Abstract
The large family of C-type lectin (CLEC) receptors comprises carbohydrate-binding proteins that require Ca2+ to bind a ligand. The prototypic receptor is the asialoglycoprotein receptor-1 (ASGR1, CLEC4H1) that is expressed primarily by hepatocytes. The early work on ASGR1, which is highly specific for N-acetylgalactosamine (GalNAc), established the foundation for understanding the overall function of CLEC receptors. Cells of the immune system generally express more than one CLEC receptor that serve diverse functions such as pathogen-recognition, initiation of cellular signaling, cellular adhesion, glycoprotein turnover, inflammation and immune responses. The receptor CLEC10A (C-type lectin domain family 10 member A, CD301; also called the macrophage galactose-type lectin, MGL) contains a carbohydrate-recognition domain (CRD) that is homologous to the CRD of ASGR1, and thus, is also specific for GalNAc. CLEC10A is most highly expressed on immature DCs, monocyte-derived DCs, and alternatively activated macrophages (subtype M2a) as well as oocytes and progenitor cells at several stages of embryonic development. This receptor is involved in initiation of TH1, TH2, and TH17 immune responses and induction of tolerance in naïve T cells. Ligand-mediated endocytosis of CLEC receptors initiates a Ca2+ signal that interestingly has different outcomes depending on ligand properties, concentration, and frequency of administration. This review summarizes studies that have been carried out on these receptors.
Keywords: lectin, asialoglycoprotein receptor-1, ASGR1, CLEC10A, macrophage, dendritic cell, T cell, tolerance, calcium, IL-10, IL-12
1. Introduction
ASGR1 and CLEC10A are members of a large group of proteins called ‘lectins’ that non-enzymatically bind carbohydrate structures. In plants these proteins usually occur in storage tissues such as seeds. Research over the past four decades identified a vast number of lectin receptors in mammalian tissues, which function as surveillance of the extensive glycome in normal tissues, recognition of environmental danger signals, cell-cell adhesion, ligand-specific endocytosis, glycoprotein turnover, and mediators of inflammation and immune responses. Five major families of lectin-type receptors have been cataloged: the I-type Siglec receptors are specific for glycan ligands that contain terminal sialic acid [1,2,3]; galectins bind ligands that contain β-galactoside moieties [4,5]; F-type lectins bind fucose [6]; and R-type lectins resemble the protein ricin [7]. By far the largest and most diverse family, indeed a superfamily, consists of C-type lectins that in most but not all cases require Ca2+ to bind the ligand within a conserved carbohydrate-recognition domain (CRD) [8,9,10]. Starting from the late 1980s, research on these lectins identified 17 sub-groups based on structure of the CRD and specificity of the sugar ligand [10,11,12].
Among the large family of Ca2+-dependent lectin receptors, two are of particular interest. The prototypic C-Type Lectin Domain Family 4 Member H1 (CLEC4H1, asialoglycoprotein receptor-1, ASGR1) and C-Type Lectin Domain Family 10 Member A (CLEC10A, macrophage galactose-type lectin, MGL, CD301) contain homologous CRDs that bind N-acetylgalactosamine (GalNAc) with much higher affinity than galactose (Gal). ASGR1 is expressed nearly exclusively by hepatic parenchymal cells but also by cells in the dermis of human skin [13] and at a much lower level in human peripheral blood monocytes [14]. Most of the early functional information on C-type receptors was developed with ASGR1, which functions to survey blood cells and plasma proteins for removal when sialic acid, the terminal sugar of attached glycans, is lost. CLEC10A is expressed at several critical stages of human development, with the highest expression in oocytes [15]. The totipotent 8-cell stage of embryogenesis expresses CLEC10A at a level 70-fold higher than later embryonic stem cells [16], and a three-fold increase in expression occurs upon differentiation of mouse multipotent stem cells to myeloid committed progenitor cells [17]. CLEC10A is also expressed on immature dendritic cells (DCs) [15,18], monocyte-derived DCs (mo-DCs) [19,20], alternatively activated M2a macrophages [21,22], and at low levels in human intermediate (CD14++CD16+) monocytes [23]. GalNAcα1-3Galβ is the most potent ligand for human circulating primary DCs [24]. CLEC10A mediates various immune responses dependent on the microenvironment and the nature of the ligand [19,25,26].
2. ASGR1
Gilbert Ashwell and his colleagues discovered ASGR1 during studies of the remarkably active uptake of serum glycoproteins by liver cells [27,28,29,30,31]. While most serum proteins contain glycan chains with terminal sialic acid, loss of this terminal sugar over time and exposure of penultimate Gal residues leads to rapid internalization by liver cells and degradation of these asialoglycoproteins. Orosomucoid (α1-acid glycoprotein) was the most actively internalized protein after removal of terminal sialic acid residues by treatment with neuraminidase [28]. Orosomucoid contains 183 amino acids (~20 kDa) but its mass is doubled by five N-linked glycan structures [32,33]. Ashwell’s team revealed the major characteristics of ASGR1, which include (i) a preference for terminal α-D-GalNAc (for example, a 60-fold greater affinity for GalNAc over Gal is expressed by the rat ASGR1) [34,35], (ii) increased affinity as a function of multivalency of the ligand, (iii) the number of receptors per cell, (iv) the overall rate of endocytosis (cellular uptake of the protein), and (v) receptor recycling.
ASGR contains two subunits, of which the predominant subunit, ASGR1 (CLEC4H1), contains 291 amino acids, whereas the less abundant ASGR2 (CLEC4H2) contains 311 amino acids, but the two are surprisingly different in sequence and in binding specificity. ASGR2 has only a two-fold selectivity for GalNAc over Gal, which is evident from the sequence of the CRD, and is easily dissociated from ASGR1 oligomers [35]. Various oligomeric structures were described, from the functional receptor occurring as homo-oligomers [36], a 2:2 heterotetramer [37], to a 3:1 heterotetramer [35]. Hardy et al. [38] observed two independent classes of binding sites for asialo-orosomucoid, described by KD values of 0.44 nM and 9.7 nM. A synthetic ligand that contained six nonreducing Gal residues provided KD values of 0.63 nM and 25.3 nM for the two sites, which, respectively, accounted for 817,000 and 1.23 × 106 sites per rabbit hepatocyte. These studies were performed at 2 °C to prevent internalization of the receptor. Steer and Ashwell [31] had estimated 1.2 × 106 total binding sites for asialo-orosomucoid per rat hepatocyte, whereas 200,000 to 500,000 receptors were detected on the surface of these cells [39,40]. The number of receptors on the surface at any one time depends on the extent of endocytosis and recycling [40,41].
The endocytic process was studied extensively by Schwartz et al. with asialo-orosomucoid [39,40,41,42,43]. Although Gal itself is a low affinity ligand for the receptor, multivalency—five terminal Gal residues per protein molecule or more, depending upon the extent of branching of the glycan—enhances avidity by orders of magnitude [43]. Even without a ligand, the receptor is internalized with a half-time of 5 to 6 min. In the presence of ligand, internalization is more rapid, with a t½ of 2.5 to 3 min, and the receptor returns to the cell surface in 5 to 7 min [44]. This remarkably active process of continuous recycling involves internalization with clathrin-coated pits [45], dissociation of ligand and receptor in the endosome concomitant with vesicle uncoating, and return of the receptor to the surface. The ligand is funneled through the vacuolar system to its fateful degradation in lysosomes [42,43]. Products of degradation of the asialo-orosomucoid appear in the medium about an hour after initial receptor binding [41]. Interestingly, there was a rapid loss of ASGR1 from the surface when cells were treated with weak bases such as chloroquine or primaquine, which inhibit acidification of the endosome and the return of the internalized receptor to the cell surface [44].
ASGR1 requires three bound Ca2+ ions to bind a ligand, and thus, dissociation of ligand in the endosome must be preceded by dissociation of the cation from the receptor. Whereas the initial insertion of Ca2+ occurs during folding of the protein within the endoplasmic reticulum, the continuous recycling of the receptor over several hours indicates that the CRD is recharged repeatedly with Ca2+ upon exposure to the extracellular fluid. This intuition is supported by the refolding of recombinant CRD of ASGR1 after expression in Escherichia coli. The inclusion body that resulted was solubilized in 8 M urea containing β-mercaptoethanol and then dialyzed against buffer containing 25 mM CaCl2 [46]. Although the three disulfide bonds that stabilize the CRD structure are apparently maintained during recycling, they form correctly from the completely unfolded structure after bacterial expression.
The extracellular concentration of Ca2+ is 1 to 2 mM, whereas the cytosolic concentration is less than 0.1 µM. During endocytosis, the Ca2+ concentration within the endosome drops rapidly [47], and as it is lowered below about 330 µM, the KD of the two ‘high’ affinity Ca2+ binding sites in the CRD and with a reduced pH of 6.9, the ligand dissociates. This condition is achieved in about 3 min [47,48]. These events are dependent on acidification of the endosome by vacuolar H+-ATPase (V-ATPase) on the endosomal membrane [49,50]. Acidification of the endosome is a prerequisite to the reduction in the endosomal Ca2+ concentration, which suggests that an exchange mechanism is engaged to transport Ca2+ into the cytosol [51,52,53]. Although the cell has several mechanisms to control cytosolic Ca2+ concentrations near 0.1 µM, a transient elevation in the concentration induced by ligand-mediated recycling of the receptor initiates Ca2+-dependent signaling pathways within the cell.
3. Targeting Liver Cells via ASGR1
Extensive studies have been performed to find ligand conjugates that will provide specific transfer of drugs into hepatocytes through ASGR1. A basic structure of a tri-GalNAc ligand was initially synthesized by Lee and Lee [54]. Other teams further investigated multivalency and modifications of the ligand, and even polymers of GalNAc [55], to deliver various ‘cargoes’ into the liver [56,57,58]. Khorev et al. [59] designed trivalent structures with polypropylene or peptide spacers to extend the GalNAc residues into a space that would fit a trimeric receptor complex. The IC50 of binding of these ligands to the receptor follows the increasing avidity with valency, with mono-, di-, tri-, and tetravalent structures found to have KD values of approximately 1 × 10−3, 1 × 10−6, 5 × 10−9, and 1 × 10−9 M, respectively. Cargoes attached to these structures for delivery into liver cells include radiolabeled human serum albumin or asialofetuin to measure liver function [60,61]. The structure-activity relationship between the optimal number of GalNAc residues and the linkage to the cargo have been extensively evaluated [62]. Recent advances involved GalNAc to deliver siRNAs, anti-microRNAs and antisense oligonucleotides for gene silencing in hepatocytes [62,63,64,65,66]. Initial clinical phase I trials found conjugates of a tri-GalNAc construct with antisense oligonucleotides to be highly potent and with a high margin of safety [67,68]. Ligand structures based on peptides, Gal or pullulan (a polysaccharide consisting of maltotriose units) were developed to deliver chemotherapeutic drugs such as doxorubicin and paclitaxel to treat hepatic cancers [69,70,71]. Complex structures in which GalNAc is attached to the tyrosine hydroxyl oxygen of proteins or peptides also express a KD in the nanomolar range [72]. Studies of the size of the ligand indicated that particles with a diameter greater than 70 nm could not be processed by ASGR1 [73].
4. CLEC10A
In the late 1980s a lectin similar to the hepatic ASGR was found on mouse macrophages and designated the macrophage lectin specific for Gal and GalNAc (macrophage asialoglycoprotein-binding protein or M-ASGP-BP) [74]. This receptor was also called the mouse macrophage Gal and GalNAc-specific lectin (MMGL) [75]. Irimura’s team extended these studies, which were described in an extensive series of reports, and found that the mouse contains two related lectins, one specific for Gal (macrophage Gal-type lectin, MGL1) that is expressed predominantly by macrophages, while the other specific for GalNAc (MGL2) is expressed primarily by DCs [76,77]. A human protein (hMGL) was found that is homologous to the mouse MGL2 [78,79]. A powerful demonstration of the differential binding specificities of MGL1, MGL2, and hMGL was provided by Artigas et al. [80], who showed that hMGL effectively bound a glycopeptide containing one or two GalNAc adducts (e.g., the Tn antigen, D-GalNAcα1-OSer/Thr) but not when Gal was added to GalNAc to form Gal-GalNAc-OSer/Thr (the Thomsen-Friedenreich or T antigen). The human receptor (hMGL or simply MGL) bound to the glycan array in a similar fashion as murine MGL2, although the latter also bound to the T antigen with low affinity.
