Abstract
Hybrid sterility is a hallmark of speciation, but the underlying molecular mechanisms remain poorly understood. Here, we report that speciation may regularly proceed through a stage at which gene flow is completely interrupted, but hybrid sterility occurs only in male hybrids whereas female hybrids reproduce asexually. We analyzed gametogenic pathways in hybrids between the fish species Cobitis elongatoides and C. taenia, and revealed that male hybrids were sterile owing to extensive asynapsis and crossover reduction among heterospecific chromosomal pairs in their gametes, which was subsequently followed by apoptosis. We found that polyploidization allowed pairing between homologous chromosomes and therefore partially rescued the bivalent formation and crossover rates in triploid hybrid males. However, it was not sufficient to overcome sterility. In contrast, both diploid and triploid hybrid females exhibited premeiotic genome endoreplication, thereby ensuring proper bivalent formation between identical chromosomal copies. This endoreplication ultimately restored female fertility but it simultaneously resulted in the obligate production of clonal gametes, preventing any interspecific gene flow. In conclusion, we demonstrate that the emergence of asexuality can remedy hybrid sterility in a sex-specific manner and contributes to the speciation process.
Keywords: meiosis, speciation, gynogenesis, hybridization, polyploidy, clonality, Cobitis, endoreplication
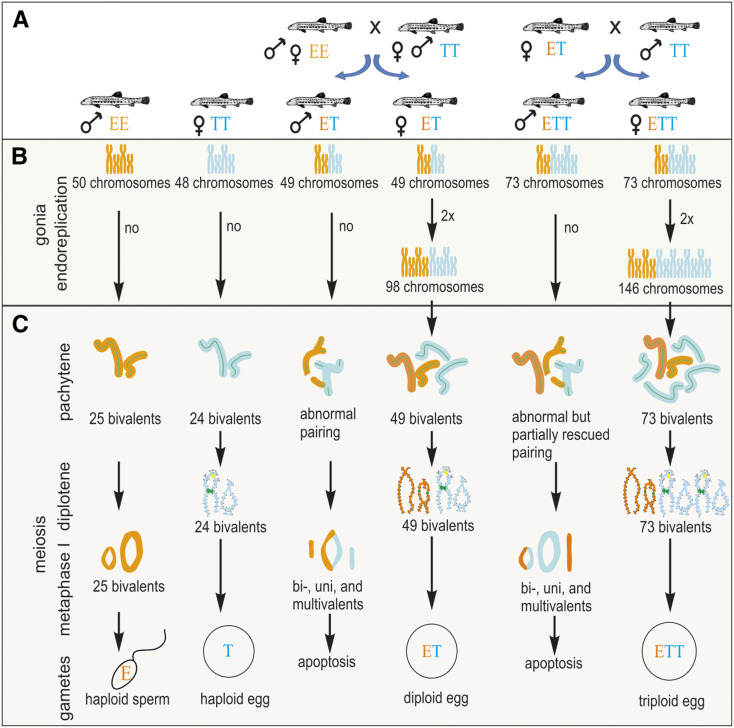
MOST multicellular organisms pass their genes to the next generation through specialized cells, the gametes. Although, the molecular machinery controlling the production of reduced gametes is highly conserved among Metazoans (Bernstein and Bernstein 2010), gametogenesis has been repeatedly modified over the course of evolution, giving rise to many clonal or asexual taxa. Such organisms are excellent models to address fundamental questions pertaining to persistence of sex. Nevertheless, surprisingly few answers have been posited for even the most basic questions. For instance, unreduced gametes may be produced by a wide range of cytogenetic mechanisms, ranging from completely ameiotic processes (apomixis) to those with meiosis and even recombination retained (automixis) [reviewed by Stenberg and Saura (2009) and Lenormand et al. (2016)]. However, it is unclear why some mechanisms differ even among closely related species while other gametogenic pathways have evolved more commonly across unrelated asexual taxa. A common way of achieving clonality is via the premeiotic endoreplication of chromosomes during the proliferation of germ cells, which allows the pairing of identical chromosomal copies during meiotic division and leads to the production of unreduced gametes and a lack of variability among offspring (Figure 1) (Macgregor and Uzzel 1964; Lutes et al. 2010).
Figure 1.
Schematic overview of gametogenic pathways and aberrations in parental species and hybrids of both sexes. (A) Scheme of reproductive interactions among studied genomotypes and the types of resulting progeny. (B) Hypothetical gametogenic pathways before meiosis. (C) Observed stages of meiosis. Note that for methodological reasons, we studied the pachytene stage in both sexes, but that diplotene and metaphase I stages could be observed only in females and males, respectively.
It also remains unclear what mechanisms cause the switch from sexual to asexual reproduction. Since many asexual organisms are interspecific hybrids, it seems likely that a link between asexual reproduction and interspecific hybridization exists. To explain this link, Ernst (1918) proposed that the type of a hybrid’s reproduction depends on divergence between its parental species, following a continuum from sexually reproducing hybrids between closely related parents to obligate asexual hybrids between distant parental species. More recently, Moritz et al. (1989) formulated the “balance hypothesis,” which assumes that asexuality may arise when hybridizing species accumulate a sufficient number of incompatibilities between genes to disrupt meiosis in hybrids, yet not enough to seriously compromise their fertility. On the other hand, De Storme and Mason (2014) suggested that the production of unreduced gametes might be a consequence of decreased sequence homology preventing the pairing and segregation of orthologous chromosomes in a hybrid. Carman (1997) suggested that hybrid asexuality results from asynchronous expression of genes brought together by hybridization between species with differently timed developmental programs.
Although empirical support for these hypotheses remains scarce, recent analysis of diversification and speciation in spined loaches (Cobitis, Teleostei) has brought direct evidence that emergence of asexuality is a direct consequence of interspecific hybridization and is also correlated with the phylogenetic divergence of parental species (Choleva et al. 2012, Janko et al. 2018). Namely, we found that early stages of Cobitis speciation were accompanied by the production of sexually reproducing hybrids mediating interspecific gene flow. At later stages, hybridization between diverged species did not lead to sexually reproducing hybrids anymore, but instead gave rise to exclusively sterile males and clonal females. Since production of unrecombined gametes in hybrids efficiently restricts interspecific gene exchange (Lampert et al. 2007; Janko et al. 2018), these data demonstrate that the production of clonal gametes represents a special form of postzygotic barrier that tends to evolve at lower divergences than complete sterility or hybrid inviability (Janko et al. 2018). Comparative analysis of genetic divergences and reproductive modes across Actinopterygian fishes further showed that the “divergence window” allowing asexuality to arise is quite wide, ranging from closely related species pairs with < 6% divergence in mitochondrial DNA to those with > 15% divergence (Janko et al. 2018).
Thus, a century after Ernst (1918), it has become clear that theories relating the initiation of asexuality to hybridization have many analogies to speciation models that involve postzygotic reproductive incompatibilities. Indeed, both concepts emphasize the role of accumulating divergence between hybridizing species, and both seek the causal mechanisms underlying the emergence of asexuality as well as hybrid sterility. Namely, the balance hypothesis (Moritz et al. 1989) matches the Dobzhansky–Muller genic speciation models by postulating the prominent role of gene-to-gene interactions. On the other hand, the model of De Storme and Mason (2014) is similar to the original perspective of Bateson (1909) of nongenic residues as implemented in current chromosomal speciation models (Faria and Navarro 2010), and Carman (1997) envisaged the roles of diverged regulatory networks in the establishment of hybrid asexuality, which is similar to the perspective of Tulchinsky et al. (2014) regarding postzygotic trans-regulatory incompatibility.
An important feature common to both the accumulation of postzygotic reproductive incompatibilities and hybrid asexuality is their tendency to emerge in a sex-specific manner. Indeed, early stages of speciation are often characterized by a prominent decrease in fertility of only one hybrid sex, which may be related to the sex-determination system (Haldane 1922). Although in classical speciation cases both hybrid sexes ultimately achieve sterility at advanced stages of divergence, it remains unclear whether that proceeds by the same routes in males and females (e.g., Bhattacharyya et al. 2013; Torgasheva and Borodin 2016). Similarly, in the majority of asexually reproducing organisms, only the females are able to overcome the postzygotic barriers via alteration in gametogenesis. However, it is unclear whether female bias in asexual taxa results from true sex-specificity of clonal gametogenic pathways or from observational bias, as asexual males can escape researchers’ attention more easily than females because they usually cannot generate progeny on their own [for exceptions, see e.g., androgenetic taxa (Mantovani and Scali 1992)].
