- Lakso M., Sauer B., Mosinger B., Lee E. J., Manning R. W. et al. , 1992. Targeted oncogene activation by site-specific recombination in transgenic mice. Proc. Natl. Acad. Sci. USA 89: 6232–6236. 10.1073/pnas.89.14.6232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T., 2013. Generating mosaics for lineage analysis in flies. WIREs Dev Biol 3: 69–81. 10.1002/wdev.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandoski M., and Martin G. R., 1997. Cre-mediated chromosome loss in mice. Nat. Genet. 17: 223–225. 10.1038/ng1097-223 [DOI] [PubMed] [Google Scholar]
- Livet J., Weissman T. A., Kang H., Draft R. W., Lu J. et al. , 2007. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450: 56–62. 10.1038/nature06293 [DOI] [PubMed] [Google Scholar]
- Moon A. M., Boulet A. M., and Capecchi M. R., 2000. Normal limb development in conditional mutants of Fgf4. Development 127: 989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Jiménez C., Ayuso C., Dobrzynska A., Torres-Mendéz A., de la Cruz Ruiz P. et al. , 2017. An efficient FLP-based toolkit for spatiotemporal control of gene expression in Caenorhabditis elegans. Genetics 206: 1763–1778. 10.1534/genetics.117.201012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarkar-Jaiswal S., Manivannan S. N., Zuo Z., and Bellen H. J., 2017. A cell cycle-independent, conditional gene inactivation strategy for differentially tagging wild-type and mutant cells. eLife 6: e26420 10.7554/eLife.26420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., 2000. Cre recombinase: the universal reagent for genome tailoring. Genesis 26: 99–109. [PubMed] [Google Scholar]
- Otsuji T., Matsumura H., Suzuki T., Nakatsuji N., Tada T. et al. , 2008. Rapid induction of large chromosomal deletions by a cre/inverted loxP system in mouse ES cell hybrids. J. Mol. Biol. 378: 328–336. 10.1016/j.jmb.2008.01.065 [DOI] [PubMed] [Google Scholar]
- Pei W., Feyerabend T. B., Rössler J., Wang X., Postrach D. et al. , 2017. Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature 548: 456–460. 10.1038/nature23653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes-Quero S., Heredia L., Casquero-García V., Fernández-Chacón M., Luo W. et al. , 2017. Dual ifgMosaic: a versatile method for multispectral and combinatorial mosaic gene-function analysis. Cell 170: 800–814.e18. 10.1016/j.cell.2017.07.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M., Bayley C., Stockton T., and Ow D. W., 1994. Cre recombinase-mediated site-specific recombination between plant chromosomes. Proc. Natl. Acad. Sci. USA 91: 1706–1710. 10.1073/pnas.91.5.1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Solis R., Liu P., and Bradley A., 1995. Chromosome engineering in mice. Nature 378: 720–724. 10.1038/378720a0 [DOI] [PubMed] [Google Scholar]
- Richier B., and Salecker I., 2014. Versatile genetic paintbrushes: Brainbow technologies. WIREs Dev Biol 4: 161–180. 10.1002/wdev.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Kato H., Yamaza H., Masuda K., Nguyen H. T. N. et al. , 2017. Engineering of systematic elimination of a targeted chromosome in human cells. BioMed Res. Int. 2017: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnütgen F., Doerflinger N., Calléja C., Wendling O., Chambon P. et al. , 2003. A directional strategy for monitoring Cre-mediated recombination at the cellular level in the mouse. Nat. Biotechnol. 21: 562–565. 10.1038/nbt811 [DOI] [PubMed] [Google Scholar]
- Smith A. J., De Sousa M. A., Kwabi-Addo B., Heppell-Parton A., Impey H. et al. , 1995. A site-directed chromosomal translocation induced in embryonic stem cells by Cre-loxP recombination. Nat. Genet. 9: 376–385 [corrigenda: Nat. Genet. 12: 110 (1996)]. 10.1038/ng0495-376 [DOI] [PubMed] [Google Scholar]
- Snippert H. J., van der Flier L. G., Sato T., van Es J. H., van den Born M. et al. , 2010. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143: 134–144. 10.1016/j.cell.2010.09.016 [DOI] [PubMed] [Google Scholar]
- Stuart G. W., McMurray J. V., and Westerfield M., 1988. Replication, integration and stable germ-line transmission of foreign sequences injected into early zebrafish embryos. Development 103: 403–412. [DOI] [PubMed] [Google Scholar]
- Stuart G. W., Vielkind J. R., McMurray J. V., and Westerfield M., 1990. Stable lines of transgenic zebrafish exhibit reproducible patterns of transgene expression. Development 109: 577–584. [DOI] [PubMed] [Google Scholar]
- Tada M., Matsumura H., Kurose Y., Nakatsuji N., and Tada T., 2009. Target chromosomes of inducible deletion by a Cre/inverted loxP system in mouse embryonic stem cells. Chromosome Res. 17: 443–450. 10.1007/s10577-009-9035-0 [DOI] [PubMed] [Google Scholar]
- Thomas R., Marks D. H., Chin Y., and Benezra R., 2017. Whole chromosome loss and associated breakage–fusion–bridge cycles transform mouse tetraploid cells. EMBO J. 37: 201–218. 10.15252/embj.201797630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titen S. W. A., and Golic K. G., 2008. Telomere loss provokes multiple pathways to apoptosis and produces genomic instability in Drosophila melanogaster. Genetics 180: 1821–1832. 10.1534/genetics.108.093625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titen S. W. A., and Golic K. G., 2010. Healing of euchromatic chromosome breaks by efficient de novo telomere addition in Drosophila melanogaster. Genetics 184: 309–312. 10.1534/genetics.109.109934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Yau Y.-Y., Perkins-Balding D., and Thomson J. G., 2010. Recombinase technology: applications and possibilities. Plant Cell Rep. 30: 267–285. 10.1007/s00299-010-0938-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman T. A., and Pan Y. A., 2015. Brainbow: new resources and emerging biological applications for multicolor genetic labeling and analysis. Genetics 199: 293–306. 10.1534/genetics.114.172510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. L., Shearin H. K., and Stowers R. S., 2019. Conditional synaptic vesicle markers for Drosophila. G3 (Bethesda) 9: 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhu R.-Y., Mitchell L. A., Ma L., Liu R. et al. , 2018. In vitro DNA SCRaMbLE. Nat. Commun. 9: 1935 10.1038/s41467-018-03743-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H. B., 2005. Gene trap and gene inversion methods for conditional gene inactivation in the mouse. Nucleic Acids Res. 33: e14 10.1093/nar/gni016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Nguyen M.-T., Nakamura E., Yang J., and Mackem S., 2012. Cre-mediated recombination can induce apoptosis in vivo by activating the p53 DNA damage-induced pathway. Genesis 50: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Kim Y.-M., Li S., and Zhuang Y., 2010. Generation and analysis of partially haploid cells with cre-mediated chromosome deletion in the lymphoid system. J. Biol. Chem. 285: 26005–26012. 10.1074/jbc.M110.139196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H., Espinosa J. S., Su H. H., Muzumdar M. D., and Luo L., 2005. Mosaic analysis with double markers in mice. Cell 121: 479–492. 10.1016/j.cell.2005.02.012 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are represented fully within the article.