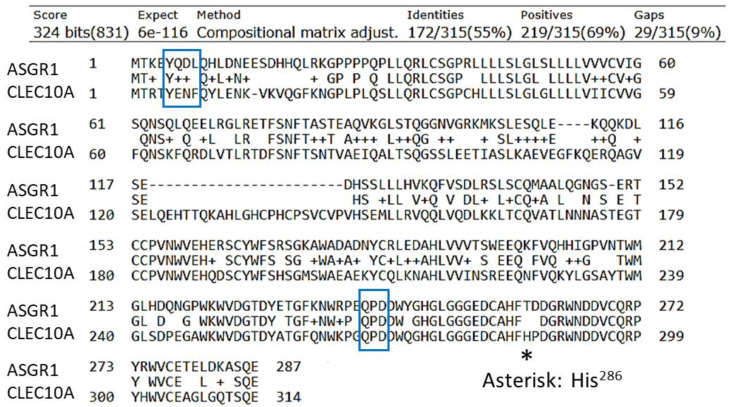
A receptor homologous but not identical to ASGR1 was discovered that is expressed by mo-DCs and was designated DC-ASGPR [81]. This receptor is 55% identical to ASGR1 but 82% identical in the CRD surrounding the QPD (Gln-Pro-Asp) sequence (boxed in Figure 1) that is correlated with Gal/GalNAc specificity [82,83]. DC-ASGPR is completely homologous to CLEC10A isoform 1 (MGL). (The National Center for Biotechnology Information does not include DC-ASGPR; the reported short form of DC-ASGPR [81] is C-type lectin domain family 10 member A isoform 2). CLEC10A has three major isoforms that are generated by alternative splicing. Isoform 1 contains 316 amino acids, whereas isoforms 2 and 3 contain 292 and 256 amino acids, respectively. Higashi et al. [79] found a total of seven isoforms in immature DCs and all contained the endocytic motif YENF (boxed in Figure 1) in the cytosolic domain. The gene for CLEC10A is located on chromosome 17 (17p13.1) and resides in a sequence of CLEC10A—ASGR2 with ASGR1 nearby.
Figure 1.
Comparison of the sequences of CLEC10A (isoform 1) and ASGR1. CLEC10A has two additional amino acids at the C-terminus, SH. ASGR1 has four additional amino acids, PPLL, at the C-terminus. The endocytosis motif (positions 5 to 8) and the QPD sequence that correlates with specificity for Gal/GalNAc are boxed.
These receptors have a strong preference for α-GalNAc over β-GalNAc, with very low binding to Gal [80,84]. CLEC10A is the only C-type lectin in the human immune system that exclusively binds terminal GalNAc residues [18,84,85]. CLEC10A was also found to bind tumor-associated sialylated-GalNAc antigens, Neu5Acα2,6-Tn, and NeuGcα2,6-Tn with similar affinities [86]. As with ASGR1, CLEC10A actively undergoes endocytosis. Most commonly, this process is analyzed by the disappearance of a tagged anti-CLEC10A antibody from the surface of DCs. The t½ of internalization of a bound antibody is about 5 min at 37 °C with mo-DCs [81,87,88]. Both ASGR1 and CLEC10A are type II transmembrane proteins, with the N-terminus in the cytosol. CLEC10A contains an endocytosis motif YENF near the N-terminus whereas the motif in ASGR1 is YQDL (boxed in Figure 1). Phosphorylation of the tyrosine residue (Y5, see Figure 1) is required for internalization [88] and subsequent signaling [89].
Within the immune system, CLEC10A is expressed by immature DCs [79]; CD1c+ DCs, for which CLEC10A is a specific marker [87]; mo-DCs [20,25,81]; M2a (alternatively activated) macrophages [21,90]; intermediate monocytes [23]; and at very low levels in many other cell types including monocytes, eosinophils, and erythroblasts [91,92,93]. CD1c+ blood DCs were recently subdivided into ‘non-inflammatory’ CD1c+A (DC2) and ‘inflammatory’ CD1c+B (DC3) sub-types, both of which express CLEC10A [94,95,96]. Moreover, CLEC10A is expressed by DCs that differentiate from monocytes that are recruited to inflammatory environments such as rheumatoid arthritis or ovarian ascites [20,97]. Expression of CLEC10A by DCs in the skin is most likely to detect pathogens containing GalNAc or Gal [98,99] and leads to strong Th2 or Th17 immune responses. Migration of the endocytic, active immature DCs to lymph nodes for interaction with T cells and initiation of an immune response has been found to require several days, during which time the DCs mature and express the co-stimulatory surface factors and MHC class II complexes that are required to activate T cells [100,101,102]. However, maturation of DCs leads to down-regulation of expression of CLEC10A [87,103].
Widespread interest has been focused on CLEC10A as an immuno-active receptor on immature DCs and alternatively activated macrophages [18,22,92]. As a pathogen-recognition receptor, CLEC10A binds to GalNAc in the teichoic acid of Staphylococcus aureus cell wall [104] and the surface of parasites such as helminths [84,105,106]. A common antigen on carcinoma cells, the Tn antigen (D-GalNAcα1-OSer/Thr) [78,79,107,108,109], binds to CLEC10A with relatively low affinity (KD = 8–12 µM) [85,110]. However, more complex GalNAc-containing ligands interact with additional groups within the binding site, which consequently increase the affinity by several orders of magnitude [111]. This feature was clearly demonstrated by Marcelo et al. [112], who found that a change from His286 to Thr286 (His259 in the sequence of their recombinant MGL) in the sequence His284-Phe-His286 (HFH286 in Figure 1) changed the specificity and lowered the affinity of Tn-peptide ligands. In particular, the sequence His284-Phe-Thr286 still recognized the Tn antigen but the receptor had much reduced ability to bind longer structures such as the blood group A structure. Moreover, whereas sialyl-Tn was bound by CLEC10A [86], the His286 to Thr286 mutation still bound the Tn antigen but eliminated binding of the sialylated ligand [112]. A ‘second’ binding site was thereby inferred that includes His286, which forms H-bonds with the underlying peptide or sugars in the ligand in addition to GalNAc [112]. Thus, the CRD of CLEC10A interacts with the carbohydrate structure and also the peptide backbone. Hepatic ASGR1 contains the analogous His257-Phe-Thr259 sequence (Figure 1), which may prevent the liver from actively ingesting young, sialylated red blood cells. The sequence in ASGR2 is Glu257-Val-Gln259 and the absence of His seems to reduce the specificity for GalNAc [111].
The highly glycosylated, cell membrane protein tyrosine phosphatase, CD45, which bears the Tn structure at positions 137 and 140 in exon B of the sequence [113], was identified as an endogenous ligand of MGL (CLEC10A) [103]. CD45 is expressed as several isoforms, with the full-length protein (CD45RABC) containing exons A, B, and C in the extracellular domain [114]. Binding of CLEC10A to exon B-containing isoforms causes attenuation of T cell activity, apoptosis, and immunosuppression [103]. However, activation of T cells leads to expression of shorter isoforms of CD45 such as CD45RO and CD45RA that lack exon B [115,116].
High avidity ligands for CLEC10A have been designed by increasing the number of GalNAc residues on MUC-derived peptides [19,25,111], which provide KD values in the nanomolar range. Tn glycosylation of the MUC6 protein strongly affected its immunogenicity by diminishing a TH1 response while promoting a TH17/IL-17 response [117]. Multivalent Tn structures have been synthesized, built on a tri-lysine core, that contain 4 to 12 terminal GalNAc residues and have KD values of 50 to 100 nM for binding to MGL [98,118,119,120], or simply polymers of GalNAc that have KD values in the nanomolar range [55]. Multivalent Tn glycopeptides induced strong stimulation of CD4+ TH2 responses, with secretion of IL-4, IL-5, IL-13, and IL-10 and high levels of antibodies specific for the tumor-associated Tn antigen [98,118]. These ligand constructs that include the Tn antigen take advantage of the natural sugar as the specific ligand for CLEC10A [118,119,120].
5. A Ligand Mimetic
A tetravalent peptide with arms that mimic GalNAc was designed as a ligand for CLEC10A. The peptide has a KD of binding to the receptor in the low nanomolar range, with an optimal concentration in cell cultures of 10 nM [121]. The arms of the peptide have the sequence NQHTPR (Asn-Gln-His-Thr-Pro-Arg), which is linked to a tri-lysine core through the sequence GGGS (Gly-Gly-Gly-Ser). The 6-mer sequence (sv6D) is the C-terminal half of a 12-mer sequence (svL4) originally identified through a screen of a phage display library with the GalNAc-specific lectin from Helix pomatia. The 12- and 6-mer peptides effectively suppress ovarian ascites, which is an inflammatory environment that recruits monocytes that differentiate into DCs that express CLEC10A [20].
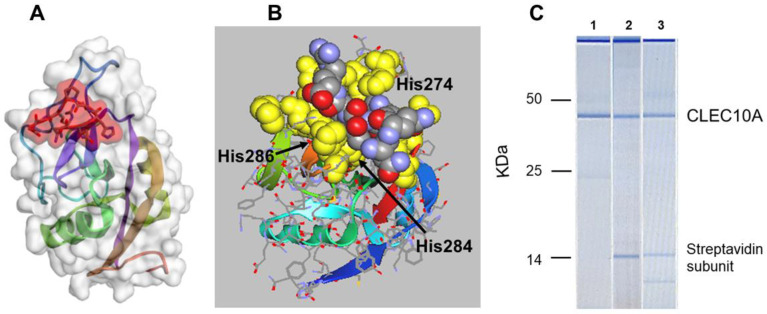
Although non-antigenic in the mouse, antibodies were raised against sv6D conjugated to keyhole limpet hemocyanin (KLH). This antibody preparation recognized GalNAc conjugated to polyacrylamide, which demonstrated the authenticity of the peptide as a mimetic of GalNAc [121]. The predicted characteristics of binding of the peptide to CLEC10A and ASGR1 are similar to those of GalNAc. In silico modeling suggested that the peptide is larger than a single GalNAc unit but binds to CLEC10A in the same site as the sugar (Figure 2A,B). The QPD (Gln-Pro-Asp269) sequence that correlates with specificity for GalNAc/Gal, in contrast to EPN (Glu-Pro-Asn) that determines mannose binding in the mannose receptor [82,83,122], lies at one end of the binding pocket in CLEC10A. The guanidino group of Arg is in close contact with the sidechain carboxyl of Asp269 in the model of the receptor (Figure 2B). His286 provides preference for GalNAc in an engineered mannose binding protein [82,111,122] and lies under the peptide bound to CLEC10A. His274 is in contact with the peptidic His, while Trp271 lies behind the His-Thr-Pro-Arg sequence of the peptide. Interestingly, the oxygen atoms of the peptide are oriented outward while the ‘back’ of the peptide, in contact with the receptor, is largely hydrophobic and shielded from the Ca2+ ion by the Trp271 and His274. The predicted binding energy, ΔG′ = −41.6 kJ/mol, is derived from peptide/protein interactions [123]. Although the model predicts that the peptide does not directly interact with the Ca2+ ions, binding occurs in a strictly Ca2+-dependent manner [121].
Figure 2.
(A) In silico docking of an arm of sv6D (NQHTPR) to ASGR1 (accession number 1DV8) with CABS-dock (RMSD = 0.7611 Å) [124]. The peptide is enclosed in red shading. (B) The structure of CLEC10A was generated with SWISS-MODEL Deep View from the structure of ASGR1 [125]. Docking was modeled with CABS-dock (RMSD = 1.421 Å) and downloaded into ArgusLab. The position of sv6D in the binding pocket is shown after additional molecular dynamics. The peptide is colored (carbon, grey; nitrogen, blue; oxygen, red) while the binding site is yellow. The QPD sequence (Gln-Pro-Asp) is at the upper left of the binding site. The positions of His274, His284, and His286 of the binding site are indicated (see Figure 1). (C) A lysate of human mo-DCs was incubated with (1) mouse anti-human CLEC10A, which was recovered with magnetic beads coated with Protein A; (2) biotinylated sv6D; or (3) biotinylated svL4, which were recovered with magnetic beads coated with streptavidin. Proteins were eluted from the beads and subjected to electrophoresis with a BioAnalyzer (Agilent) in the presence of dithiothreitol. Molecular mass markers are indicated for IgG heavy chain (50 kDa), IgG light chain (25 kDa), and a streptavidin C1 subunit (13.6 kDa). The top band is an instrument marker.
A demonstration of the specificity of binding of peptides to CLEC10A in mo-DCs is shown in Figure 2C. Biotinylated peptide ligands of CLEC10A, sv6D, and svL4 [121], were incubated with a lysate of cells and then retrieved with streptavidin-coated magnetic beads, which pulled out a single protein. The calculated molecular mass of unglycosylated CLEC10A is 35,446 Da. With the two identified N-linked glycans, the mass is expected to be about 41 kDa. These cells are known to express DC-SIGN [126,127] and the mannose receptor [128], but CLEC10A was the only protein detected by this technique. In particular, a protein with the mass of DC-SIGN (45,775 Da plus the single N-linked glycan, or about 48 kDa) was not detected. CLEC10A was not detected in lysates of THP-1 monocytes (data not shown), but this protein was detected at very low levels on the surface of unstimulated THP-1 cells by flow cytometry [91]. Analyses of expression by single-cell RNA sequencing indicated a low level in primary monocytes [20,94].