Therefore, current understanding of causal relationships between hybridization, asexuality, and speciation does not include mechanistic insight into similarities and differences between gametogenic pathways of the hybrid’s sexes. Interestingly, hybrid brothers of asexual females are usually sterile or absent (e.g., Park et al. 2011; Spangenberg et al. 2017; but see Graf and Polls-Pelaz 1989), which implies the existence of some sex-specific differences in chromosomal segregation pathways. In this respect, the most compelling information comes from the Misgurnus anguillicaudatus complex, which consists of several undescribed species, some of which produce hybrid males with gametogenesis failing due to the improper pairing of orthologous chromosomes, whereas hybrid females possess the ability to produce unreduced gametes due to premeiotic endoreplication (Kuroda et al. 2018, 2019). It is noteworthy that the production of clonal gametes is restricted to only some hybrid females, usually diploid. Other types produce reduced gametes or even several gamete types simultaneously (Zhang et al. 1998; Itono et al. 2006; Morishima et al. 2008).
In the present study, we analyzed the meiotic pathways of both hybrid sexes across two distinct ploidy levels. We investigated the mechanisms underlying asexuality and hybrid sterility using the freshwater fish of the Cobitis taenia hybrid species complex (Teleostei). This model taxon, which is distantly related to M. anguillicaudatus, is particularly advantageous for evolutionary studies owing to its well-resolved taxonomy and reconstructed speciation history. Comparison with M. anguillicaudatus allows general conclusions to be drawn about the roles of hybridization, speciation, polyploidy, and asexuality. The C. taenia hybrid complex consists of several species; two central to our study, C. elongatoides (diploid genome, denoted as “EE,” has 50 chromosomes) and C. taenia (diploid genome, denoted as “TT,” has 48 chromosomes), diverged ∼9 MYA (Janko et al. 2018) and were subject to intensive introgressive hybridization until the Pleistocene, when gene flow ceased (Janko et al. 2018). Nevertheless, these species can still hybridize and produce viable hybrids. Reproductive experiments and histological observations have shown that C. elongatoides × C. taenia hybrid females (ET, 49 chromosomes) reproduce clonally through sperm-dependent gynogenesis, producing only female hybrid progeny (i.e., they produce unreduced eggs but require sperm to activate cellular division; summarized in Figure 1A; Janko et al. 2007a, 2018; Choleva et al. 2012; Juchno et al. 2017; Juchno and Boroń 2018). The occasional fusion of sperm with hybrid egg pronuclei results in triploid progeny of both sexes with EET (74 chromosomes) or ETT (73 chromosomes) genomic constitutions, but only females are able to clonally propagate (Figure 1A). In contrast to their clonal sisters, the diploid (ET) and triploid (EET and ETT) male hybrids have drastically reduced reproductive capabilities, and appear to be incapable of either fertilizing normal haploid eggs or triggering the development of clonal eggs (Figure 1A) (Vasil’ev et al. 2003; Choleva et al. 2012; Juchno and Boroń 2018).
Our study resolves the link between asexuality and speciation by revealing the mechanistic basis of the sterility of hybrid males and the asexuality of hybrid females. It also elucidates the effects of genome duplications and polyploidy on hybrid gametogenesis. We show that a sex-specific cytogenetic mechanism underlying parthenogenetic reproduction may mitigate the effects of hybrid sterility in females, but provides an efficient barrier to interspecific gene flow and thereby contributes to speciation.
Materials and Methods
Samples
The identity, ploidy, and genome composition of every specimen in this study were evaluated using standard species-diagnostic markers (Janko et al. 2007b). In total, 15 males were analyzed: 3 C. taenia (TT), 3 C. elongatoides (EE), 5 diploid hybrid (ET), and 4 triploid hybrid (ETT) individuals. After genotype identification, the gonads of each male were inspected using classic histology examination, transmission electron microscopy, meiotic chromosome analyses, pachytene chromosome analyses, and DNA flow cytometry. As for females, we analyzed 12 individuals: 4 TT, 4 EE, 3 diploid hybrid ET, and 5 triploid hybrid ETT individuals. All females and the triploid ETT males were collected from the wild. Diploid ET males represent F1 generations from experimental crosses because hybrid males are not able to reproduce in nature. Thus, only triploid males are occasionally found as a result of ET females backcrossing to parental species and accidentally accepted sperm, while true diploid F1 males have never been observed in nature.
Histology and SEM
Fragments of testes for light and electron microscopy were fixed at 4° in 2.5% glutaraldehyde with phosphate buffer (pH 7.4) and postfixed in 1% osmium tetroxide using the same buffer. After dehydration with an ethanol series, the tissues were embedded in Epon 812. Semithin sections were stained with methylene blue, while ultrathin sections of the selected areas were contrasted with uranyl acetate and lead citrate, and analyzed using a JOELJEM-100Sx transmission electron microscope. Apoptotic cells were detected with the QIA33 | FragEL DNA Fragmentation Detection Kit.
DNA flow cytometry
Genome sizes of cell populations from the testes were estimated by measurement of the cell nuclei using a BD FACSAria II flow cytometer. A detailed description of the method is given in Supplemental Methods.
Mitotic and meiotic metaphase chromosomes
Mitotic and meiotic metaphase chromosome spreads were obtained from the kidneys and testes of sexual and hybrid males without colchicine treatment according to standard procedures (Macgregor and Varley 1983). To confirm the numbers and morphologies of chromosomes and bivalent formations, chromosome metaphases were initially stained with Giemsa staining buffer (5%, 10 min).
Pachytene chromosomes and immunofluorescent staining
Pachytene chromosome spreads were prepared from testes according to Moens (2006) and from ovaries following Araya-Jaime et al. (2015). Synaptonemal complexes (SCs) were visualized using immunofluorescent staining with antibodies against SYCP3 (ab14206; Abcam) and SYCP1 (a gift from Sean M. Burgess). The recombination sites were visualized by antibody staining against the MLH1 (ab15093; Abcam) proteins. Detailed descriptions of pachytene chromosome preparation and immunofluorescent staining are given in File S1.
FISH and comparative genomic hybridization
Telomeric FISH using a Cy3-labeled peptide nucleic acid (PNA) probe was performed on metaphase and pachytene chromosomes according to the manufacturer’s instructions (Telomere PNA FISH Kit/Cy3, Dako, Glostrup, Denmark). After telomeric FISH, slides were destained and comparative genomic hybridization (CGH) was performed according to Majtánová et al. (2016). A detailed description of CGH methodology is given in File S1.
Diplotene chromosomes
Chromosomes during the diplotene stage (lampbrush chromosomes) were microsurgically isolated from growing oocytes of sexual and hybrid females according to Callan (1986) with modifications suggested by Gall et al. (1991). Descriptions of bivalent morphology and construction of lampbrush chromosome maps were performed according to Callan (1986) in CorelTM DRAW graphics suite X8 software. Details of diplotene chromosome preparation are given in File S1.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Supplemental material available at figshare: https://doi.org/10.25386/genetics.11988192.
