Abstract
OBJECTIVE
To identify county and facility factors associated with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) outbreaks in skilled nursing facilities (SNFs).
DESIGN
Cross‐sectional study linking county SARS‐CoV‐2 prevalence data, administrative data, state reports of SNF outbreaks, and data from Genesis HealthCare, a large multistate provider of post‐acute and long‐term care. State data are reported as of April 21, 2020; Genesis data are reported as of May 4, 2020.
SETTING AND PARTICIPANTS
The Genesis sample consisted of 341 SNFs in 25 states, including a subset of 64 SNFs that underwent universal testing of all residents. The non‐Genesis sample included all other SNFs (n = 3,016) in the 12 states where Genesis operates that released the names of SNFs with outbreaks.
MEASUREMENTS
For Genesis and non‐Genesis SNFs: any outbreak (one or more residents testing positive for SARS‐CoV‐2). For Genesis SNFs only: number of confirmed cases, SNF case fatality rate, and prevalence after universal testing.
RESULTS
One hundred eighteen (34.6%) Genesis SNFs and 640 (21.2%) non‐Genesis SNFs had outbreaks. A difference in county prevalence of 1,000 cases per 100,000 (1%) was associated with a 33.6 percentage point (95% confidence interval (CI) = 9.6–57.7 percentage point; P = .008) difference in the probability of an outbreak for Genesis and non‐Genesis SNFs combined, and a difference of 12.5 cases per facility (95% CI = 4.4–20.8 cases; P = .003) for Genesis SNFs. A 10‐bed difference in facility size was associated with a 0.9 percentage point (95% CI = 0.6–1.2 percentage point; P < .001) difference in the probability of outbreak. We found no consistent relationship between Nursing Home Compare Five‐Star ratings or past infection control deficiency citations and probability or severity of outbreak.
CONCLUSIONS
Larger SNFs and SNFs in areas of high SARS‐CoV‐2 prevalence are at high risk for outbreaks and must have access to universal testing to detect cases, implement mitigation strategies, and prevent further potentially avoidable cases and related complications. J Am Geriatr Soc 68:2167–2173, 2020.
Keywords: COVID‐19, facility, nursing home, long‐term care, SARS‐CoV‐2, skilled nursing
Short abstract
See related Special Article by Ouslander et al. in this issue.
INTRODUCTION
The COVID‐19 pandemic, caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has disproportionately affected long‐term care residents in the United States, who represent 1% to 31% of cases across states, but 33% to 82% of deaths. 1 As of June 25, at least 52,000 long‐term care residents have died, but incomplete data mean these are undercounts. 1 Long‐term care residents account for 36% to 62% of total deaths in Canada and European countries. 2
Skilled nursing facilities (SNFs) provide a mix of post‐acute and long‐term care to medically complex older adults who are particularly vulnerable to SARS‐CoV‐2. 3 , 4 Asymptomatic transmission seems to be the major contributing factor to SNF outbreaks due to the high‐touch care and communal living environment. 5 , 6 Surveillance studies of two facilities with severe outbreaks documented high prevalence of SARS‐CoV‐2 within just 3 weeks of their index cases, demonstrating rapid dissemination. 4 , 6 Even with universal visitor bans, cessation of group activities, and regular symptom monitoring of residents and staff, 7 at least 10,400 U.S. facilities have had outbreaks as of June 25. 1
SARS‐CoV‐2 represents the most serious challenge to SNFs in decades because of its lethality in older adults and ease of transmission. A major barrier to developing appropriate clinical and operational responses to mitigate the pandemic's effects is the lack of comprehensive information on the determinants of outbreak. In the absence of national data, we took advantage of SARS‐CoV‐2 tracking data from a large post‐acute and long‐term care provider, and linked these with publicly available state, county, and other administrative data. We describe the evolving prevalence of SARS‐CoV‐2 in SNFs and examine county‐ and facility‐level factors associated with outbreaks.
METHODS
Design, Setting, and Participants
We rely on publicly available state and federal data, as well as data from Genesis HealthCare, one of the nation's largest post‐acute and long‐term care providers with nearly 400 skilled nursing, assisted living, independent living, and behavioral health facilities in 25 states. We limited our analysis to SNFs and dropped 10 facilities designated as COVID‐19‐only, yielding a sample of 341 SNFs.
Since mid‐March 2020, all Genesis facilities have maintained detailed line listings of presumed and laboratory‐confirmed SARS‐CoV‐2 cases and deaths among residents and staff. The resulting daily line listings summarize cumulative counts of cases and deaths for every Genesis SNF. This includes 64 SNFs in 10 states (Alabama, Colorado, Massachusetts, Maryland, New Hampshire, New Jersey, New Mexico, Pennsylvania, Rhode Island, and West Virginia) that had all residents universally tested for SARS‐CoV‐2 as of May 4, 2020. A Data Use Agreement and institutional review board approval from Brown University enabled us to download the daily line lists directly from Genesis via a secure file transfer protocol.