6. Roles for CLEC10A in Health and Disease
Vlismas et al. [16] observed that human embryos at the totipotent eight-cell stage expressed CLEC10A at least 70-fold higher than the levels in embryonic stem and pluripotent cells. Klimmeck et al. [17] found that differentiation of hematopoetic multipotent progenitor cells to myeloid committed progenitors in the mouse was accompanied by a several-fold increase in expression of MGL2 (CLEC10A). The function of the receptor at these early stages of development is not known. Interestingly, a large population of ‘undifferentiated’ cells occurs in the peritoneum of Balb/c mice, which essentially disappeared when the animals were injected with svL4, a peptide ligand mimetic of MGL2. Concomitant with loss of the undifferentiated cells were increases in populations of mature immune cells [121]. In contrast, the undifferentiated population was minimal in C57BL/6 mice but increased dramatically within 24 h after an injection of svL4. After a second injection of svL4, the loss of this population was accompanied by increases in mature immune cells. The undifferentiated population was Lineage negative and did not stain for markers of mature cells. It was concluded that a common myeloid progenitor population resides in the peritoneum of Balb/c mice that differentiates to mature immune cells upon subcutaneous injection of a ligand for CLEC10A, and that this population can be induced in C57BL/6 mice [121]. Therefore, it appears that CLEC10A mediates proliferation of progenitor cells at least at two steps in the developmental pathway of mature immune cells in the peritoneal cavity of mice. The consequence of treatment with the peptide is expansion of the innate immune system, which may thus serve a foundational immunological role. The question is the specific action of CLEC10A/MGL2 in these processes.
An initial consequence of introduction of a ligand of CLEC10A is stimulation of endocytosis, with a concomitant influx of Ca2+. In studies with fibroblasts, endocytosis of fluorescent dyes (non-specific ligands) contributed a significant amount of Ca2+ to the cellular interior at a rate as much as 2 µM min−1 [47]. Acidification of the endosome, driven by vacuolar H+-ATPase (V-ATPae) [49,50] is a prerequisite to the reduction in the endosomal Ca2+ concentration, which suggests an exchange mechanism is engaged to transport Ca2+ into the cytosol [51,52,53]. Umemoto et al. [129] discovered that an influx of Ca2+ stimulated mitochondrial ATP production, which led to cell division of hematopoietic stem cells in C57BL/6 mice. These cells were described as ‘stem cells’ (Lin- Sca-1−), which may be analogous to the myeloid committed progenitor cells described by Klimmeck et al. [17].
Several studies found a correlation between expression of CLEC10A and positive or negative outcomes in disease. Dusoswa et al. [109] found that glioblastoma tumor cells express relatively high levels of the Tn antigen. Moreover, CLEC10A is expressed at higher levels by tumor-associated macrophages and microglial cells in the brain. When a murine glioma cell line was engineered to further increase the expression of Tn, growth of tumors from the implanted cell line was enhanced. The increased number of infiltrating macrophages that expressed MGL (CLEC10A) also expressed PD-L1, both of which are immunosuppressive factors and were considered the cause of enhanced tumor growth. Similarly, Kurze et al. [130] found increased expression of Tn antigen in breast cancer tissues in mice treated with 4-hydroxy-tamoxifen, which increased phagocytic activity of macrophages that expressed CLEC10A within tumor tissue. However, in this case, the increase in Tn was associated with improved survival attributed to removal of damaged and dead cells.
Interestingly, house mite-induced dermatitis in mice is exacerbated in MGL1 (CD301a)-deficient strains of mice [13]. Repair of a point mutation in the gene for MGL1 by CRISPR attenuated severity of the dermatitis. Reporter cells that expressed MGL1 responded to a glycoprotein extracted from the mites that contained Tn and T structures, which ameliorated secretion of inflammatory cytokines. Whereas dermatitis caused a dramatic thickening of the epidermis, treatment with this glycoprotein reduced dermatitis induced by lipopolysaccharide in wild-type mice and restored the epidermis to a more normal thickness. Kanemaru et al. [13] proposed that ASGR1 is the human ortholog that defends against dermatitis. In a similar condition, MGL1 was upregulated in the lungs of a murine model of pneumonic sepsis caused by infection with the gram-negative bacterium Klebsiella pneumoniae. Deficiency of MGL1 resulted in significantly greater mortality, which indicated that MGL1 is required for resolution of pulmonary inflammation. Thus, MGL1 appeared to play a protective function in this bacterial infection [131], although the mechanism by which the receptor performs this function is not known.
7. Activation of T Cells
Tolerance defines a state of T cells. Whether T cells become tolerant is determined by the signal(s) given by DCs. Because CLEC10A is expressed by DCs and is often described as a “tolerogenic” receptor [18,109,119,132,133], it is useful to briefly review the outlines of T cell activation.
After phagocytosis by a pathogen-recognition receptor, peptide products generated by digestion in the endolysosomal system within DCs are loaded onto major histocompatibility complex (MHC) class II complexes and transported on vesicles to the cell surface. A functional presentation of these digestion products to the T cell receptor (TCR) on naïve CD4+ T cells requires three signals in correct sequence [134]. Signal 1 is recognition by the TCR of the antigen (’danger signal’) [135] presented by the MHC class II complex. Consequently, the TCR on T cells that recognize the antigen is phosphorylated on its cytoplasmic ITAM (immunoreceptor tyrosine-based activation motif) sequence, which leads to an increase in intracellular Ca2+. Signal 2 is expression of co-stimulatory factors CD40/CD80/CD86 by DCs as they migrate to draining lymph nodes where they interact with CD28 on the T cell. Furthermore, signal 3 is an inflammatory stimulus via secretion by DCs of cytokines such as IL-12, the principal cytokine for a TH1 response, type I interferon (IFN-α/β), or IL-27 [136] that amplify T cell differentiation and expansion [137,138,139]. Secretion of the type I interferons is an expression of a danger signal emitted by stressed cells or cells with damaged DNA that provide the essential context for an immune response, without which T cell receive a tolerogenic signal from DCs [140,141].
Upon binding of an antigen (signal 1), the ITAM sequence in the cytoplasmic domain of the CD3 subunit of the TCR is phosphorylated by the Src protein-Tyr kinases Lck and Fyn, which leads to subsequent phosphorylation of ZAP70 [142]. ZAP70 activates phospholipase C-γ1 (PLC-γ1}, which hydrolyzes phosphatidylinositol 3-P (PIP3) to diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3), both of which are required to activate T cells. PIP3 is generated by PIP3 kinase, which is activated by CD28 on T cells by interaction with its ligands CD80/CD86 on DCs (signal 2). IP3 binds to its receptor on the endoplasmic reticulum and causes release of Ca2+, which binds to calmodulin and activates the Ca2+-dependent Ser/Thr phosphatase, calcineurin, which in turn dephosphorylates NFAT [142,143,144]. Translocation of dephosphorylated NFAT into the nucleus allows binding of the transcription activation factor to specific promoter regions and induction of expression of activation genes. DAG activates protein-Tyr kinase C-θ (PKCθ), which phosphorylates MAP kinases, including JNK2, which phosphorylates the Jun/Fos subunits of AP-1. AP-1 binds to and stabilizes the association of NFAT within regulatory elements of activating genes. PLC-γ1 and the Ca2+ arm lead to activation of NFAT, whereas PCKθ activity is required for AP-1, which in combination with NFAT induces expression of activation genes [144]. AP-1 is the key link between chromatin opening and activation of naïve T cells [145]. Reduced levels of AP-1 lead to loss of expression of activation genes and sustained unresponsiveness of the cells to subsequent stimulation by promoting expression of inhibitory genes by NFAT alone [144,145,146,147]. Whereas antigen presentation to the TCR activates the Ca2+ arm of T cell activation, treatment with anti-PD-1 compensates for a weak or absent signal 2.
Tolerance is achieved by several mechanisms. Lack of co-stimulatory signals such as CD86 leads to the absence of active AP-1, which leads to anergy and deletion, or bona fide tolerance, as described by Steinman [148]. Heissmeyer et al. [142] showed that a sustained elevated Ca2+ signaling induces a state of unresponsiveness in T cells by calcineurin-mediated degradation of PLC-γ1 and PCKθ. Without AP-1, NFAT locks in an inhibitory transcriptional pattern of genes [142,144,145,146,147], which leads to anergy. A lengthier process, described as ‘exhaustion,’ is associated with loss of effector T cell function and altered transcriptional patterns [149]. Whereas anergy is achieved within a few days, development of exhaustion requires weeks. The exhausted state can be overcome by checkpoint blockade [150].
An important program within the immune system’s repertoire is an actively regulated, antigen-dependent state of immunosuppression or an anti-inflammatory response that evolved to keep the immune system from over-reacting [141,151,152,153]. Upon ligation of CLEC10A on DCs, a signal transduction pathway is activated that leads to phosphorylation of CREB and expression of the IL-10 gene [89]. IL-10 binds to the receptor IL-10R on T cells, which is associated with Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2). Phosphorylation of these kinases leads to phosphorylation of tyrosine residues in the cytosolic domain of IL-10Rα, to which the transcriptional factor STAT3 is recruited. Activation of STAT3 by phosphorylation allows its translocation to the nucleus and initiation of expression of a set of genes that provide an anti-inflammatory response [154]. Interestingly, treatment of mo-DCs with a multivalent GalNAc-containing dendrimer significantly reduced expression of several genes encoding enzymes in energy metabolism and the rate of glycolysis [120]. This effect may also moderate the strength of the signals provided to T cells by DCs.
8. The Ca2+ Connection
Ca2+ is a critical regulatory messenger for many cellular activities [154,155]. Constitutive recycling of endocytic receptors such as ASGR1 [41], the heightened rate with a bound ligand, and transport of Ca2+ from the endosome to the cytosol [47,48,49,50,51], leads to an elevated cytosolic concentration [47]. Moreover, IgG-mediated phagocytosis through the receptor FcγRIIIb is accompanied by an increase in intracellular Ca2+ [156,157]. The maintenance of low intracellular concentrations of Ca2+ is determined by transport channels on the plasma membrane, the endoplasmic reticulum, mitochondria, and lysosomes [93,158,159,160]. Given that cellular responses may differ as a function of the Ca2+ concentration, we searched for clues that suggest how CLEC10A and other C-type receptors either induce tolerogenic or activation signals. The evidence suggests that high level engagement of the receptor (high occupancy) induces a high rate of Ca2+ influx and stimulation of a signal transduction pathway that leads to tolerance, whereas minimal occupancy (low concentrations of ligand) leads to activation. Full engagement of CLEC10A likely occurs when an antibody against the receptor is introduced, which leads to secretion of IL-10 [18,25,89,132,161]. A multivalent glycopeptide that contained 12 Tn (GalNAc) termini, added at a concentration of 5 µM, also induced secretion of IL-10 and TNF-α [98]. Other ligands of MGL (CLEC10A) often used to study DC function are the glycoproteins MUC1 and MUC6 or fragments of these proteins that have been modified to contain additional Tn antigens that bind the CRD. When added at a concentration of about 1 µM, Napoletano et al. [25] concluded that MGL (CLEC10A) engagement with the peptide containing 9 Tn adducts promoted DC activation. The responses were strongly dependent upon the type of ligand provided, with the Tn-MUC glycopeptide having advantages over the anti-MGL antibody for DC activation [26]. These studies show that a range of pathways, from activation to tolerance of DCs, can be induced with the appropriate conditions. Primary among these conditions is the ligand and the frequency and concentration of administration, i.e., the extent of engagement of the receptor [25,26] and the resulting Ca2+ response.
IL-10 is the main tolerogenic cytokine that keeps the immune system under control [153,154,161,162]. Increased secretion of IL-10 but decreased secretion of IFN-γ and IL-12 is indicative of a switch to an immunosuppressive (tolerogenic) profile. Within the reciprocal relationship between the anti-inflammatory IL-10 and pro-inflammatory IL-12 [163], extensive evidence has demonstrated that IL-10 is produced in response to an increase in intracellular Ca2+ [164,165,166,167,168]. In contrast, low concentrations of intracellular Ca2+ result in production of IL-12 [169]. Ligand-induced endocytosis of CLECs transfers Ca2+ bound to the receptor and in the extracellular fluid into the cell, where mechanisms described earlier transport Ca2+ from the endosome into the cytosol. Phosphorylation of tyrosine in the motif (YENF) in the cytoplasmic domain of CLEC10A [88], which is described as an endocytic motif as well as a hemITAM [170], initiates activation of a Src/Syk signal transduction pathway that includes PLCγ2, ERK1/2, p90RSK, and CREB and leads to expression of IL-10 [89]. A similar response was found in macrophages in which a Dectin-1/calmodulin-mediated activation of protein kinase II and Pyk2 led to phosphorylation of CREB and production of IL-10 [171]. Phosphorylation of the transcriptional factor, CREB, by calmodulin-dependent protein kinases mediates the response to Ca2+ and production of IL-10 [172,173].