Results
The female story
Analyses of chromosomal pairing:
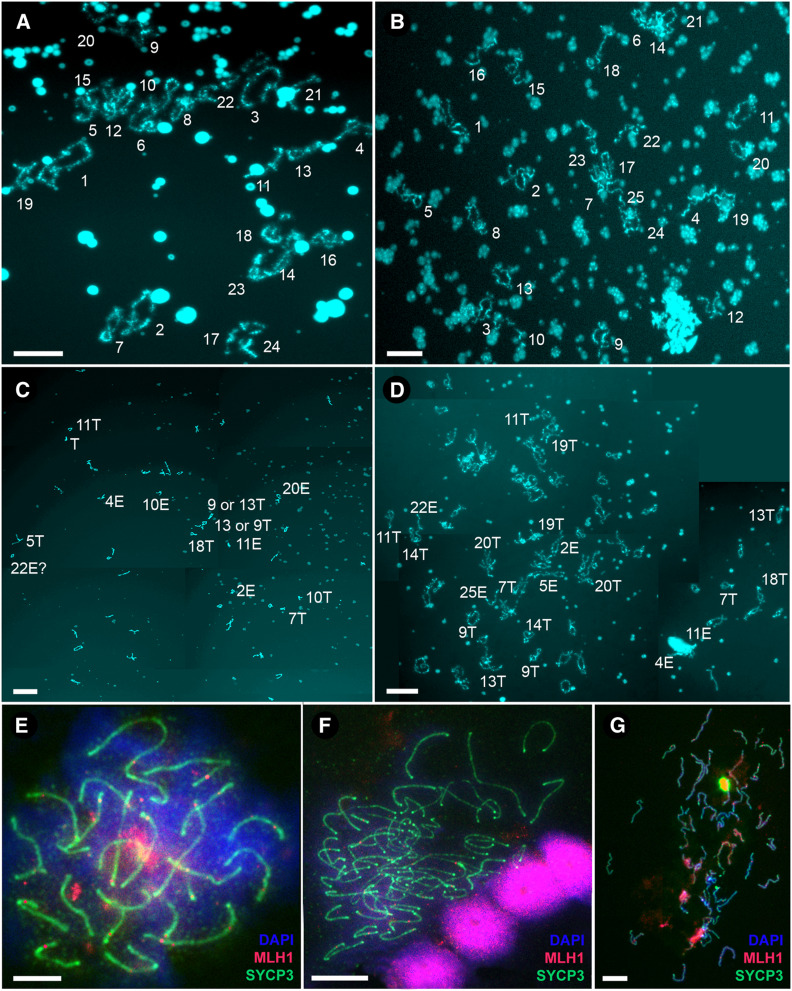
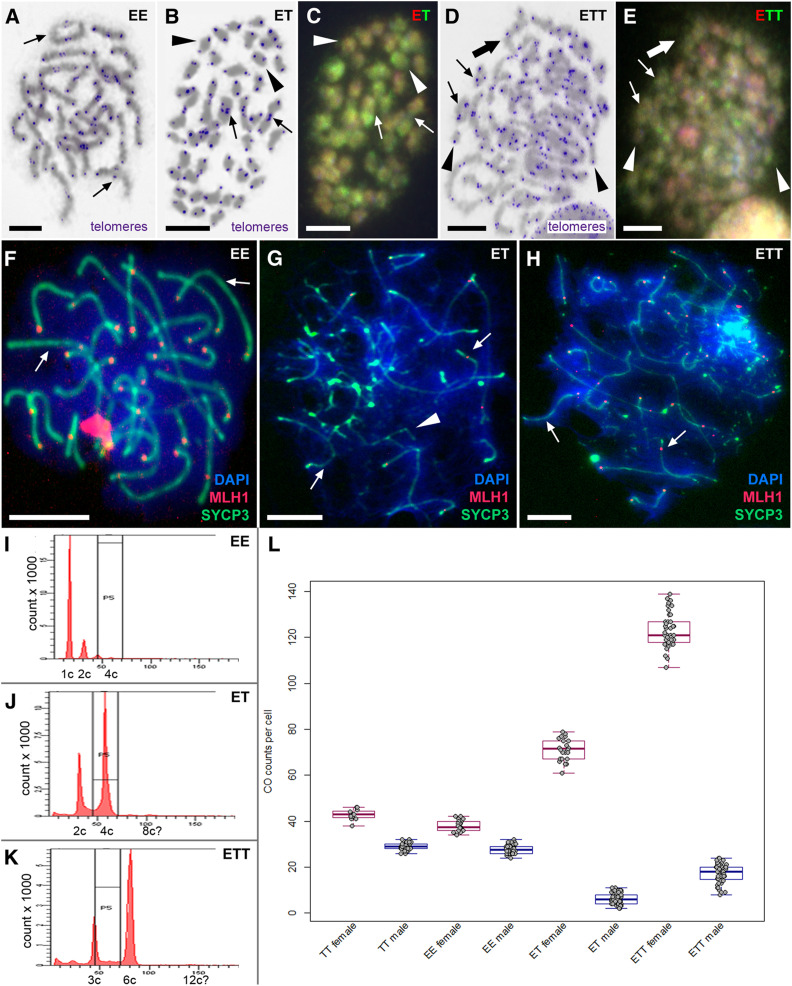
To investigate the number of bivalents and chromosomal pairs during female meiotic division, we analyzed the chromosomal spreads from diplotene and pachytene oocytes. In both parental species, we observed, as expected, the same number of chromosomes as in their somatic cells (i.e., 48 in C. taenia and 50 in C. elongatoides), which paired into bivalents whose numbers equaled exactly one-half of these counts (i.e., 24 and 25 bivalents, respectively; Figure 2, A, B, and E). To confirm bivalent formation during the pachytene stage of the sexual species we stained the axial (SYCP1) and lateral (SYCP3) elements of the SCs. In contrast, the number of bivalents in diploid ET and triploid ETT diplotene and pachytene nuclei always equaled the total number of chromosomes in their somatic cells (i.e., 49 bivalents in ET and 73 bivalents in ETT; Figure 2, C, D, F, and G and Figure 3B), suggesting that the numbers of chromosomes in oocytes were doubled as compared with somatic cells. Since we only observed properly paired bivalents in oocytes of hybrid females with no uni- or multivalents, such an increase in chromosomal number indicates the presence of premeiotic genome doubling during gametogenesis.
Figure 2.
Female meiotic spreads at diplotene and pachytene stages. (A–D) The spreads of lampbrush chromosomes from diplotene oocytes of C. taenia with 24 bivalents (A), C. elongatoides with 25 bivalents (B), diploid ET hybrid with 49 bivalents (C), and triploid ETT hybrid with 73 bivalents (D). Lampbrush chromosomes are numbered numerically according to their size and morphology (see Figure S1 for a detailed map of lampbrush chromosomes). Subscripts in italics indicate the distinguishable lampbrush chromosomes unequivocally corresponding to C. elongatoides “e” and C. taenia “t,” respectively. Bar, 50 µm. (E–G) Spread pachytene oocytes of C. elongatoides (E), diploid ET (F), and triploid ETT (G) hybrid females stained with DAPI (blue); synaptonemal complexes were immunolabeled with antibodies against SYCP3 protein (green) and MLH1 protein (red). Bar, 10 µm.
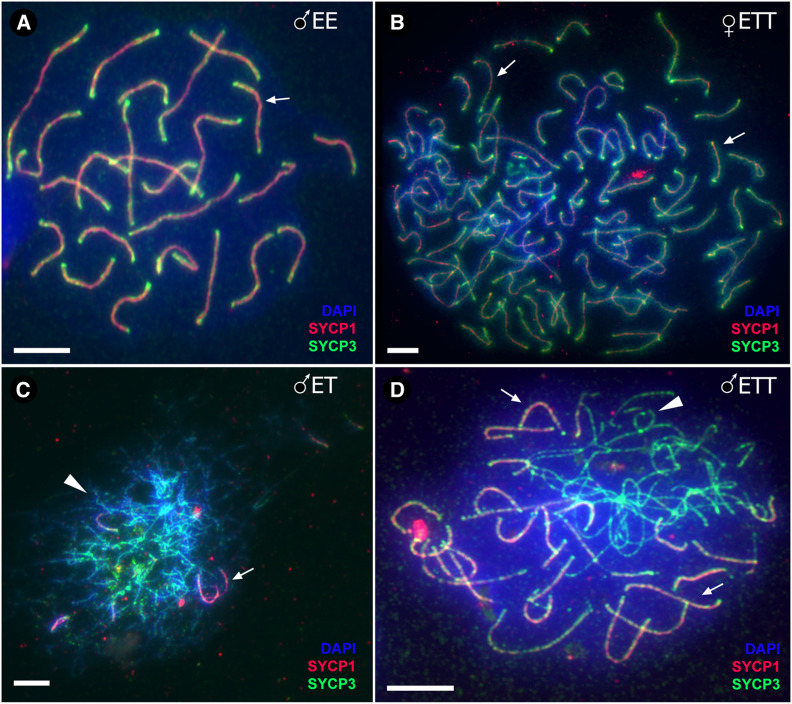
Figure 3.
Meiotic spreads at pachytene stages. (A) Male C. elongatoides, (B) triploid ETT hybrid female, (C) diploid ET hybrid male and (D) triploid ETT hybrid male. Synaptonemal complexes visualized by immunolabeling with antibodies against SYCP3 protein (green) and SYCP1 (red), stained with DAPI (blue). Synapsed chromosomes show both SYCP3 and SYCP1 localization (indicated by arrows), while asynapsed chromosomes exhibit only SYCP3 staining (arrowheads). Bar, 10 mm.
To test homologous vs. orthologous pairing in hybrid females we focused on diplotene chromosomes (lampbrush chromosomes). Throughout this study, we will use the term “homologs” to define homologous chromosomes arisen from a single species and the term “orthologs” to define homologous chromosomes arisen from two species. We identified the ancestry of particular chromosomes through bivalent morphology. That was possible because each parental species possesses several bivalents that may be used as species-specific markers due to unique distributions of characteristic structures (e.g., loops with specific morphology, spheres, and nucleoli loci). In total, we recognized nine marker bivalents for C. elongatoides and eight for C. taenia (Figure 2, A and B and Figure S1, upper panel). In diploid ET hybrid females, we observed all 9 C. elongatoides-specific bivalents and 8 C. taenia-specific bivalents, whereas in triploid ETT females we observed 9 C. elongatoides-specific bivalents and 16 C. taenia-specific bivalents (Figure 2, C and D and Figure S1, lower panel). The similarity of hybrid female bivalents to those of parental species suggests that they are formed by homologous chromosomes, originating from the same species (i.e., E×E and T×T bivalents, respectively).
Analysis of crossover rate:
We estimated the occurrence of crossovers (COs) in females using two methods. First, we used immunofluorescence staining of MLH1 sites and counted the foci in mid- and late-pachytene nuclei (DNA mismatch repair protein) (Baudat and de Massy 2007; Balcova et al. 2016). Second, we analyzed the occurrence of meiotic COs in diplotene oocyte nuclei by counting chiasmata between two bivalents (Callan 1986). Both methods appeared equally reliable as we observed equivalent numbers of CO sites using both chiasmata and MLH1 sites in the females analyzed.