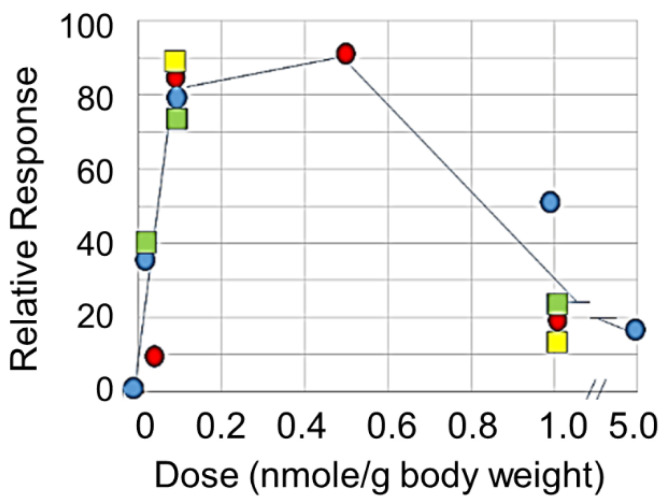
This question can be further addressed with small ligands with which relative occupancy of the receptor can be manipulated. Eggink et al. [121] designed small peptide mimetics of GalNAc that bind to CLEC10A with high avidity (see Figure 2). Injection of the mimetic svL4 into glioma tumor-bearing mice at 1 nmole/g had little effect on reduction of tumor growth [174]. However, tumor size was an order of magnitude less in animals given 0.1 nmole/g doses for two weeks as compared with the untreated control (unpublished results). Tetravalent, short peptide mimetics of GalNAc, induced an initial (4 h after injection) TH1 type cytokine response in female Balb/c mice bearing an implanted breast tumor, in which the levels of IL-12 and IFN-γ were increased to a much greater extent than IL-10 [121]. The peptide dramatically extended survival of mice with an ovarian cancer cell line implanted in the peritoneal cavity, and doses of 0.1 nmole/g were more effective than 1 nmole/g [121]. In vitro experiments with human peripheral blood myeloid cells (PBMCs) showed that incubation with 10 nM sv6D caused a rapid (within 10 min) and extensive (up to 80%) dephosphorylation of a large number of signal transduction intermediates, followed by a rapid re-phosphorylation (unpublished results). This effect has the appearance of ‘resetting’ the signal transduction kinase intermediates to deliver an activation response. In contrast, 100 nM peptide did not cause a decrease in phosphorylation and only a slight rise in the phosphorylation state of several intermediates after 15 min. Activation by mo-DCs of secretion of IFN-γ by T cells was optimal at a concentration of 10 nM [121]. As shown in Figure 3, effects of peptides svL4 and sv6D on several processes in vivo, including proliferation of peritoneal cells, inhibition of ascites accumulation in a model of ovarian cancer, and reduction in the growth of glioma tumors, followed a bell-shaped curve. With the mouse, in vivo doses between 0.1 and 1.0 nmole/g seemed to correspond to the in vitro range of 10 to 100 nM.
Figure 3.
Effect of dose on response in C57BL/6 mice. The peptides were injected subcutaneously and measured endpoints included proliferation of progenitor peritoneal cells in healthy mice (red circles), inhibition of growth of glioma tumors with cells implanted in the brain (blue circles), and survival of mice with implanted ID8 ovarian cancer cells treated with svL4 (green squares) or sv6D (yellow squares).
Mayes et al. [175] presented a critical analysis of the potential dual activities of agonist immunotherapeutic antibodies. In contrast to antagonist drugs whose effects rise to a maximum at complete receptor occupancy with a sigmoidal dose-response curve, agonist drugs often induce maximal effects near 50% receptor engagement, with a reduction in effect at higher concentrations. Activities of the receptors are also strongly affected by the extent of clustering induced by the ligand. Westerfield and Barrera [176] proposed a mechanism for receptor activation based on ligand-induced clustering that determines efficiency and sensitivity. At high receptor occupancy, the receptor may aggregate to a greater extent, which would exclude CD45, the ubiquitous protein tyrosine phosphatase on immune cells, and allow the phosphorylated receptors to prolong the initiation signal. Clustering (dimerization) of the platelet receptor CLEC2 was required for phosphorylation by Syk/Src kinases of the hemITAM sequence (YITL) within the cytoplasmic domain and activation of downstream signaling, which involves PLCγ2 [177], a pathway similar to that for CLEC10A [89]. Ligand binding induced an increase in cytosolic Ca2+ that reached a maximal level about 90 sec after adding ligand, which was dependent upon the fluidity/stability of the membrane [177]. CLEC10A occurs on cell membranes as a trimer [178] and may form larger clusters with multivalent ligands. A bell-shaped pharmacodynamic curve for effects in the mouse as shown in Figure 3 has also been found with other Ca2+-mediated processes [179,180] and may be a common feature of the effects of C-type lectin receptors.
9. Conclusions
The cell biology of lectin-like receptors was established by the extensive early research with ASGR1, which set the stage for the work on C-type lectin receptors that followed. The role of C-type lectin receptors in the responses of immune cells to pathogens has been a critical motivator for research on these proteins. Although CLEC10A has been a research interest for many years, and a large amount of data has been obtained, the apparent pleiotropic effects induced by different environments and various ligands leave the action of this receptor as a conundrum. To achieve a deeper understanding of the activities of these receptors, it will be important to determine the extent to which activation of specific signal transduction pathways by C-type lectin receptors are dependent upon the cytosolic concentration of Ca2+ and the kinetics of transients of the cation [93]. There is a large body of evidence on the regulation of cellular events by Ca2+ on which these approaches can be based.
Acknowledgments
This work was supported by Susavion Biosciences, Inc.
Funding
This research received no external funding.
Conflicts of Interest
J.K.H. is a co-founder of Susavion Biosciences, Inc., in which he holds shares. The sponsors had no role in the design, collection, analysis, or interpretation of data, the writing of this article, or the decision to submit it for publication.
References
- 1.Pillai S., Netravali I.A., Cariappa A., Mattoo H. Siglecs and Immune Regulation. Annu. Rev. Immunol. 2012;30:357–392. doi: 10.1146/annurev-immunol-020711-075018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macauley M.S., Crocker P.R., Paulson J.C. Siglec-Mediated Regulation of Immune Cell Function in Disease. Nat. Rev. Immunol. 2014;14:653–666. doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan S., Paulson J.C. Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol. 2020;38:365–395. doi: 10.1146/annurev-immunol-102419-035900. [DOI] [PubMed] [Google Scholar]
- 4.Rabinovich G.A., Toscano M.A. Turning ‘Sweet’ on Immunity: Galectin-Glycan Interactions in Immune Tolerance and Inflammation. Nat. Rev. Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 5.Di Lella S., Sundblad V., Cerliani J.P., Guardia C.M., Estrin D.A., Vasta G.R., Rabinovich G.A. When Galectins Recognize Glycans: From Biochemistry to Physiology and Back Again. Biochemistry. 2011;50:7842–7857. doi: 10.1021/bi201121m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasta G.R., Amzel L.M., Bianchet M.A., Cammarata M., Feng C., Saito K. F-Type Lectins: A Highly Diversified Family of Fucose-Binding Proteins with a Unique Sequence Motif and Structural Fold, Involved in Self/Non-Self-Recognition. Front. Immunol. 2017;8:1648. doi: 10.3389/fimmu.2017.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings R.D., McEver R.P. Chapter 31: R-Type Lectins. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., editors. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2017. pp. 2015–2017. [Google Scholar]
- 8.Drickamer K., Taylor M.E. Recent Insights into Structures and Functions of C-Type Lectins in the Immune System. Curr. Opin. Struct. Biol. 2015;34:26–34. doi: 10.1016/j.sbi.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor M.E., Drickamer K. Mammalian Sugar-Binding Receptors: Known Functions and Unexplored Roles. FEBS J. 2019;286:1800–1814. doi: 10.1111/febs.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sancho D., Reis e Sousa C. Signaling by Myeloid C-Type Lectin Receptors in Immunity and Homeostasis. Ann. Rev. Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drickamer K. Two Distinct Classes of Carbohydrate-recognition Domains in Animal Lectins. J. Biol. Chem. 1988;263:9557–9560. [PubMed] [Google Scholar]
- 12.Weis W.I., Taylor M.E., Drickamer K. The C-Type Lectin Superfamily in the Immune System. Immunol. Rev. 1998;163:19–34. doi: 10.1111/j.1600-065X.1998.tb01185.x. [DOI] [PubMed] [Google Scholar]
- 13.Kanemaru K., Noguchi E., Tahara-Hanaoka S., Mizuno S., Tateno H., Denda-Nagai K., Irimura T., Matsuda H., Sugiyama F., Takahashi S., et al. Clec10a Regulates Mite-Induced Dermatitis. Sci. Immunol. 2019;4:eaax6908. doi: 10.1126/sciimmunol.aax6908. [DOI] [PubMed] [Google Scholar]
- 14.Harris R.L., van den Berg C.W., Bowen D.J. ASGR1 and ASGR2, the Genes that Encode the Asialoglycoprotein Receptor (Ashwell Receptor), Are Expressed in Peripheral Blood Monocytes and Show Interindividual Differences in Transcript Profile. Mol. Biol. int. 2012;2012:283974. doi: 10.1155/2012/283974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UniProt.org UniProtKB-Q8IUN9(CLC10_HUMAN) [(accessed on 25 May 2020)]; Available online: www.uniprot.org.