We found clear interspecific differences between C. taenia and C. elongatoides sexual females. On average, C. taenia females had ∼42.8 COs per cell and C. elongatoides females had ∼38 COs per cell (Figure 4L). Female COs were evenly distributed throughout bivalent lengths. CO frequency in the ET hybrid females was significantly lower than predicted by simple extrapolation of sexual counts to endoreplicated hybrid nuclei; we observed only ∼71.3 COs against ∼80.8 expected (one sample Student’s t-test P < 10−5). ETT hybrid females possessed ∼123.4 COs per cell, which almost exactly fits the expectation value (123.6).
Figure 4.
Male meiotic spreads at metaphase I and pachytene stages. (A, B, and D) Giemsa-stained chromosomes (gray) with FISH-labeled telomeres (blue) in C. elongatoides, ET and ETT hybrid males, respectively. (C and E) The same metaphases as in (B and D) of hybrids after comparative genomic hybridization revealing the origins of individual chromosomes (red colour chromosomes correspond to C. elongatoides and green ones to C. taenia). Thin arrows indicate exemplary cases of bivalents, arrowheads exemplary univalents, and thick arrows exemplary cases of multivalents. (F–H) Meiotic spreads at pachytene stage of C. elongatoides (F), diploid ET hybrid (G), and triploid ETT hybrid (H) males stained with DAPI (blue). Synaptonemal complexes were immunolabeled with antibodies against SYCP3 protein (green) and MLH1 protein (red). Arrows indicate exemplary bivalents, arrowheads show examples of abnormal pairing and failures of bivalent formation. Bar, 10 mm. (I–K) Flow cytometry results of testes of C. elongatoides (I), diploid ET hybrid males (J), and triploid ETT hybrid males (K). (L) Diagram shows the average frequencies of crossovers (COs) per cell in studied genomotypes (males and females indicated in blue and red, respectively).
The male story
Histological examination of testes:
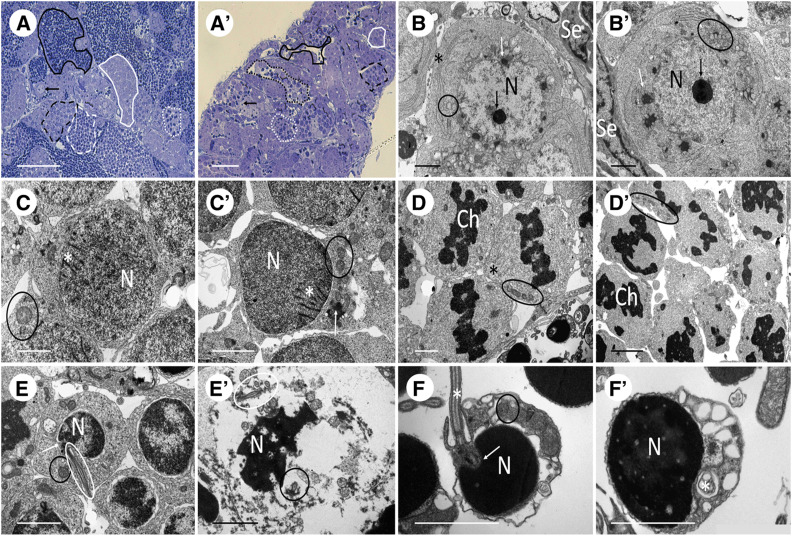
Histological examinations of diploid ET and triploid ETT males performed in this study, and on diploid ET males examined by Juchno and Boroń (2018), demonstrated the reproductive incapacity of hybrid males, reflected by defective development of hybrid testes. Testes of sexual as well as hybrid males contained properly developed Sertoli and Leydig cells; nevertheless, hybrid testes evidenced asynchronous development of germinal cells in cysts (Figure 5, A and A’ and in detail in Figure S2). First, at the beginning of prophase I (leptotene/pachytene), hybrid spermatogonia and spermatocytes displayed proper development with organelles typical for that stage, i.e., as mitochondria and the Golgi apparatus, “nuage,” nucleus, and nucleolus (Figure 5, B, B’, C, and C’). However, in metaphase I, the chromatin of all spermatocytes of sexual males was equatorially distributed, while the spermatocytes of hybrid males displayed irregular chromatin distribution (Figure 5, D and D’).
Figure 5.
Comparison of spermatogenesis between male representatives of sexual diploid species and hybrid genomotypes. As the variation between two species and between two hybrid genomotypes is negligible, for simplicity we selected C. elongatoides and ETT males as representatives of both groups. (A and A’) Semithin sections show spermatogonia (arrow), spermatocyte in zygotene/leptotene (white firm line) and pachytene (black dashed line) of prophase I, spermatocyte in metaphase I (white dotted line), spermatids (white dashed line), and spermatozoa (black firm line), while testes of hybrid displays defective development with only a few single spermatozoa and germ cells of one cyst at different stages (black dotted line). (B and B’) Spermatogonia A with nucleus (N), nucleolus (black arrow), Golgi apparatus (asterisk), mitochondria (black circle), nuage (white arrow), and Sertoli cell (Se). (C and C’) Spermatocyte in zygotene/leptotene of prophase I with nucleus (N), mitochondria (black circle), nuage (white arrow), and synaptonemal complexes (white asterisk). (D and D’) Spermatocytes in metaphase I show compact chromatin (Ch) in equatorial position with spindle fibers (black asterisk) and mitochondria (black circle) (in contrast, hybrid exhibits irregular distribution of chromatin and no spindle fiber formation). (E and E’) Spermatids in sexual diploid display nucleus (N), mitochondria (black circle), basal body (white arrow), and flagellum (white circle), while spermatids of hybrids are usually fragmented and contain numerous axonemes/flagella. (F and F’) spermatozoa are composed of nucleus (N), mitochondria (black circle), basal body (white arrow), and flagellum (white asterisk) (hybrids exhibit very rare occurrence of spermatozoa with generally bigger nucleus).
After metaphase I, hybrids usually displayed fragmented postmeiotic germ cells with numerous nuclear vesicles and multiple axonemes/flagella (Figure 5, E and E’). Rarely, they displayed asynchronously developing cysts with a few typical spermatozoa, containing multiple axonemes or flagella. The nuclei of those spermatozoa were generally larger (Figure 5, F and F’), which may indicate greater DNA content or abnormal chromatin packaging. Hybrid testes also contained many degenerating germinal cells, which were more often found among spermatocytes than among spermatogonia, and we documented frequent apoptotic processes there (for a detailed sexual hybrid comparison see Figure S3).
Flow cytometric analysis:
The testes of hybrid males lacked 1C cells corresponding to haploid sperm nuclei, which are characteristic of sexual males (Figure 4I). Instead, ET hybrid males possessed diploid (2C) and double-diploid (4C) cell populations in their gonads [this situation was analogous in ETT males with prominent triploid (3C) and double-triploid (6C) cell populations] (Figure 4, J and K). These 2C and 3C cell populations in ET and ETT males, respectively, correspond to spermatogonia and somatic cells, while 4C and 6C cells, respectively, probably correspond to primary spermatocytes, which accumulated due to problems during chromosomal pairing. It is noteworthy that flow cytometry of hybrid male gonads revealed a minor cell population (< 3%) with 8C and 12C DNA content in diploid and triploid hybrids, respectively (Figure 4, J and K).
Analyses of chromosomal pairing:
To uncover the initial cause of spermatogenetic aberrations we focused on male meiosis. We analyzed ∼250 meiotic metaphase I spreads in each male, and noticed the same number of chromosomes as in somatic tissues, i.e., no polyploid or aneuploid cells were observed. Using FISH with telomere-specific probes, we detected 25 and 24 bivalents in C. elongatoides and C. taenia males, respectively. However, in hybrids we observed aberrant pairing, with a majority of uni- and multivalents, and only a few bivalents, present (Figure 4, A, B, and D). On average we observed ∼34 univalents, 5 bivalents, and 1 multivalent among the 49 chromosomes of diploid ET males. In ETT triploids, the proportion of bivalents was considerably higher as these males contained an average of ∼23 univalents, 12 bivalents, and 8 multivalents among 73 chromosomes. Observed patterns suggest that premeiotic genome endoreplication of the germ cell genome is not a prevailing gametogenic alteration during hybrid male meiosis.
To determine the origin of paired chromosomes in hybrids we applied CGH on meiotic figures. We observed the pairing of orthologous chromosomes (E×T) in bivalents and multivalents in ET males (Figure 4C). We also noticed that both genomes contributed roughly equally to the formation of univalents (i.e., on average we observed ∼16 T-like and ∼18 E-like univalents). Metaphases of triploid ETT males possessed a higher proportion of bivalents that were formed by T×T chromosome pairing (probably homologs), but several instances of orthologous E×T chromosomal pairing were also observed in each metaphase plate. Some multivalents appeared to contain chromosomes of both species, and univalents were usually composed of E-like chromosomes (Figure 4E).