- 16.Vlismas A., Bletsa R., Mavrogianni D., Mamali G., Pergamali M., Dinopoulo V., Partsinevelos G., Drakakis P., Loutradis D., Kiessling A.A. Microarray Analyses Reveal Marked Differences in Growth Factor and Receptor Expression Between 8-Cell Human Embryos and Pluripotent Stem Cells. Stem Cells Dev. 2016;25:160–177. doi: 10.1089/scd.2015.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klimmeck D., Hansson J., Raffel S., Vakhrushev S.Y., Trumpp A., Krijgsveld J. Proteomic Cornerstones of Hematopoietic Stem Cell Differentiation: Distinct Signatures of Multipotent Progenitors and Myeloid Committed Cells. Mol. Cell. Proteom. 2012;11:286–302. doi: 10.1074/mcp.M111.016790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Kooyk Y., Ilarregui J.M., van Vliet S.J. Novel Insights into the Immunomodulatory Role of the Dendritic Cell and Macrophage-expressed C-type Lectin MGL. Immunobiology. 2015;220:185–192. doi: 10.1016/j.imbio.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Napoletano C., Rughetti A., Tarp M.P.A., Coleman J., Bennett E.P., Picco G., Sale P., Denda-Nagai K., Irimura T., Mandel U., et al. Tumor-Associated Tn-MUC1 Glycform is Internalized through the Macrophage Galactose-type C-type Lectin and Delivered to the HLA Class I and II Compartments in Dendritic Cells. Cancer Res. 2007;67:8358–8367. doi: 10.1158/0008-5472.CAN-07-1035. [DOI] [PubMed] [Google Scholar]
- 20.Tang-Huau T.L., Gueguen P., Goudot C., Durand M., Bohec M., Baulande S., Pasquier B., Amigorena S., Sequra E. Human in vivo-Generated Monocyte-derived Dendritic Cells and Macrophages Cross-present Antigens through a Vacuolar Pathway. Nat. Commun. 2018;9:2570. doi: 10.1038/s41467-018-04985-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang W., Xu W., Xiong S. Macrophage Differentiation and Polarization via Phosphatidylinositol 3-Kinase/Akt-ERK Signaling Pathway Conferred by Serum Amyloid P Component. J. Immunol. 2011;187:1764–1777. doi: 10.4049/jimmunol.1002315. [DOI] [PubMed] [Google Scholar]
- 22.Röszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediat. Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong K.L., Tai J.J.-Y., Wong W.-C., Han H., Sem X., Yeap W.-H., Kourilsky P., Wong S.-C. Gene Expression Profiling Reveals the Defining Features of the Classical, Intermediate, and Nonclassical Human Monocyte Subsets. Blood. 2011;118:e16–e31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 24.Rapoport E.M., Moiseeva E.V., Aronov D.A., Khaidukov S.V., Pazynina G.V., Tsygankova S.V., Ryzhov I.M., Belyanchikov I.M., Tyrtysh T.V., McCullough K.C., et al. Glycan-Binding Profile of DC-like Cells. Glycoconj. J. 2020;37:129–138. doi: 10.1007/s10719-019-09897-9. [DOI] [PubMed] [Google Scholar]
- 25.Napoletano C., Zizzari I.G., Rughetti A., Rahimi H., Irimura T., Clausen H., Wandall H.H., Belleudi F., Bellati F., Pierelli L., et al. Targeting of Macrophage Galactose-type C-type Lectin (MGL) Induces DC Signaling and Activation. Eur. J. Immunol. 2012;42:936–945. doi: 10.1002/eji.201142086. [DOI] [PubMed] [Google Scholar]
- 26.Zizzari I.G., Napoletano C., Battisti F., Rahimi H., Caponnetto S., Pierelli L., Nuti M., Rughetti A. MGL Receptor and Immunity: When the Ligand can Make the Difference. J. Immunol. Res. 2015;2015:450695. doi: 10.1155/2015/450695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morell A.G., Irvine R.A., Sternlieb I., Scheinberg I.H., Ashwell G. Physical and Chemical Studies on Ceruloplasmin. V. Metabolic Studies on Sialic Acid-Free Ceruloplasmin In Vivo. J. Biol. Chem. 1968;243:155–159. [PubMed] [Google Scholar]
- 28.Morell A.G., Gregoriadis G., Scheinberg I.H., Hickman J., Ashwell G. The Role of Sialic Acid in Determining the Survival of Glycoproteins in the Circulation. J. Biol. Chem. 1971;246:1461–1467. [PubMed] [Google Scholar]
- 29.Pricer W.E., Jr., Ashwell G. The Binding of Desialylated Glycoproteins by Plasma Membranes of Rat Liver. J. Biol. Chem. 1971;246:4825–4833. [PubMed] [Google Scholar]
- 30.Sarkar M., Liao J., Kabat E.A., Tanabe T., Ashwell G. The Binding Site of Rabbit Hepatic Lectin. J. Biol. Chem. 1979;254:3170–3174. [PubMed] [Google Scholar]
- 31.Steer C.J., Ashwell G. Studies on a Mammalian Hepatic Binding Protein Specific for Asialoglycoproteins. Evidence for Receptor Recycling in Isolated Hepatocytes. J. Biol. Chem. 1980;255:3008–3013. [PubMed] [Google Scholar]
- 32.Schönfeld D.L., Ravelli R.B.G., Mueller U., Skerra A. The 1.8-Å Crystal Structure of α1-Acid Glycoprotein (Orosomucoid) Solved by UV RIP Reveals the Broad Drug-binding Activity of This Human Plasma Lipocalin. J. Mol. Biol. 2008;384:393–405. doi: 10.1016/j.jmb.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Nishi K., Ono T., Nakamura T., Fukunaga N., Izumi M., Watanabe H., Senaga A., Maruyama T., Yamagata Y., Curry S., et al. Structural Insights into Differences in Drug-binding Selectivity between Two Forms of Human α1-Acid Glycoprotein Genetic Variants, the A and F1*S Forms. J. Biol. Chem. 2011;286:14427–14434. doi: 10.1074/jbc.M110.208926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feinberg H., Torgersen D., Drickamer K., Weis W.I. Mechanism of pH-Dependent N-Acetylgalactosamine Binding by a Functional Mimic of the Hepatic Asialoglycoprotein Receptor. J. Biol. Chem. 2000;275:35176–35184. doi: 10.1074/jbc.M005557200. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz N.I., Drikamer K. Differential Ligand Binding by Two Subunits of the Rat Liver Asialoglycoprotein Receptor. Glycobiology. 1996;6:551–559. doi: 10.1093/glycob/6.5.551. [DOI] [PubMed] [Google Scholar]
- 36.Henis Y.I., Katzir Z., Shia M.A., Lodish H.F. Oligomeric Structure of the Human Asialoglycoprotein Receptor: Nature and Stoichiometry of Mutual Complexes Containing H1 and H2 Polypeptides Assessed by Fluorescence Photobleaching Recovery. J. Cell Biol. 1990;111:1409–1418. doi: 10.1083/jcb.111.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bider M.D., Wahlberg J.M., Kammerer R.A., Spiess M. The Oligomerization Domain of the Asialoglycoprotein Receptor Preferentially Forms 2:2 Heterotetramers In Vitro. J. Biol. Chem. 1996;271:31996–32001. doi: 10.1074/jbc.271.50.31996. [DOI] [PubMed] [Google Scholar]
- 38.Hardy M.R., Townsend R.R., Parkhurst S.M., Lee Y.C. Different Modes of Ligand Binding to the Hepatic Galactose/N-Acetylgalactosamine Lectin on the Surface of Rabbit Hepatocytes. Biochemistry. 1985;24:22–28. doi: 10.1021/bi00322a004. [DOI] [PubMed] [Google Scholar]
- 39.Schwartz A.L., Rup D., Lodish H.F. Difficulties in the Quantitation of Asialoglycoprotein Receptors on the Rat Hepatocyte. J. Biol. Chem. 1980;255:9033–9036. [PubMed] [Google Scholar]
- 40.Schwartz A.L., Marshak-Rothstein A., Rup D., Lodish H.F. Identification and Quantitation of the Rat Hepatocyte Asialoglycoprotein Receptor. Proc. Natl. Acad. Sci. USA. 1981;78:3348–3352. doi: 10.1073/pnas.78.6.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwartz A.L., Fridovich S.E., Lodish H.F. Kinetics of Internalization and Recycling of the Asialoglycoprotein Receptor in a Hepatoma Cell Line. J. Biol. Chem. 1982;257:4230–4237. [PubMed] [Google Scholar]
- 42.Geuze H.J., Slot J.W., Strous G.J.A., Lodish H.F., Schwartz A.L. Intracellular Site of Asialoglycoprotein Receptor-Ligand Uncoupling: Double-Label Immunoelectron Microscopy during Receptor-Mediated Endocytosis. Cell. 1983;32:277–287. doi: 10.1016/0092-8674(83)90518-4. [DOI] [PubMed] [Google Scholar]
- 43.Ciechanover A., Schwartz A.L., Lodish H.F. Sorting and Recycling of Cell Surface Receptors and Endocytosed Ligands: The Asialoglycoprotein and Transferrin Receptors. J. Cell. Biochem. 1983;23:107–130. doi: 10.1002/jcb.240230111. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz A.L., Bolognesi A., Fridovich S.E. Recycling of the Asialoglycoprotein Receptor and the Effect of Lysosomotropic Amines in Hepatoma Cells. J. Cell Biol. 1984;98:732–738. doi: 10.1083/jcb.98.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mettlen M., Chen P.-H., Srinivasan S., Danuser G., Schmid S.L. Regulation of Clathrin-Mediated Endocytosis. Annu. Rev. Biochem. 2018;87:871–896. doi: 10.1146/annurev-biochem-062917-012644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meier M., Bider M.D., Malashkevich V.N., Spiess M., Burkhard P. Crystal Structure of the Carbohydrate Recognition Domain of the H1 Subunit of the Asialoglycoprotein Receptor. J. Mol. Biol. 2000;300:857–865. doi: 10.1006/jmbi.2000.3853. [DOI] [PubMed] [Google Scholar]
- 47.Gerasimenko J.V., Tepikin A.V., Petersen O.H., Gerasimenko O.V. Calcium uptake via endocytosis with rapid release from acidifying endosomes. Curr. Biol. 1998;8:1335–1338. doi: 10.1016/S0960-9822(07)00565-9. [DOI] [PubMed] [Google Scholar]
- 48.Onizuka T., Shimizu H., Moriwaki Y., Nakano T., Kanai S., Shimada I., Takahashi H. NMR Study of Ligand Release from Asialoglycoprotein Receptor Under Solution Conditions in Early Endosomes. FEBS J. 2012;279:2645–2656. doi: 10.1111/j.1742-4658.2012.08643.x. [DOI] [PubMed] [Google Scholar]
- 49.Maxson M.E., Grinstein S. The Vacuolar-type H+-ATPase at a Glance—More than a Proton Pump. J. Cell Sci. 2014;127:4987–4993. doi: 10.1242/jcs.158550. [DOI] [PubMed] [Google Scholar]
- 50.Pamarthy S., Kulshrestha A., Katara G.K., Beaman K.D. The Curious Case of Vacuolar ATPase: Regulation of Signaling Pathways. Mol. Cancer. 2018;17:41. doi: 10.1186/s12943-018-0811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morgan A.J., Platt F.M., Lloyd-Evans E., Galione A. Molecular Mechanisms of Endolysosomal Ca2+ Signalling in Health and Disease. Biochem. J. 2011;439:349–374. doi: 10.1042/BJ20110949. [DOI] [PubMed] [Google Scholar]
- 52.Shumilina E., Huber S.M., Lang F. Ca2+ Signaling in the Regulation of Dendritic Cell Functions. Am. J. Physiol. Cell. Physiol. 2011;300:C1205–C1214. doi: 10.1152/ajpcell.00039.2011. [DOI] [PubMed] [Google Scholar]
- 53.Plattner H., Verkhratsky A. Inseparable Tanden: Evolution Chooses ATP and Ca2+ to Control life, Death and Cellular Signlling. Philos. Trans. R. Soc. B. 2016;371:20150419. doi: 10.1098/rstb.2015.0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee R.T., Lee Y.C. Facile Synthesis of a High-Affinity Ligand for Mammalian Hepatic Lectin Containing Three Terminal N-Acetylgalactosamine Residues. Bioconj. Chem. 1997;8:762–765. doi: 10.1021/bc9700796. [DOI] [PubMed] [Google Scholar]
- 55.Tanaka J., Gleinich A.S., Zhang Q., Whitfeld R., Kempe K., Haddleton D.M., Davis T.P., Perrier S., Mitchell D.A., Wilson P. Specific and Differential Binding of N-Acetylgalactosamine Glycopolymers to the Human Macrophage Galactose Lectin and Asialoglycoprotein Receptor. Biomolecules. 2017;18:1624–1633. doi: 10.1021/acs.biomac.7b00228. [DOI] [PubMed] [Google Scholar]
- 56.Westerlind U., Westman J., Törnquist E., Smith C.I.E., Oscarson S., Lahmann M., Norberg T. Ligands of the Asialoglycoprotein Receptor for Targeted Gene Delivery, Part 1: Synthesis of and Binding Studies with Biotinylated Cluster Glycosides Containing N-Acetylgalactosamine. Glycoconj. J. 2004;21:227–241. doi: 10.1023/B:GLYC.0000045095.86867.c0. [DOI] [PubMed] [Google Scholar]
- 57.Rensen P.C.N., van Leeuwen S.H., Sliedregt L.A.J.M., van Berkel T.J.C., Biessen E.A.L. Design and Synthesis of Novel N-Acetylgalactosamine-Terminated Glycolipids for Targeting of Lipoproteins to the Hepatic Asialoglyoprotein Receptor. J. Med. Chem. 2004;47:5798–5808. doi: 10.1021/jm049481d. [DOI] [PubMed] [Google Scholar]
- 58.Lepenies B., Lee J., Sonkaria S. Targeting C-Type Lectin Receptors with Multivalent Carbohydrate Ligands. Adv. Drug Del. Rev. 2013;65:1271–1281. doi: 10.1016/j.addr.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 59.Khorev O., Stokmaier D., Schwardt O., Cutting B., Ernst B. Trivalent, Gal/GalNAc-Containing Ligands Designed for the Asialoglycoprotein Receptor. Bioorg. Med. Chem. 2008;16:5216–5231. doi: 10.1016/j.bmc.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 60.Yang W., Zhang X., Liu Y. Asialoglycoprotein Receptor-Targeted Radiopharmaceuticals for Measurement of Liver Function. Curr. Med. Chem. 2014;21:4–23. doi: 10.2174/09298673113209990213. [DOI] [PubMed] [Google Scholar]
- 61.Abe M., Lai J., Kortylewicz Z.P., Nagata H., Fox I.J., Enke C.A., Baranowska-Kortylewicz J. Radiolabeled Constructs for Evaluation of the Asialoglycoprotein Receptor Status and Hepatic Functional Reserves. Bioconj. Chem. 2003;14:997–1006. doi: 10.1021/bc034081a. [DOI] [PubMed] [Google Scholar]
- 62.Prakash T.P., Yu J., Migawa M.T., Kinberger G.A., Wan W.B., Østergaard M.E., Vasquez G., Low A., Chappell A., Schmidt K. Comprehensive Structure-Activity Relationship of Triantennary N-Acetylgalactosamine Conjugated Antisense Oligonucleotides for Targeted Delivery to Hepatocytes. J. Med. Chem. 2016;59:2718–2733. doi: 10.1021/acs.jmedchem.5b01948. [DOI] [PubMed] [Google Scholar]
- 63.Yamamoto T., Sawamura M., Wada F., Harada-Shiba M., Obika S. Serial Incorporation of a Monovalent GalNAc Phosphoramidite Unit into Hepatocyte-Targeting Antisense Oligonucleotides. Bioorg. Med. Chem. 2016;24:26–32. doi: 10.1016/j.bmc.2015.11.036. [DOI] [PubMed] [Google Scholar]
- 64.Nair J.K., Willoughby J.L.S., Chan A., Charisse K., Alam M.R., Wang Q., Hoekstra M., Kandasamy P., Kel’in A.V., Milstein S., et al. Multivalent N-Acetylgalactosamine-Conjugated siRNA Localizes in Hepatocytes and Elicits Robust RNAi-Mediated Gene Silencing. J. Am. Chem. Soc. 2014;136:16958–16961. doi: 10.1021/ja505986a. [DOI] [PubMed] [Google Scholar]
- 65.Huang Y. Preclinical and Clinical Advances of GalNAc-Decorated Nucleic Acid Therapeutics. Mol. Ther. Nucleic Acids. 2017;6:116–132. doi: 10.1016/j.omtn.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kinberger G.A., Prakash T.P., Yu J., Vasquez G., Low A., Chappell A., Schmidt K., Murray H.M., Gaus H., Swayze E.E., et al. Conjugation of Mono- and Di-GalNAc Sugars Enhances the Potency of Antisense Oligonucleotides via ASGR Mediated Delivery to Hepatocytes. Bioorg. Med. Chem. Lett. 2016;26:3690–3693. doi: 10.1016/j.bmcl.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y., Yu R.Z., Henry S., Geary R.S. Pharmacokinetics and Clinical Pharmacology Considerations of GalNAc3-Conjugated Antisense Oligonucleotides. Exp. Opin. Drug Metab. Toxicol. 2019;15:475–485. doi: 10.1080/17425255.2019.1621838. [DOI] [PubMed] [Google Scholar]
- 68.Crooke S.T., Baker B.F., Zia S., Yu R.Z., Viney N.J., Wang Y., Tsimikas S., Geary R.S. Integrated Assessment of the Clinical Performance of GalNAc3-Conjugated 2′-O-Methoxyethyl Chimeric Antisense Oligonucleotides. 1. Human Volunteer Experience. Nucleic Acid Ther. 2019;29:16–32. doi: 10.1089/nat.2018.0753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guan H., McGuire M.J., Li S., Brown K.C. Peptide-Targeted Polyglutamic Acid Doxorubicin Conjugates for the Treatment of αvβ6-Positive Cancers. Bioconjug. Chem. 2008;19:1813–1821. doi: 10.1021/bc800154f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang L., Wang Y., Ling X., Chaurasiya B., Yang C., Du Y., Tu J., Xiong Y., Sun C. Efficient Delivery of Paclitaxel into ASGPR Over-expressed Cancer Cells Using Reversibly Stabilized Multifunctional Pullulan Nanoparticles. Carbohydr. Polym. 2017;159:178–187. doi: 10.1016/j.carbpol.2016.11.094. [DOI] [PubMed] [Google Scholar]
- 71.Pranatharthiharan S., Patel M.D., Malshe V.C., Pujari V., Gorakshakar A., Madkaikar M., Ghosh K., Devarajan P.V. Asialoglycoprotein Receptor Targeted Delivery of Doxorubicin Nanoparticles for Hepatocellular Carcinoma. Drug Deliv. 2017;24:20–29. doi: 10.1080/10717544.2016.1225856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibadullin R., Farnsworth D.W., Barchi J.J., Jr., Gildersleeve J.C. GalNAc-Tyrosine is a Ligand of Plant Lectins, Antibodies, and Human and Murine Macrophage Galactose-Type Lectins. ACS Chem. Biol. 2017;12:2172–2182. doi: 10.1021/acschembio.7b00471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rensen P.C.N., Sliedregt L.A.J.M., Ferns M., Kieviet E., van Rossenberg S.M.W., van Leeuwen S.H., van Berkel T.J.C., Biessen E.A.L. Determination of the Upper Size Limit for Uptake and Processing of Ligands by the Asialoglycoprotein Receptor on Hepatocytes In Vitro and In Vivo. J. Biol. Chem. 2001;276:37577–37584. doi: 10.1074/jbc.M101786200. [DOI] [PubMed] [Google Scholar]
- 74.Ii M., Kurata H., Itoh N., Yamashina I., Kawasaki T. Molecular Cloning and Sequence Analysis of cDNA Encoding the Macrophage Lectin Specific for Galactose and N-Acetylgalactosamine. J. Biol. Chem. 1990;265:11295–11298. [PubMed] [Google Scholar]
- 75.Sato M., Kawakami K., Osawa T., Toyoshima S. Molecular Cloning and Expression of cDNA Encoding a Galactose/N-Acetylgalactosamine-Specific Lectin on Mouse Tumoricidal Macrophages. J. Biochem. 1992;111:331–336. doi: 10.1093/oxfordjournals.jbchem.a123758. [DOI] [PubMed] [Google Scholar]
- 76.Tsuiji M., Fujimori M., Ohashi Y., Higashi N., Onami T.M., Hedrick S.M., Irimura T. Molecular Cloning and Characterization of a Novel Mouse Macrophage C-type Lectin, mMGL2, Which Has a Distinct Carbohydrate Specificity from mMGL1. J. Biol. Chem. 2002;277:28892–28901. doi: 10.1074/jbc.M203774200. [DOI] [PubMed] [Google Scholar]
- 77.Denda-Nagai K., Aida A., Saba K., Suzuki K., Moriyama S., Oo-puthinan S., Tsuiji M., Morikawa A., Kumamoto Y., Sugiura D., et al. Distribution and Function of Macrophage Galactose-type C-type Lectin 2 (MGL2/CD301b) J. Biol. Chem. 2010;285:19193–19204. doi: 10.1074/jbc.M110.113613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suzuki N., Yamamoto K., Toyoshima S., Osawa T., Irimura T. Molecular Cloning and Expression of cDNA Encoding Human Macrophage C-Type Lectin. Its Unique Carbohydrate Binding Specificity for the Tn Antigen. J. Immunol. 1996;156:128–135. [PubMed] [Google Scholar]
- 79.Higashi N., Fujioka K., Denda-Nagai K., Hashimoto S.-I., Nagai S., Sato T., Fujita Y., Morikawa A., Tsuiji M., Miyata-Takeuchi M., et al. The Macrophage C-type Lectin Specific for Galactose/N-Acetylgalactosamine Is an Endocytic Receptor Expressed on Monocyte-derived Immature Dendritic Cells. J. Biol. Chem. 2002;277:20686–20693. doi: 10.1074/jbc.M202104200. [DOI] [PubMed] [Google Scholar]
- 80.Artigas G., Monteiro J.T., Hinou H., Nishimura S.I., Lepenies B., Garcia-Martin F. Glycopeptides as Targets for Dendritic Cells: Exploring MUC1 Glycopeotides Binding Profile toward Macrophage Galactose-Type Lectin (MGL) Orthologs. J. Med. Chem. 2017;60:9012–9021. doi: 10.1021/acs.jmedchem.7b01242. [DOI] [PubMed] [Google Scholar]
- 81.Valladeau J., Duvert-Frances V., Pin J.-J., Kleijmeer M.J., Ait-Yahia S., Ravel O., Vincent C., Vega F., Jr., Helms A., Gorman D., et al. Immature Human Dendritic Cells Express Asialoglycoprotein Receptor Isoforms for Efficient Receptor-Mediated Endocytosis. J. Immunol. 2001;167:5767–5774. doi: 10.4049/jimmunol.167.10.5767. [DOI] [PubMed] [Google Scholar]
- 82.Drickamer K. Engineering Galactose-binding Activity into a C-type Mannose-binding Protein. Nature. 1992;360:183–186. doi: 10.1038/360183a0. [DOI] [PubMed] [Google Scholar]
- 83.Iobst S.T., Drickamer K. Selective Sugar Binding to the Carbohydrate Recognition Domain of the Rat Hepatic and Macrophage Asialoglycoprotein Receptors. J. Biol. Chem. 1996;271:6686–6693. doi: 10.1074/jbc.271.12.6686. [DOI] [PubMed] [Google Scholar]
- 84.van Vliet S.J., van Liempt E., Saeland E., Aarnoudse C.A., Appelmelk B., Irimura T., Geijtenbeek T.B.H., Blixt O., Alvarez R., van Die I., et al. Carbohydrate Profiling Reveals a Distinctive Role for the C-Type Lectin MGL in the Recognition of Helminth Parasites and Tumor Antigens by Dendritic Cells. Int. Immunol. 2005;17:661–669. doi: 10.1093/intimm/dxh246. [DOI] [PubMed] [Google Scholar]
- 85.Iida S.-I., Yamamoto K., Irimura T. Interaction of Human Macrophage C-type Lectin with O-linked N-Acetylgalactosamine Residues on Mucin Glycopeptides. J. Biol. Chem. 1999;274:10697–10705. doi: 10.1074/jbc.274.16.10697. [DOI] [PubMed] [Google Scholar]
- 86.Mortezai N., Behnken H.N., Kurze A.-K., Ludewig P., Buck F., Meyer B., Wagener C. Tumor-Associated Neu5Ac-Tn and Neu5Gc-Tn Antigens bind to C-Type Lectin CLEC10A (CD301, MGL) Glycobiology. 2013;23:844–852. doi: 10.1093/glycob/cwt021. [DOI] [PubMed] [Google Scholar]
- 87.Heger L., Balk S., Lühr J.J., Heidkamp G.F., Lehmann C.H.K., Hatscher L., Purbojo A., Hartmann A., Garcia-Martin F., Nishimura S.-I., et al. CLEC10A Is a Specific Marker for Human CD1c+ Dendritic Cells and Enhances Their Toll-Like Receptor 7/8-Induced Cytokine Secretion. Front. Immunol. 2018;9:744. doi: 10.3389/fimmu.2018.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van Vliet S.J., Aarnoudse C.A., Broks-van den Berg V.C.M., Boks M., Geijtenbeek T.B.H., van Kooyk Y. MGL-Mediated Internalization and Antigen Presentation by Dendritic Cells: A Role for Tyrosine-5. Eur. J. Immunol. 2007;37:2075–2081. doi: 10.1002/eji.200636838. [DOI] [PubMed] [Google Scholar]
- 89.Gu C., Wang L., Zurawski S., Oh S.K. Signaling Cascade through DC-ASGPR Induces Transcriptionally Active CREB for IL-10 Induction and Immune Regulation. J. Immunol. 2019;203:389–399. doi: 10.4049/jimmunol.1900289. [DOI] [PubMed] [Google Scholar]
- 90.Wang L.-X., Zhang S.-X., Wu H.-J., Rong X.-L., Guo J. M2b Macrophage Polarization and Its Roles in Diseases. J. Leukoc. Biol. 2019;106:345–358. doi: 10.1002/JLB.3RU1018-378RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Forrester M.A., Wassall H.J., Hall L.S., Cao H., Wilson H.M., Barker R.N., Vickers M.A. Similarities and Differences in Surface Receptor Expression by THP-1 Monocytes and Differentiated Macrophages Polarized Using Seven Different Conditioning Regimens. Cell. Immunol. 2018;332:58–76. doi: 10.1016/j.cellimm.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Vliet S.J., Saeland E., van Kooyk E. Sweet Preferences of MGL: Carbohydrate Specificity and Function. Trends Immunol. 2008;29:83–90. doi: 10.1016/j.it.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 93.Cote R., Eggink L.L., Hoober J.K. CLEC Receptors, Endocytosis, and Calcium Signaling. AIMS Aller Immunol. 2017;1:207–231. doi: 10.3934/Allergy.2017.4.207. [DOI] [Google Scholar]