Immunostaining for lateral (SYCP3) and central (SYCP1) proteins of SCs during pachytene revealed normal pairing in C. taenia and C. elongatoides males (24 and 25 bivalents, respectively). However, in ET and ETT hybrid males, chromosomal pairing aberrations were initiated during the early stages of bivalent formation. Specifically, ET males generally contained only short and partially formed SCs, often branching and forming loops, indicating improper pairing of chromosomes. Rarely, we observed one or two standard SCs in these males (Figure 4G, Figure 3C, and Figure S4A). In triploid ETT males we also observed many malformed SCs but, contrary to ET diploids, between 8 and 15 properly developed SCs were usually present per cell (Figure 3D). This corroborates the hypothesis that certain T×T bivalents could be formed properly in ETT triploids (Figure 4H and Figure S4B). In abnormal bivalents, SYCP3 was localized to subtelomeric regions, while inner fragments of chromosomes usually lacked the SYCP3 signals (Figure S4). We conclude that hybrid males have aberrant pairing, with only a few chromosomes being able to form bivalents. In triploid ETT males, pairing is partly rescued but not all T×T chromosomes are paired, as we also detected E×T bivalent and multivalent formation.
Analysis of CO rate:
In general, CO frequencies were lower in sexual males than in sexual females. We also found significant differences in CO frequencies between species with generally higher values in C. taenia. On average, C. taenia males had ∼28.9 COs per cell while C. elongatoides males had ∼27.7 per cell (Figure 4L). In contrast to females, male CO sites were mostly located close to the telomeric regions of bivalents, similar to studies on Danio rerio (Kochakpour and Moens 2008).
The decrease in CO frequency was more drastic in hybrid males, which possessed only ∼6.2 (diploid ET) and ∼17.2 (triploid ETT) MLH1 foci per cell (Figure 4L). Although the CO frequency was partially increased in triploids, it was still much lower than expected from T×T chromosome pairing in ETT hybrid males.
The differences between sexes and species/hybrids were corroborated by the mixed effect of the Generalized Linear Model GLMpoisson, which showed that the model incorporating both such parameters fitted the data significantly better than single-parameter models [formula = CO∼sex + species + (1 | individual_ID); P(test = χ2) = 0.04].
Discussion
Asexual reproduction in females and hybrid sterility in males are closely connected
Although the initial divergence of C. elongatoides and C. taenia was followed by intensive historical introgressions, both species are currently isolated (Janko et al. 2018), and the present study identified gametogenic aberrations that exist in both male and female hybrids, and represent efficient postzygotic barriers to gene flow. Interestingly, the cytogenetic background of such barriers radically differs between hybrid sexes (Figure 1, B and C) as females produce clonal gametes via premeiotic endoreplication, but males most likely lack this mechanism and are sterile. Flow cytometry indicated minor populations of octoploid or dodecaploid testicular cells in ET and ETT males, respectively (Figure 4, J and K), which may be theoretically interpreted as a result of the premeiotic endoreplication of spermatocytes (Yoshikawa et al. 2009). However, given that we did not observe any testicular cells with a doubled number of chromosomes despite cytogenetic analysis of hundreds of metaphase I nuclei, this hypothesis seems unlikely. The occurrence of unusually high-ploidy cells in males may indicate that meiotic arrest occurred following chromosome replication (Li et al. 2015). Alternatively, these cells may represent fused or unseparated spermatozoa given their larger heads, and multiple axonemes or flagella.
Therefore, we conclude that premeiotic endoreplication, as observed in hybrid females, is absent or at least undetectable in male hybrids. Such results are in agreement with artificial crossing experiments of parental species, which showed that hybrid males are sterile and hybrid females produce clonal eggs (Choleva et al. 2012). The major aberration associated with male sterility results from a failure in chromosomal pairing and/or reduction in crossovers during meiotic division, which prevents the proper segregation of orthologs. These aberrations likely enforce the meiotic arrest at pachytene–metaphase I followed by apoptosis in the majority of spermatocytes. As hybrid males suffer from the improper formation of SCs and drastically decreased crossover rates, these aberrations likely take place during the early stages of homolog pairing. Such stages are characterized by preliminary double-strand break (DSB)-independent pairing that restricts the searching area for homologous recognition, and subsequent DSB formation and coalignment (Zickler 2006). In cypriniform fishes, the homology search is initiated in subtelomeric regions, followed by SYCP3 upload and zipping toward the interstitial chromosomal segments (Blokhina et al. 2018). In the case of sufficient homology between two chromosomes, zipping is finalized by uploading the SYCP1 protein accomplishing bivalent formation (Blokhina et al. 2018). Therefore, it is possible that the low homology between the orthologous chromosomes of contemporary species prevented sufficient pre-DSB coalignment in their hybrids.
Gametogenic aberrations in interspecific hybrids may take various forms spanning from mitotic arrest in germ cells before meiosis (Yoshikawa et al. 2018) to sperm production without meiotic division (Shimizu et al. 1997). However, several studies have reported patterns analogous to those of the Cobitis males (Balcova et al. 2016; Torgasheva and Borodin 2016; Spangenberg et al. 2017; Kuroda et al. 2019), suggesting that compromised pairing and a reduced CO frequency can be relatively common aberrations underlying hybrid sterility. Apart from few model taxa, the proximate molecular mechanisms underlying hybrid sterility remain unknown, but previous research has traditionally focused on genic models (i.e., Dobzhansky–Muller-type incompatibilities) rather than on the role of divergence in DNA base composition (Bateson’s model). Nevertheless, a recent study on house mouse hybrid males suggested that both aspects could operate together, owing to the complementary effects of the allelic form of the PRDM9 gene and the sequence nonhomology of its binding sites, resulting in asymmetrical DSBs (Gregorova et al. 2018). The importance of sequence homology in hybrid sterility is also highlighted by evidence indicating that the fertility of mouse hybrid males greatly increased when homology was restored to the segments of chromosomes that were mainly involved in mispairing (Gregorova et al. 2018).
Observations of Cobitis lead us to the same conclusion, since triploidization partially rescued bivalent formation and increased the CO rate owing to the proper pairing of C. taenia-like homologs. Similarly, in Darevskia lizards, the CO rate of triploid male hybrids matches the values typical for parental species contributing double chromosome sets into hybrids (Spangenberg et al. 2017). Kuroda et al. (2019) have also reported that the vast majority of bivalents in triploid hybrid M. anguillicaudatus males consist of homologs rather than orthologs, but as in the case of Cobitis, several orthologous bivalents are also present.
These findings support the importance of sequence homology in hybrid gametogenesis, but also indicate that the remedy of hybrid sterility may, at least partially, be delivered by polyploidization, because the addition of extra copies of genomes may facilitate the proper pairing of homologs. In this context, it is indeed noteworthy that many animal and plant hybrids are polyploids, and their bivalents are formed from conspecific rather than orthologous chromosomes during meiosis (Grandont et al. 2013; Stenberg and Saura 2013). This indicates that genome doubling may stabilize hybrid gametogenesis. Indeed, if sterility is largely caused by the problem of orthologous chromosome pairing, then ploidy elevation of germ cells owing to premeiotic endoreplication could provide an elegant solution by supplying each chromosome a peer to properly pair with. Such a mechanism should work even without whole-genome duplication in the entire organism. Asexuality may thus represent a surprisingly efficient remedy for hybrid sterility that arises during species differentiation.
Mechanisms of bivalent formation differ between males and females
The improper pairing of homologs vs. orthologs may be detrimental to neopolyploid lineages (Lenormand et al. 2016), and organisms may employ various pathways to avoid the formation of multivalents and orthologous pairing. These range from the reduction of the CO rate between orthologs to reverted meiosis in some hybrids (Lukhtanov et al. 2018) and the evolution of genic control selectively preferring homologous pairing (Griffiths et al. 2006), or compensatory mechanisms increasing COs between properly paired chromosomes (Bomblies et al. 2016).
Our study demonstrates that pairing affinities of homologous and orthologous chromosomes may differ between sexes. In particular, hybrid females always formed perfect sets of E×E and T×T bivalents without any observed mismatches. In contrast, triploid ETT males always possessed some T-like univalents and E×T bivalents, even though each C. taenia-derived chromosome had a conspecific homolog. Similar observations come from M. anguillicaudatus (Kuroda et al. 2018, 2019). A possible explanation for such dissimilarity between hybrid males and females may stem from differences in localizations of CO sites. In males, the CO sites occur close to telomeric regions, while in females, the CO sites are more numerous and localized to interstitial segments of chromosomes. This may increase the incidence of high-homology interactions, and hence, unlike their brothers, females would potentially have more stringent disruption of low-homology or ectopic pairings, thereby avoiding any orthologous pairing.