- 94.Villani A.-C., Satija R., Reynolds G., Sarkizova S., Shekkar K., Fletcher J., Griesbeck M., Butler A., Zheng S., Lazo S., et al. Single-Cell RNA-seq Reveals New Types of Human Blood Dendritic Cells, Monocytes, and Progenitors. Science. 2017;356:eaah4573. doi: 10.1126/science.aah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Minoda Y., Virshup I., Rojas I.L., Haigh O., Wong Y., Miles J.J., Wells C.A., Radford K.J. Human CD141+ Dendritic Cell and CD1c+ Dendritic Cell Undergo Concordant Early Genetic Programming after Activation in Humanized Mice In Vivo. Front. Immunol. 2017;8:1419. doi: 10.3389/fimmu.2017.01419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brown C.G., Gudjonson H., Pritykin Y., Deep D., Lavalléd V.-P., Mendoza A., Fromme R., Mazutis L., Ariyan C., Leslie C., et al. Transcriptional Basis of Mouse and Human Dendritic Cell Heterogeneity. Cell. 2019;179:846–863. doi: 10.1016/j.cell.2019.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tang-Huau T.-L., Segura E. Human In Vivo-Differentiated Monocyte-Derived Dendritic Cells. Sem. Cell Dev. Biol. 2018;86:44–49. doi: 10.1016/j.semcdb.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 98.Freire T., Zhang X., Dériaud E., Ganneau C., Vichier-Guerre S., Azria E., Launay O., Lo-Man R., Bay S., Leclerc C. Glycosidic Tn-based Vaccines Targeting Dermal Dendritic Cells Favor Germinal Center B-Cell Development and Potent Antibody Response in the Absence of Adjuvant. Blood. 2010;116:3526–3536. doi: 10.1182/blood-2010-04-279133. [DOI] [PubMed] [Google Scholar]
- 99.Murakami R., Denda-Nagai K., Hashimoto S.-I., Nagai S., Hattori M., Irimura T. A Unique Dermal Dendritic Cell Subset that Skews the Immune Response Toward Th2. PLoS ONE. 2013;8:e73270. doi: 10.1371/journal.pone.0073270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garg A., Oran A., Wajchman J., Sasaki S., Maris C.H., Kapp J.A., Jacob J. Genetic Tagging Shows Increased Frequency and Longevity of Antigen-presenting, Skin-derived Dendritic Cells In Vivo. Nat. Immunol. 2003;4:907–912. doi: 10.1038/ni962. [DOI] [PubMed] [Google Scholar]
- 101.Tomura M., Hata A., Matsuoka S., Shand F.H.W., Nakahishi Y., Ikebuchi R., Ueha S., Tsutsui H., Inaba K., Matsushima K., et al. Tracking and Quantification of Dendritic Cell Migration and Antigen Trafficking Between the Skin and Lymph Nodes. Sci. Rep. 2014;4:6030. doi: 10.1038/srep06030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kitano M., Yamazaki C., Takumi A., Ikeno T., Hemmi H., Takahashi N., Shimizu K., Fraser S.E., Hoshino K., Kaisho T., et al. Imaging of the Cross-Presenting Dendritic Cell Subsets in the Skin-Draining Lymph Node. Proc. Natl. Acad. Sci. USA. 2016;113:1044–1049. doi: 10.1073/pnas.1513607113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Vliet S.J., Gringhuis S.I., Geijtenbeek T.B.H., van Kooyk Y. Regulation of Effector T Cells by Antigen-Presenting Cells via Interaction of the C-Type Lectin MGL with CD45. Nat. Immunol. 2006;7:1200–1208. doi: 10.1038/ni1390. [DOI] [PubMed] [Google Scholar]
- 104.Mnich M.E., van Dalen R., Gerlach D., Hendriks A., Xia G., Peschel A., van Strijp J.A.G., van Sorge N.M. The C-Type Lectin Receptor MGL Senses N-Acetylgalactosamine on the Unique Staphylococcus aureus ST395 Wall Teichoic Acid. Cell. Microbiol. 2019:e13072. doi: 10.1111/cmi.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raes G., Brys L., Dahal B.K., Brandt J., Grooten J., Brombacher F., Vanham G., Noel W., Bogaert P., Boonefaes T., et al. Macrophage Galactose-Type C-Type Lectins as Novel Markers for Alternatively Activated Macrophages Elicited by Parasitic Infections and Allergic Airway Inflammation. J. Leukoc. Biol. 2005;77:321–327. doi: 10.1189/jlb.0304212. [DOI] [PubMed] [Google Scholar]
- 106.Everts B., Smits H.H., Hokke C.H., Yazdanbakhsh M. Helminths and Dendritic Cells: Sensing and Regulating via Pattern Recognition Receptors, Th2 and Treg Responses. Eur. J. Immunol. 2010;40:1525–1537. doi: 10.1002/eji.200940109. [DOI] [PubMed] [Google Scholar]
- 107.Singhal A., Fohn M., Hakomori S.-I. Induction of α-N-Acetylgalactosamine-O-Serine/Threonine (Tn) Antigen-mediated Cellular Immune Response for Active Immunotherapy in Mice. Cancer Res. 1991;51:1406–1411. [PubMed] [Google Scholar]
- 108.Springer G.F. Immunoreactive T and Tn Epitopes in Cancer Diagnosis, Prognosis, and Immunotherapy. J. Mol. Med. 1997;75:594–602. doi: 10.1007/s001090050144. [DOI] [PubMed] [Google Scholar]
- 109.Dusoswa A., Verhoeff J., Abels E., Méndez-Huergo S.P., Croci D.O., Kuijper L.H., de Miguel E., Wouters V.M.C.J., Best M.G., Rodriguez E., et al. Glioblastomas Exploit Truncated O-Linked Glycans for Local and Distant Immune Modulation via the Macrophage Galactose-Type Lectin. Proc. Natl. Acad. Sci. USA. 2020;117:3693–3703. doi: 10.1073/pnas.1907921117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marcelo F., Garcia-Martin F., Matsushita T., Sardinha J., Coelho H., Oude-Vrielink A., Koller C., André S., Cabrita E.J., Gabium H.-J., et al. Delineating Binding Modes of Gal/GalNAc and Structural Elements of the Molecular Recognition of Tumor-Associated Mucin Glycopeptides by the Human Macrophage Galactose-Type Lectin. Chem. Eur. J. 2014;20:16147–16155. doi: 10.1002/chem.201404566. [DOI] [PubMed] [Google Scholar]
- 111.Diniz A., Coelho H., Dias J.S., van Vliet S.J., Jiménez-Barbero J., Corzana F., Cabrita E.J., Marcelo F. The Plasticity of Carbohydrate Recognition Domain Dictates the Exquisite Mechanism of Binding of Human Macrophage Galactose-Type Lectin. Chemistry. 2019;25:13945–13955. doi: 10.1002/chem.201902780. [DOI] [PubMed] [Google Scholar]
- 112.Marcelo F., Supekar N., Corzana F., van der Horst J.C., Vuist I.M., Live D., Boons G.-J.P.H., Smith D.F., van Vliet S.J. Identification of a Secondary Binding Site in Human Macrophage Galactose-Type Lectin by Microarray Studies: Implications for the Molecular Recognition of its Ligands. J. Biol. Chem. 2019;294:1300–1311. doi: 10.1074/jbc.RA118.004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zheng J., Xiao H., Wu R. Specific Identification of Glycoproteins Bearing the Tn Antigen in Human Cells. Angew. Chem. Int. Ed. 2017;56:7107–7111. doi: 10.1002/anie.201702191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hermiston M.L., Xu Z., Weiss A. CD45: A Critical Regulator of Signaling Thresholds in Immune Cells. Annu. Rev. Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 115.Tong A., Nguyen J., Lynch K.W. Differential Expression of CD45 Isoforms is Controlled by the Combined Activity of Basal and Inducible Splicing-Regulatory Elements in Each of the Variable Exons. J. Biol. Chem. 2005;280:38297–38304. doi: 10.1074/jbc.M508123200. [DOI] [PubMed] [Google Scholar]
- 116.McNeill L., Cassady R.L., Sarkardei S., Cooper J.C., Morgan G., Alexander D.R. CD45 Isoforms in T Cell Signaling and Development. Immunol. Lett. 2004;92:125–134. doi: 10.1016/j.imlet.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 117.Freire T., Lo-Man R., Bay S., Leclerc C. Tn Glycosylation of the MUC6 Protein Modulates Its Immunogenicity and Promotes the Induction of Th17-Biased T Cell Responses. J. Biol. Chem. 2011;286:7797–7811. doi: 10.1074/jbc.M110.209742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lo-Man R., Vichier-Guerre S., Bay S., Dériaud E., Cantacuzène D., Leclerc C. Anti-Tumor Immunity Provided by a Synthetic Multiple Antiogenic Glycopeptide Displaying a Tri-Tn Glycotope. J. Immunol. 2001;166:2849–2854. doi: 10.4049/jimmunol.166.4.2849. [DOI] [PubMed] [Google Scholar]
- 119.van Vliet S.J., Bay S., Vuist I.M., Kalay H., Garcia-Vallejo J.J., Leclere C., van Kooyk Y. MGL Signaling Augments TLR2-Mediated Responses for Enhanced IL-10 and TNF-α Secretion. J. Leukoc. Biol. 2013;94:315–323. doi: 10.1189/jlb.1012520. [DOI] [PubMed] [Google Scholar]
- 120.Zaal A., Li R.J.E., Lübbers J., Bruijns S.C.M., Kalay H., van Kooyk Y., van Vliet S.J. Activation of the C-Type Lectin MGL by Terminal GalNAc Ligands Reduces the Glycolytic Activity of Human Dendritic Cells. Front. Immunol. 2020;11:305. doi: 10.3389/fimmu.2020.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Eggink L.L., Roby K.F., Cote R., Hoober J.K. An Innovative Immunotherapeutic Strategy for Ovarian Cancer: CLEC10A and Glycomimetic Peptides. J. Immunother. Cancer. 2018;6:28. doi: 10.1186/s40425-018-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kolathar A.R., Leung A.K., Isecke R., Brossmer R., Drickamer K., Weis W.I. Mechanism of N-Acetylgalactosamine Binding to a C-type Animal Lectin Carbohydrate-recognition Domain. J. Biol. Chem. 1998;273:19502–19508. doi: 10.1074/jbc.273.31.19502. [DOI] [PubMed] [Google Scholar]
- 123.London N., Movshovitz-Attias D., Shueler-Furman O. The Structural Basis of Peptide-Protein Binding Strategies. Structure. 2010;18:188–199. doi: 10.1016/j.str.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 124.Blaszczyk M., Kurcinski M., Kouza M., Wieteska L., Debinski A., Kolinski A., Kmiecik S. Modeling of protein-peptide interactions using the CABS-dock web server for binding site search and flexible docking. Methods. 2016;93:72–83. doi: 10.1016/j.ymeth.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 125.Biasini M., Bienert S., Waterhouse A., Arnold K., Studer G., Schmidt T., Kiefer F., Cassarino T.G., Bertoni M., Bordoli L., et al. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Geijtenbeek T.B.H., Torensma R., van Vliet S.J., van Duijnhoven G.C.F., Adema G.J., van Kooyk Y., Figdor C.G. Identification of DC-SIGN, a Novel Dendritic Cell-Specific ICAM-3 Receptor that Supports Primary Immune Responses. Cell. 2000;100:575–585. doi: 10.1016/S0092-8674(00)80693-5. [DOI] [PubMed] [Google Scholar]
- 127.Engering A., Geijtenbeek T.B.H., van Vliet S.J., Wijers M., van Liempt E., Demaurex N., Lanzavecchia A., Fransen J., Figdor C.G., Piguet V., et al. The Dendritic Cell-specific Adhesion Receptor DC-SIGN Internalizes Antigen for Presentation to T Cells. J. Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 128.Segura E., Touzot M., Bohineust A., Cappuccio A., Chiocchia G., Hosmalin A., Dalod M., Soumelis V., Amigorena S. Human Inflammatory Dendritic Cells Induce Th17 Differentiation. Immunity. 2013;38:336–348. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 129.Umemoto T., Hashimoto M., Matsumura T., Nakamura-Ishizu A., Suda T. Ca2+-Mitochondria Axis Drives Cell Division in Hematopoietic Stem Cells. J. Exp. Med. 2018;215:2097–2113. doi: 10.1084/jem.20180421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kurze A.-K., Buhs S., Eggert D., Oliveira-Ferrer L., Müller V., Niendorf A., Wagener C., Nollau P. Immature O-Glycans Recognized by the Macrophage Glycoreceptor CLEC10A (MGL) are Induced by 4-Hydroxy-tamoxifen, oxidative stress and DNA-Damage in Breast Cancer Cells. Cell Commun. Signal. 2019;17:107. doi: 10.1186/s12964-019-0420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jondle C.N., Sharma A., Simonson T.J., Larson B., Mishra B.B., Sharma J. Macrophage Galactose Lectin-1 (MGL-1) Deficiency Is Associated with Increased Neutrophilia and Hyper Inflammation in Gram-Negative Pneumonia. J. Immunol. 2016;196:3088–3096. doi: 10.4049/jimmunol.1501790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Vliet S.J., van Liempt E., Geijtenbeek T.B.H., van Kooyk Y. Differential Regulation of C-Type Lectin Expression on Tolerogenic Dendritic Cell Subsets. Immunobiology. 2006;211:577–585. doi: 10.1016/j.imbio.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 133.van Vliet S.J., Vuist I.M., Lenos K., Tefsen B., Kalay H., Garcia-Vallejo J.J., van Kooyk Y. Human T Cell Activation Results in Extracellular Signal-regulated Kinase (ERK)-Calcineurin-Dependent Exposure of Tn Antigen on the Cell Surface and Binding of the Macrophage Galactose-type Lectin (MGL) J. Biol. Chem. 2013;288:27519–27532. doi: 10.1074/jbc.M113.471045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sckisel G.D., Bouchlaka M.N., Monjazeb A.M., Crittenden M., Curti B., Wilkins D.E.C., Alderson K.A., Sungur C.M., Ames E., Mirsoian A., et al. Out-of-Sequence Signal 3 Paralyzes Primary CD4+ T-Cell-Dependent Immunity. Immunity. 2015;43:240–250. doi: 10.1016/j.immuni.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Matzinger P. An Innate Sense of Danger. Semin. Immunol. 1998;10:399–415. doi: 10.1006/smim.1998.0143. [DOI] [PubMed] [Google Scholar]