Premeiotic endoreplication as a widespread solution to females’ hybrid fertility
The observation that endoreplication prevails in females but not in males is an important outcome of the present study, which has, to our knowledge, previously only been demonstrated in distantly related Misgurnus hybrids. In speciation research, the asymmetric accumulation of gametogenic aberrations is a well-known and intensively studied phenomenon (e.g., Haldane’s rule). Torgasheva and Borodin (2016) recently proposed that both hybrid sexes may follow the same route to sterility caused by incorrect homologous pairing, just at a different pace. Analysis of the Cobitis asexual complex somewhat challenges this view as it indicates that even when postzygotic barriers have been accomplished in both hybrid sexes, the underlying mechanisms may have very different backgrounds because hybrid females remain fertile but restrict gene flow by producing clonal gametes.
The mechanisms that control endoreplication and the reasons for the specificity to females remain unclear. Although we, just like in other previous studies, did not observe the particular moment of endoreplication nor reveal its causal stimulus, our comparison with the related genus Misgurnus leads us to propose that it is not determined by hormonal levels but more likely by genetic sex determination. Specifically, while Kuroda et al. (2019) reported the sterility of naturally occurring hybrid males, Yoshikawa et al. (2009) artificially reverted diploid hybrid Misgurnus females into males and reported the production of unreduced spermatozoa via endoreplication. These results suggest that diploid hybrids genetically determined as females possess the endoreplication ability, even when expressing male phenotypes.
The sex-determination system in the C. taenia complex is unknown, but male heterogamety (X1X2Y or X0) has been proposed in congeneric species (Saitoh 1989; Vasil’eva and Vasil’ev 1998), making it tempting to speculate that intersexual differences in fertility and asexuality relate to the genetic sex-determination system. However, we stress that even if sterile Cobitis males are heterogamous, the analogy to Haldane’s famous rule is only superficial. This is because females should also face the improper pairing of orthologous chromosomes, but their fertility is restored by the gametogenic modification that complements hybrid sterility by pairing the endoreplicated sister chromosomes.
Besides the possible effects of the hybrid sex-determination system, the ploidy level and proper dosage of hybridizing genomes appear as other important parameters controlling the emergence of asexuality. Indeed, the emergence and stability of asexual lineages correlates with particular ploidy levels (Moritz et al. 1989), and the reproductive strategies of hybrids between the same species may differ drastically, depending on whether they are diploid or polyploid. For example, diploid Poeciliopsis hybrids are hybridogenetic, whereas their triploid counterparts are clonal gynogens. In contrast, diploid C. hankugensis × Iksokimia longicorpa hybrids are gynogenetic, but their triploid counterparts reproduce via meiotic hybridogenesis (Vrijenhoek 1998; Saitoh et al. 2004). Interestingly, Morishima et al. (2012) reported that artificially induced tetraploid Misgurnus hybrids lost their capacity for endoreplication, which characterizes their diploid hybrid ancestors. In Cobitis, the effects of ploidy are not so strict since both diploid and triploid C. elongatoides × C. taenia females have similar gametogenic pathways, and may naturally establish successful clonal lineages (Janko et al. 2007b). Tetraploid Cobitis hybrids usually appear inviable (Janko et al. 2007a; Juchno et al. 2014), but Vasil’ev et al. (2003) reported the existence of stable tetraploid hybrid clones suggesting that clonality may also exist in the tetraploid state. Overall, these results suggest that the ability of endoreplication somehow depends on ploidy and may be under dosage-dependent control of some crucial transcripts. This hypothesis is in agreement with recent theoretical and empirical research showing that the efficiency of trans-regulatory cross talk between merged genomes is affected by the ploidy level (Bottani et al. 2018, Bartoš et al. 2019).
Conclusion
The mechanisms underlying the existence of biological species have been intensively studied since Darwin’s time, as has the question of why sex is a dominant reproductive mechanism among Metazoans. Our study resolved the link between asexuality and speciation by revealing the mechanistic basis of the simultaneously arising sterility of hybrid males and asexuality of hybrid females.
Premeiotic endoreplication, as found in Cobitis hybrid females, is a relatively common pathway among asexual plant and animal hybrids (reviewed in Stenberg and Saura 2009; De Storme and Geelen 2013), but to date, its triggers have not been revealed, which arguably represents a considerable gap in our understanding of speciation and the evolution of sex. Asexuality has arisen independently upon hybridization of various sexual species and we documented that the same type of gametogenic mechanism occurs in C. elongatoides × C. taenia hybrid females as in hybrids of Misgurnus from Asia (Yoshikawa et al. 2009; Kuroda et al. 2018), whose evolutionary distance from European loaches is substantial, reaching ∼30 MYA (Majtánová et al. 2016). Thus, it appears unlikely that these two distant lineages share the same predisposition. Our findings rather indicate that interspecific crosses may induce similar types of aberrancies when parental species are sufficiently diverged to disrupt classical sexual development of their hybrids.
One reason that the majority of known asexual hybrids are formed from the parental species with a certain level of divergence may thus be that accumulated incompatibilities in key genetic pathways fail to suppress the replication of hybrid chromosomes before cytokinesis, ultimately resulting in endoreplication. An interesting alternative explanation may assume that increased divergence is not necessary per se as a trigger for asexuality. Rather, the divergence may prevent the pairing of orthologous chromosomes during asexual meiosis. In other words, endoreplication may also appear in hybrids between closely related species, but decreased homology of orthologs is necessary to prevent the formation of spurious bivalents or multivalents following endoreplication, therefore ensuring proper gametogenesis. Thorough investigation of gametogenic aberrations in asexual organisms may thus considerably increase not only our understanding of speciation, but also of meiosis and chromosomal pairing.
Acknowledgments
We thank Roger Butlin, František Marec, Lukáš Kratochvíl, and Radka Reifová for inspiring comments on the manuscript; Lukáš Choleva for his help with fish maintenance; Karel Halačka for his assistance in the field; and Sean M. Burgess for kindly sharing antibodies against SYCP1. We are grateful for financial support from the Czech Academy of Sciences (grant number RVO67985904), the Czech Science Foundation (grant numbers 17-09807S and 19-21552S), the Ministry of Education, Youth and Sports of the Czech Republic (grant number EXCELLENCE CZ.02.1.01/0.0/0.0/15_003/0000460 OP RDE), and National Science Centre, Poland [grant numbers DEC-2011/03/B/NZ8/02095 (to J.K.) and 2011/03/B/NZ8/02982 (to A.B. and D.J.]. The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript. No competing interests declared.
Author Contributions: D.D., Z.M., and K.J. designed the study and codrafted the manuscript. D.D., Z.M., and A.M. performed cytogenetic analyses. D.J., A.B., and J. Kotusz. provided material for experiments. J. Klíma, D.D., and A.M. performed flow cytometry. M.P. performed histological experiments, transmission electron microscopy, and wrote relevant parts of the text. K.J. performed statistical analyses and drafted the first version of the text. All coauthors contributed to the final version.
Footnotes
Supplemental material available at figshare: https://doi.org/10.25386/genetics.11988192.