- 136.Jakubzick C.V., Randolph G., Henson P.M. Monocyte Differentiation and Antigen-Presenting Functions. Nat. Rev. Immunol. 2017;17:349–362. doi: 10.1038/nri.2017.28. [DOI] [PubMed] [Google Scholar]
- 137.Hervas-Stubbs S., Riezu-Boj J.-I., Gonzalez I., Mancheño U., Dubrot J., Azpilicueta A., Gabari I., Palazon A., Aranguren A., Ruiz J., et al. Effects of IFN-α as a Signal-3 Cytokine on Human Naïve and Antigen-Experienced CD8+ T Cells. Eur. J. Immunol. 2010;40:3389–3402. doi: 10.1002/eji.201040664. [DOI] [PubMed] [Google Scholar]
- 138.Keppler S.J., Rosenits K., Koegl T., Vucikuja S., Aichele P. Signal 3 Cytokines as Modulators of Primary Immune Responses during Infections: The Interplay of Type I IFN and IL-12 in CD8 T Cell Responses. PLoS ONE. 2012;7:e40865. doi: 10.1371/journal.pone.0040865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Diamond M.S., Kinder M., Matsushita H., Mashayekhi M., Dunn G.P., Archambault J.M., Lee H., Arthur C.D., White J.M., Kalinke U., et al. Type I Interferon is Selectively Required by Dendritic Cells for Immune Rejection of Tumors. J. Exp. Med. 2011;208:1989–2001. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Raker V.K., Domogalla M.P., Steinbrink K. Tolerogenic Dendritic Cells for Regulatory T Cell Induction in Man. Front. Immunol. 2015;6:569. doi: 10.3389/fimmu.2015.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Domogalla M.P., Rostan P.V., Raker V.K., Steinbrink K. Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity. Front. Immunol. 2017;8:1764. doi: 10.3389/fimmu.2017.01764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Heissmeyer V., Macián F., Im S.-H., Varma R., Feske S., Venuprasad K., Gu H., Liu Y.-C., Dustin M.L., Rao A. Calcineurin Imposes T Cell Unresponsiveness Through Targeted Proteolysis of Signaling Proteins. Nat. Immunol. 2004;5:255–265. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 143.Razzadq T.M., Ozegbe P., Jury E.C., Sembi P., Blackwell N.M., Kabouridis P.S. Regulation of T-Cell Receptor Signaling by Membrane Microdomains. Immunology. 2004;113:413–426. doi: 10.1111/j.1365-2567.2004.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Müller M.R., Rao A. NFAT, Immunity and Cancer: A Transcription Factor Comes of Age. Nat. Rev. Immunol. 2010;10:645–656. doi: 10.1038/nri2818. [DOI] [PubMed] [Google Scholar]
- 145.Yukawa M., Jagannathan S., Vallabh S., Kartashov A.V., Chen X., Weirauch M.T., Barski A. AP-1 Activity Induced by Co-stimulation is Required for Chromatin Opening During T Cell Activation. J. Exp. Med. 2020;217:e20182009. doi: 10.1084/jem.20182009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Powell J.D. The Induction and Maintenance of T Cell Anergy. Clin. Immunol. 2006;120:239–246. doi: 10.1016/j.clim.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 147.Hogan P.G. Calcium-NFAT Transcriptional Signaling in T Cell Activation and T Cell Exhaustion. Cell Calcium. 2017;63:66–69. doi: 10.1016/j.ceca.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Steinman R.M., Turley S., Mellman I., Inaba K. The Induction of Tolerance by Dendritic Cells That Have Captured Apoptotic Cells. J. Exp. Med. 2000;191:411–416. doi: 10.1084/jem.191.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wherry E.J., Kurachi M. Molecular and Cellular Insights into T Cell Exhaustion. Nat. Rev. Immunol. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Saeidi A., Zandi K., Cheok Y.Y., Saeidi H., Wong W.F., Lee C.Y.Q., Cheong H.C., Yong Y.K., Larsson M., Shankar E.M. T-Cell Exhaustion in Chronic Infections: Reversing the State of Exhaustion and Reinvigorating Optimal Protective Immune Responses. Front. Immunol. 2018;9:2569. doi: 10.3389/fimmu.2018.02569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nossal G.J.V. Cellular Mechanisms of Immunologic Tolerance. Annu. Rev. Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- 152.Heath W.R., Carbone F.R. Cross-Presentation, Dendritic Cells, Tolerance, and Immunity. Annu. Rev. Immunol. 2001;19:47–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 153.Saraiva M., Vieira P., O’Garra A. Biology and Therapeutic Potential of Interleukin-10. J. Exp. Med. 2019;217:e20190418. doi: 10.1084/jem.20190418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Berridge M.J., Bootman M.D., Roderick H.L. Calcium Signaling: Dynamics, Homeostatis and Remodelling. Nat. Rev. Mol. Cell Biol. 2003;4:517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 155.Carafoli E., Krebs J. Why Calcium? How Calcium Became the Best Communicator. J. Biol. Chem. 2016;291:20849–20857. doi: 10.1074/jbc.R116.735894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marois L., Paré G., Vaillancourt M., Rollet-Labelle E., Naccache P.H. FcγRIIIb Triggers Raft-dependent Calcium Influx in IgG-mediated Responses in Human Neutrophils. J. Biol. Chem. 2011;286:3509–3519. doi: 10.1074/jbc.M110.169516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nunes P., Demaurex N. The Role of Calcium Signaling in Phagocytosis. J. Leukoc. Biol. 2010;88:57–68. doi: 10.1189/jlb.0110028. [DOI] [PubMed] [Google Scholar]
- 158.Mellado-Sánchez G., Vivanco-Cid H., Sumoza-Toledo A. Regulation of Murine Dendritic Cell Functions by Calcium Channels. SOJ Immunol. 2014;2:1–6. [Google Scholar]
- 159.Feske S. Calcium signaling in Lymphocyte Activation and Disease. Nat. Rev. Immunol. 2007;7:690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 160.Izquierdo J.H., Bonella-Abadia F., Cañas C.A., Tobón G.J. Calcium Channels, Intracellular Signaling and Autoimmunity. Reumatol. Clin. 2014;10:43–47. doi: 10.1016/j.reuma.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 161.Li D., Romain G., Flamar A.-L., Duluc D., Dullaers M., Li X.-H., Zurawski S., Bosquet N., Palucka A.K., Le Grand R., et al. Targeting Self- and Foreign Antigens to Dendritic Cells via DC-ASPGR Generates IL-10-Producing Suppressive CD4+ T Cells. J. Exp. Med. 2012;209:109–121. doi: 10.1084/jem.20110399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xia C.-Q., Kao K.J. Suppression of Interleukin-12 Production through Endogenously Secreted Interleukin-10 in Activated Dendritic Cells: Involvement of Activation of Extracellular Signal-Regulated Protein Kinase. Scand. J. Immunol. 2003;58:23–32. doi: 10.1046/j.1365-3083.2003.01268.x. [DOI] [PubMed] [Google Scholar]
- 163.Ma X., Yan W., Zheng H., Du Q., Zhang L., Ban Y., Li N., Fang Wei F. Regulation of IL-10 and IL-12 Production and Function in Macrophages and Dendritic Cells [version 1; peer review: 3 approved] F1000Research. 2015;4:1465. doi: 10.12688/f1000research.7010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lehmann M.H., Berg H. Interleukin-10 Expression is Induced by Increase of Intracellular Calcium Levels in the Monocytic Cell Line U937. Pflügers Archiv. 1998;435:868–870. doi: 10.1007/s004240050596. [DOI] [PubMed] [Google Scholar]
- 165.Kelly E.K., Wang L., Ivashkiv L.B. Calcium-Activated Pathways and Oxidative Burst Mediate Zymosan-Induced Signaling and IL-10 Production in Human Macrophages. J. Immunol. 2010;184:5545–5552. doi: 10.4049/jimmunol.0901293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rafiq K., Charitidou L., Bullens D.M.A., Kasran A., Lorré K., Ceuppens J.L., van Gool S.W. Regulation of the IL-10 Production by Human T Cells. Scand. J. Immunol. 2001;53:139–147. doi: 10.1046/j.1365-3083.2001.00851.x. [DOI] [PubMed] [Google Scholar]
- 167.Boubali S., Liopeta K., Virgillo L., Thyphronitis G., Mavrothallassitis G., Dimitracopoulos G., Paliogianni F. Calcium/Calmodulin-Dependent Protein Kinase II Regulates IL-10 Production by Human T Lymphocytes: A Distinct Target in the Calcium Dependent Pathway. Mol. Immunol. 2012;52:51–60. doi: 10.1016/j.molimm.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 168.Faries M.B., Bedrosian I., Xu S., Koski G., Roros J.G., Moise M.A., Nguyen H.Q., Engels F.H.C., Cohen P.A., Czemiecki B.J. Calcium Signaling Inhibits Interleukin-12 Production and Activates CD83+ Dendritic Cells that Induce Th2 Cell Development. Blood. 2001;98:2489–2497. doi: 10.1182/blood.V98.8.2489. [DOI] [PubMed] [Google Scholar]
- 169.Liu X., Wang N., Zhu Y., Yang Y., Chen X., Fan S., Chen Q., Zhou H., Zheng J. Inhibition of Extracellular Calcium Influx Results in Enhanced IL-12 Production in LPS-Treated Murine Macrophages by Downregulation of the CaMKKβ-AMPK-SIRT1 Signaling Pathway. Med. Inflamm. 2016;2016:6152713. doi: 10.1155/2016/6152713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Bauer B., Steinle A. HemITAM: A Single Tyrosine Motif that Packs a Punch. Sci. Signal. 2017;10:eaan3676. doi: 10.1126/scisignal.aan3676. [DOI] [PubMed] [Google Scholar]
- 171.Schülke S. Induction of interleukin-10 producing dendritic cells as a tool to suppress allergen-specific T helper 2 responses. Front. Immunol. 2018;9:455. doi: 10.3389/fimmu.2018.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sheng M., Thompson M.A., Greenberg M.E. CREB: A Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 173.Zhang L., Liu L., Thompson R., Chan C. CREB Modulates Calcium Signaling in cAMP-Induced Bone Marrow Stromal Cells (BMSCs) Cell Calcium. 2014;56:257–268. doi: 10.1016/j.ceca.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kushchayev S.V., Sankar T., Eggink L.L., Kushchayeva Y.S., Wiener P.C., Hoober J.K., Eschbacher J., Liu R., Shi F.D., Abdelwahab M.G., et al. Monocyte Galactose/N-Acetylgalactosamine-Specific C-Type Lectin Receptor Stimulant Immunotherapy of an Experimental Glioma. Part II: Combination with External Radiation Improves Survival. Cancer Manag. Res. 2012;4:325–334. doi: 10.2147/CMAR.S33355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mayes P.A., Hance K.W., Hoos A. The Promise and Challenges of Immune Agonist Antibody Development in Cancer. Nat. Rev. Drug Discov. 2018;17:509–527. doi: 10.1038/nrd.2018.75. [DOI] [PubMed] [Google Scholar]
- 176.Westerfield J.M., Barrera F.N. Membrane Receptor Activation Mechanisms and Transmembrane Peptide Tools to Elucidate Them. J. Biol. Chem. 2020;295:1792–1814. doi: 10.1074/jbc.REV119.009457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Martyanov A.A., Balabin F.A., Dunster J.L., Panteleev M.A., Gibbins J.M., Sveshnikova A.N. Control of Platelet CLEC-2-Mediated Activation by Receptor Clustering and Tyrosine Kinase Signaling. Biophys. J. 2020;118:1–15. doi: 10.1016/j.bpj.2020.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jégouzo S.A.F., Quintero-Martinez A., Ouyang X., dos Santos Á., Taylor M.E., Drickamer K. Organization of the Extracellular Portion of the Macrophage Galactose Receptor: A Trimeric Cluster of Simple Binding Sites for N-Acetylgalactosamine. Glycobiology. 2013;23:853–864. doi: 10.1093/glycob/cwt022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Finch E.A., Turner T.J., Goldin S.M. Calcium as a Coagonist of Inositol 1,4,5-Triphosphate-Induced Calcium Release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- 180.Bezprozvanny I., Watras J., Ehrlich B.E. Bell-Shaped Calcium-Response Curves of Ins(1,4,5)P3 and Calcium-Gated Channels from Endoplasmic Reticulum of Cerebellum. Nature. 1991;351:751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]