Communicating editor: D. Barbash
Literature Cited
- Araya-Jaime C., Serrano É. A., de Andrade Silva D. M. Z., Yamashita M., Iwai T. et al. , 2015. Surface-spreading technique of meiotic cells and immunodetection of synaptonemal complex proteins in teleostean fishes. Mol. Cytogenet. 8: 4 10.1186/s13039-015-0108-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avise I. J., 2008. Clonality : The genetics, Ecology, and Evolution of Sexual Abstinence in Vertebrate Animals: The Genetics, Ecology, and Evolution of Sexual Abstinence in Vertebrate Animals, Oxford University Press, Oxford: 10.1093/acprof:oso/9780195369670.001.0001 [DOI] [Google Scholar]
- Balcova M., Faltusova B., Gergelits V., Bhattacharyya T., Mihola O. et al. , 2016. Hybrid sterility locus on chromosome X controls meiotic recombination rate in mouse. PLoS Genet. 12: e1005906 10.1371/journal.pgen.1005906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoš O., Röslein J., Kotusz J., Pačes J., Pekárik L. et al. , 2019. The legacy of sexual ancestors in phenotypic variability, gene expression and homoeolog regulation of asexual hybrids and polyploids. Mol. Biol. Evol. 36: 1902–1920. 10.1093/molbev/msz114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson W., 1909. Heredity and variation in modern lights., pp. 85–101 in Darwin and Modern Science, Cambridge University Press, Cambridge. [Google Scholar]
- Baudat F., and de Massy B., 2007. Regulating double-stranded DNA break repair towards crossover or non-crossover during mammalian meiosis. Chromosome Res. Int. J. Mol. Supramol. Evol. Asp. Chromosome Biol. 15: 565–577. 10.1007/s10577-007-1140-3 [DOI] [PubMed] [Google Scholar]
- Bernstein H., and Bernstein C., 2010. Evolutionary origin of recombination during meiosis. Bioscience 60: 498–505. 10.1525/bio.2010.60.7.5 [DOI] [Google Scholar]
- Bhattacharyya T., Gregorova S., Mihola O., Anger M., Sebestova J. et al. , 2013. Mechanistic basis of infertility of mouse intersubspecific hybrids. Proc. Natl. Acad. Sci. USA 110: E468–E477. 10.1073/pnas.1219126110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokhina Y. P., Nguyen A. D., Draper B. W., and Burgess S. M., 2018. The telomere bouquet is a hub where meiotic double-strand breaks, synapsis, and stable homolog juxtaposition are coordinated in the zebrafish, Danio rerio. bioRxiv (Preprint posted September 27, 2018). 10.1101/428086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomblies K., Jones G., Franklin C., Zickler D., and Kleckner N., 2016. The challenge of evolving stable polyploidy: could an increase in “crossover interference distance” play a central role? Chromosoma 125: 287–300. 10.1007/s00412-015-0571-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottani S., Zabet N. R., Wendel J. F., and Veitia R. A., 2018. Gene expression dominance in allopolyploids: hypotheses and models. Trends Plant Sci. 23: 393–402. 10.1016/j.tplants.2018.01.002 [DOI] [PubMed] [Google Scholar]
- Brown J. D., and O’Neill R. J., 2010. Chromosomes, conflict, and epigenetics: chromosomal speciation revisited. Annu. Rev. Genomics Hum. Genet. 11: 291–316. 10.1146/annurev-genom-082509-141554 [DOI] [PubMed] [Google Scholar]
- Callan H. G., 1986. Lampbrush Chromosomes, Springer-Verlag, Berlin, Heidelberg: 10.1007/978-3-642-82792-1 [DOI] [Google Scholar]
- Carman J. G., 1997. Asynchronous expression of duplicate genes in angiosperms may cause apomixis, bispory, tetraspory, and polyembryony. Biol. J. Linn. Soc. Lond. 61: 51–94. 10.1111/j.1095-8312.1997.tb01778.x [DOI] [Google Scholar]
- Choleva L., Janko K., Gelas K. D., Bohlen J., Šlechtová V. et al. , 2012. Synthesis of clonality and polyploidy in vertebrate animals by hybridisation between two sexual species. Evolution 66: 2191–2203. 10.1111/j.1558-5646.2012.01589.x [DOI] [PubMed] [Google Scholar]
- De Storme N., and Geelen D., 2013. Sexual polyploidization in plants - cytological mechanisms and molecular regulation. New Phytol. 198: 670–684. 10.1111/nph.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Storme N., and Mason A., 2014. Plant speciation through chromosome instability and ploidy change: cellular mechanisms, molecular factors and evolutionary relevance. Curr. Plant Biol. 1: 10–33. 10.1016/j.cpb.2014.09.002 [DOI] [Google Scholar]
- Ernst A., 1918. Bastardierung als Ursache der Apogamie im Pflanzenreich. Eine Hypothese zur experimentellen Vererbungs- und Abstammungslehre, Nabu Press, Charleston, SC: 10.5962/bhl.title.8212 [DOI] [Google Scholar]
- Faria R., and Navarro A., 2010. Chromosomal speciation revisited: rearranging theory with pieces of evidence. Trends Ecol. Evol. 25: 660–669. 10.1016/j.tree.2010.07.008 [DOI] [PubMed] [Google Scholar]
- Gall J. G., Murphy C., Callan H. G., and Wu Z. A., 1991. Lampbrush chromosomes. Methods Cell Biol. 36: 149–166. 10.1016/S0091-679X(08)60276-9 [DOI] [PubMed] [Google Scholar]
- Graf J.-D., and M. Polls Pelaz, 1989 Evolutionary genetics of the Rana esculenta complex, pp. 289–302 in Evolution and Ecology of Unisexual Vertebrates, edited by R. M. Dawley and J. P. Bogart. New York State Museum, New York.
- Grandont L., Jenczewski E., and Lloyd A., 2013. Meiosis and its deviations in polyploid plants. Cytogenet. Genome Res. 140: 171–184. 10.1159/000351730 [DOI] [PubMed] [Google Scholar]
- Gregorova S., Gergelits V., Chvatalova I., Bhattacharyya T., Valiskova B. et al. , 2018. Modulation of Prdm9-controlled meiotic chromosome asynapsis overrides hybrid sterility in mice. Elife 7: e34282 10.7554/eLife.34282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S., Sharp R., Foote T. N., Bertin I., Wanous M. et al. , 2006. Molecular characterization of Ph1 as a major chromosome pairing locus in polyploid wheat. Nature 439: 749–752. 10.1038/nature04434 [DOI] [PubMed] [Google Scholar]
- Haldane J. B. S., 1922. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 12: 101–109. 10.1007/BF02983075 [DOI] [Google Scholar]
- Hotz H., Mancino G., Bucciinnocenti S., Ragghianti M., Berger L. et al. , 1985. Rana ridibunda varies geographically in inducing clonal gametogenesis in interspecies hybrids. J. Exp. Zool. 236: 199–210. 10.1002/jez.1402360210 [DOI] [Google Scholar]
- Itono M., Morishima K., Fujimoto T., Bando E., Yamaha E. et al. , 2006. Premeiotic endomitosis produces diploid eggs in the natural clone loach, Misgurnus anguillicaudatus (Teleostei: Cobitidae). J. Exp. Zool. 305A: 513–523. 10.1002/jez.a.283 [DOI] [PubMed] [Google Scholar]
- Janko K., Bohlen J., Lamatsch D., Flajšhans M., Epplen J. T. et al. , 2007a The gynogenetic reproduction of diploid and triploid hybrid spined loaches (Cobitis: Teleostei), and their ability to establish successful clonal lineages—on the evolution of polyploidy in asexual vertebrates. Genetica 131: 185–194. 10.1007/s10709-006-9130-5 [DOI] [PubMed] [Google Scholar]
- Janko K., Flajšhans M., Choleva L., Bohlen J., Lechtová V. Š. et al. , 2007b Diversity of European spined loaches (genus Cobitis L.): an update of the geographic distribution of the Cobitis taenia hybrid complex with a description of new molecular tools for species and hybrid determination. J. Fish Biol. 71: 387–408. 10.1111/j.1095-8649.2007.01663.x [DOI] [Google Scholar]
- Janko K., Pačes J., Wilkinson-Herbots H., Costa R. J., Roslein J. et al. , 2018. Hybrid asexuality as a primary postzygotic barrier between nascent species: on the interconnection between asexuality, hybridisation and speciation. Mol. Ecol. 27: 248–263. 10.1111/mec.14377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juchno D., and Boroń A., 2018. Histological evidence that diploid hybrids of Cobitis taenia and C. elongatoides (Teleostei, Cobitidae) develop into fertile females and sterile males. Hydrobiologia 814: 147–159. 10.1007/s10750-018-3530-2 [DOI] [Google Scholar]
- Juchno D., Jabłońska O., Boroń A., Kujawa R., Leska A. et al. , 2014. Ploidy-dependent survival of progeny arising from crosses between natural allotriploid Cobitis females and diploid C. taenia males (Pisces, Cobitidae). Genetica 142: 351–359 [corrigenda: Genetica 143: 127 (2015)]. 10.1007/s10709-014-9779-0 [DOI] [PubMed] [Google Scholar]
- Juchno D., Arai K., Boroń A., and Kujawa R., 2017. Meiotic chromosome configurations in oocytes of Cobitis taenia and its polyploid hybrids. Ichthyol. Res. 64: 240–243. 10.1007/s10228-016-0556-1 [DOI] [Google Scholar]
- Kearney M., Fujita M. K., and Ridenour J., 2009. Lost sex in the reptiles: constraints and correlations, pp. 447–474 in Lost Sex: The Evolutionary Biology of Parthenogenesis. Springer-Verlag, Dordrecht, The Netherlands. [Google Scholar]
- Kim I.-S., 2000. Hybridisation experiment of diploid-triploid cobitid fishes, Cobitis sinensis-longicorpus complex (Pisces : Cobitidae). Folia Zool. (Brno) 49: 17–22. [Google Scholar]
- Kochakpour N., and Moens P. B., 2008. Sex-specific crossover patterns in Zebrafish (Danio rerio). Heredity 100: 489–495. 10.1038/sj.hdy.6801091 [DOI] [PubMed] [Google Scholar]
- Kuroda M., Fujimoto T., Murakami M., Yamaha E., and Arai K., 2018. Clonal reproduction assured by sister chromosome pairing in dojo loach, a teleost fish. Chromosome Res. 26: 243–253. 10.1007/s10577-018-9581-4 [DOI] [PubMed] [Google Scholar]
- Kuroda M., Fujimoto T., Murakami M., Yamaha E., and Arai K., 2019. Aberrant meiotic configurations cause sterility in clone-origin triploid and inter-group hybrid males of the dojo loach, Misgurnus anguillicaudatus. Cytogenet. Genome Res. 158: 46–54. 10.1159/000500303 [DOI] [PubMed] [Google Scholar]
- Lampert K. P., Lamatsch D. K., Fischer P., Epplen J. T., Nanda I. et al. , 2007. Automictic reproduction in interspecific hybrids of Poeciliid fish. Curr. Biol. 17: 1948–1953. 10.1016/j.cub.2007.09.064 [DOI] [PubMed] [Google Scholar]
- Lenormand T., Engelstädter J., Johnston S. E., Wijnker E., and Haag C. R., 2016. Evolutionary mysteries in meiosis. Philos. Trans. R. Soc. B Biol. Sci. 371: 20160001. 10.1098/rstb.2016.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-J., Gao Y.-C., Zhou H., Ma H.-Y., Li J.-Q. et al. , 2015. Meiotic chromosome configurations in triploid progeny from reciprocal crosses between wild-type diploid and natural tetraploid loach Misgurnus anguillicaudatus in China. Genetica 143: 555–562. 10.1007/s10709-015-9853-2 [DOI] [PubMed] [Google Scholar]
- Lukhtanov V. A., Dincă V., Friberg M., Šíchová J., Olofsson M. et al. , 2018. Versatility of multivalent orientation, inverted meiosis, and rescued fitness in holocentric chromosomal hybrids. Proc. Natl. Acad. Sci. USA 115: E9610–E9619. 10.1073/pnas.1802610115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutes A. A., Neaves W. B., Baumann D. P., Wiegraebe W., and Baumann P., 2010. Sister chromosome pairing maintains heterozygosity in parthenogenetic lizards. Nature 464: 283–286. 10.1038/nature08818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macgregor H. C., and Uzzell T. M., 1964. Gynogenesis in salamanders related to Ambystoma jeffersonianum. Science 143: 1043–1045. 10.1126/science.143.3610.1043 [DOI] [PubMed] [Google Scholar]
- Macgregor H. C., and Varley J. M., 1983. Working with Animal Chromosomes, John Wiley & Sons, New York. [Google Scholar]
- Majtánová Z., Choleva L., Symonová R., Ráb P., Kotusz J. et al. , 2016. Asexual Reproduction Does not apparently increase the rate of chromosomal evolution: karyotype stability in diploid and triploid clonal hybrid fish (Cobitis, Cypriniformes, Teleostei). PLoS One 11: e0146872 10.1371/journal.pone.0146872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani B., and Scali V., 1992. Hybridogenesis and androgenesis in the stick-insect Bacillus rossius-grandii benazzii (Insecta, Phasmatodea). Evolution 46: 783–796. 10.1111/j.1558-5646.1992.tb02084.x [DOI] [PubMed] [Google Scholar]
- Moens P. B., 2006. Zebrafish: chiasmata and interference. Genome 49: 205–208. 10.1139/g06-021 [DOI] [PubMed] [Google Scholar]
- Morishima K., Yoshikawa H., and Arai K., 2008. Meiotic hybridogenesis in triploid Misgurnus loach derived from a clonal lineage. Heredity 100: 581–586. 10.1038/hdy.2008.17 [DOI] [PubMed] [Google Scholar]
- Morishima K., Yoshikawa H., and Arai K., 2012. Diploid clone produces unreduced diploid gametes but tetraploid clone generates reduced diploid gametes in the Misgurnus loach. Biol. Reprod. 86: 33 10.1095/biolreprod.111.093302 [DOI] [PubMed] [Google Scholar]
- Moritz C., Brown W. M., Densmore L. D., Wright J. W., Vyas D. et al. , 1989. Genetic diversity and the dynamics of hybrid parthenogenesis in Cnemidophorus (Teiidae) and Heteronotia (Gekkonidae), pp. 87–112 in Evolution and Ecology of Unisexual Vertebrates, edited by Dawley R. M., Bogart J. P.. Albany: NY State Mus. Bull. 466. [Google Scholar]
- Murphy R. W., Fu J., MacCulloch R. D., Darevsky I. S., and Kupriyanova L. A., 2000. A fine line between sex and unisexuality: the phylogenetic constraints on parthenogenesis in lacertid lizards. Zool. J. Linn. Soc. 130: 527–549. 10.1111/j.1096-3642.2000.tb02200.x [DOI] [Google Scholar]
- Park J.-Y., Kim I.-S., and Ko M.-H., 2011. Characteristics of rare males in the cobitid unisexual complex, Cobitis hankugensis-Iksookimia longicorpa. Folia Zool. Praha 60: 290–294. 10.25225/fozo.v60.i4.a4.2011 [DOI] [Google Scholar]
- Saitoh K., 1989. Multiple sex-chromosome system in a loach fish. Cytogenet. Cell Genet. 52: 62–64. 10.1159/000132840 [DOI] [PubMed] [Google Scholar]
- Saitoh K., Kim I. S., and Lee E. H., 2004. Mitochondrial gene introgression between spined Loaches via hybridogenesis. Zool. Sci. 21: 795–798. 10.2108/zsj.21.795 [DOI] [PubMed] [Google Scholar]
- Shimizu Y., Shibata N., and Yamashita M., 1997. Spermiogenesis without preceding meiosis in the hybrid medaka between Oryzias latipes and O. curvinotus. J. Exp. Zool. 279: 102–112. [DOI] [Google Scholar]
- Spangenberg V., Arakelyan M., Galoyan E., Matveevsky S., Petrosyan R. et al. , 2017. Reticulate evolution of the rock lizards: meiotic chromosome dynamics and spermatogenesis in diploid and triploid males of the genus Darevskia. Genes (Basel) 8: 149 10.3390/genes8060149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg P., and Saura A., 2009. Cytology of asexual animals, pp. 63–74 in Lost Sex, edited by Schön I., Martens K., and Dijk P.. Springer, Netherlands: 10.1007/978-90-481-2770-2_4 [DOI] [Google Scholar]
- Stenberg P., and Saura A., 2013. Meiosis and its deviations in polyploid animals. Cytogenet. Genome Res. 140: 185–203. 10.1159/000351731 [DOI] [PubMed] [Google Scholar]
- Torgasheva A. A., and Borodin P. M., 2016. Cytological basis of sterility in male and female hybrids between sibling species of grey voles Microtus arvalis and M. levis. Sci. Rep. 6: 36564 10.1038/srep36564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulchinsky A. Y., Johnson N. A., Watt W. B., and Porter A. H., 2014. Hybrid incompatibility arises in a sequence-based bioenergetic model of transcription factor binding. Genetics 198: 1155–1166. 10.1534/genetics.114.168112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil’eva E. D., and Vasil’ev V. P., 1998. Sibling species in genus Cobitis (Cobitidae). Cobitis rossomeridionalis sp. nova. J. Ichthyol. 38: 580–590. [Google Scholar]
- Vasil’ev V. P., Akimova N. V., Emel’yanova N. G., Pavlov D. A., and Vasil’eva E. D., 2003. Reproductive capacities in the polyploid males of spined loaches from the unisexual-bisexual complex, occurred in the Moscow River. Folia Biol. (Praha) 51: 67–73. [PubMed] [Google Scholar]
- Vrijenhoek R. C., 1998. Clonal organisms and the benefits of sex, pp. 151–172 in Advances in molecular ecology, edited by Carvalho G. R. IOS Press, Amsterdam. [Google Scholar]
- Yoshikawa H., Morishima K., Kusuda S., Yamaha E., and Arai K., 2007. Diploid sperm produced by artificially sex-reversed clone loaches. J. Exp. Zool. Part Ecol. Genet. Physiol. 307: 75–83. 10.1002/jez.a.337 [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Morishima K., Fujimoto T., Saito T., Kobayashi T. et al. , 2009. Chromosome doubling in early spermatogonia produces diploid spermatozoa in a natural clonal fish. Biol. Reprod. 80: 973–979. 10.1095/biolreprod.108.075150 [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Xu D., Ino Y., Yoshino T., Hayashida T. et al. , 2018. Hybrid sterility in fish caused by mitotic arrest of primordial germ cells. Genetics 209: 507–521. 10.1534/genetics.118.300777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Arai K., and Yamashita M., 1998. Cytogenetic mechanisms for triploid and haploid eggs formation in the triploid loach, Misgurnus anguillicaudatus. J. Exp. Zool. 281: 608–619. [DOI] [Google Scholar]
- Zickler D., 2006. From early homologue recognition to synaptonemal complex formation. Chromosoma 115: 158–174. 10.1007/s00412-006-0048-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. Supplemental material available at figshare: https://doi.org/10.25386/genetics.11988192.