In addition to Genesis data, we compiled a list of non‐Genesis SNFs with at least one resident case from state departments of health and press reports citing state officials. As of April 21, 2020, when the list was compiled, only 12 of the states in which Genesis operates were releasing the names of SNFs with outbreaks (California, Colorato, Connecticut, Kentucky, New Hampshire, New Jersey, New Mexico, Nevada, Rhode Island, Tennessee, Vermont, and Washington). Facilities were hand matched to their Medicare provider number to link to the Certification and Survey Provider Enhanced Reports (CASPER) and other administrative data. CASPER data contain facility characteristics on all Medicare/Medicaid certified SNFs. We downloaded Nursing Home Compare report cards for the fourth quarter of 2019, which include Five‐Star ratings and deficiency citations; and matched facilities to 2018 data from http://ltcfocus.org/, a public database built using aggregated resident‐level Minimum Data Set information. 8 Facility‐level data were merged to the county‐level Area Health Resources File and SARS‐CoV‐2 prevalence data from a Johns Hopkins University repository. 9
Outcome Measures
We examined four facility‐level outcomes. First, for both Genesis and non‐Genesis SNFs, we created a binary indicator for whether the SNF had at least one resident with SARS‐CoV‐2 (case) as of April 21, 2020. Second, for Genesis SNFs, we counted the number of confirmed cases per SNF as of May 4, 2020. Third, we calculated the facility‐level case fatality rate (cumulative deaths/cumulative cases) as of May 4, 2020. Finally, for the 64 Genesis SNFs that underwent universal testing as of May 4, 2020, we report the percentage of residents testing positive on the date of testing, and the number of cases identified that were not on the prior day's line listing.
Independent Variables
Independent variables included SNF and county‐level characteristics. Aggregated SNF demographic characteristics from LTCfocus.org included mean resident age, percentage of residents who were Black (derived from the Minimum Data Set), and percentage of residents with dementia (as documented on the Minimum Data Set and reported by SNFs at time of survey). Measures of SNF quality included the most recent Nursing Home Compare Five‐Star ratings and an indicator of whether the SNF had an infection prevention and control deficiency citation (F880) in the prior year. Star ratings were grouped into low (1 and 2), average (3), and high (4 and 5) categories or treated as continuous measures for some analyses of Genesis SNFs to conserve statistical power. County characteristics included population size, population density, and percentage of the population that was Black. From the Johns Hopkins data, we obtained county SARS‐CoV‐2 prevalence (confirmed cases per 100,000 population) and the date of the first case in the county.
Analytic Approach
We compared characteristics of SNFs with at least one resident case using t‐tests for continuous variables and chi‐squared tests for categorical variables. Analyses were performed separately for Genesis and non‐Genesis SNFs to determine whether the predictive factors were similar. We then combined Genesis and non‐Genesis SNFs in multivariate analyses, using a linear probability model with state fixed effects, to estimate the influence of facility and county characteristics on the likelihood of having a SARS‐CoV‐2 case.
We next conducted a series of analyses on Genesis SNFs only, using data not reliably reported by states. First, we illustrated the relationship between SNF SARS‐CoV‐2 cases per 100 beds and SARS‐CoV‐2 cases per 100,000 population in the county, designating SNFs in the top 5% of U.S. counties as being in “high” prevalent counties. Second, we calculated case fatality rates among SNFs with at least five cases.
Third, we used robust Poisson regression to estimate the association between facility characteristics and the number of cases among SNFs with at least one case. By controlling for facility size, date of first county case, and whether the SNF was universally tested, this analysis effectively tests for factors related to virus dissemination within facilities. Regression results are presented as marginal effects, or the average change in predicted case count given a one unit change in an independent variable, holding the distribution of all other covariates constant. We separately plotted the estimated number of cases per facility, adjusting only for bed size, universal testing, county prevalence, and the date of first county case.
Finally, we examined a subset of 64 Genesis SNFs that underwent universal testing. SARS‐CoV‐2 prevalence was measured as the percentage of residents testing positive on the date of testing. We also examined variation in SNF prevalence by county prevalence on the date of testing, and whether the SNF had any resident cases before testing.
All multivariate analyses included standard errors clustered by state to account for potential correlation between facility outcomes within the same state. Data were analyzed with Stata MP 16.0 (StataCorp).
RESULTS
Table 1 presents characteristics of the 341 Genesis and 3,016 non‐Genesis SNFs. As of April 21, 2020, 118 (35%) Genesis SNFs and 640 (21.2%) non‐Genesis SNFs had at least one SARS‐CoV‐2 case (an outbreak). Among both Genesis and non‐Genesis SNFs, those with outbreaks were larger, had higher overall and registered nurse staffing Five‐Star ratings, and had greater proportions of Black residents, compared with facilities without outbreaks. SNFs with outbreaks were more frequently located in counties with higher SARS‐CoV‐2 prevalence, higher population density, and larger Black populations.
Table 1.
Characteristics of Skilled Nursing Facilities with at Least One Resident with SARS‐CoV‐2, as of April 21, 2020
| Characteristic | Genesis skilled nursing facilities | Other skilled nursing facilities | ||||
|---|---|---|---|---|---|---|
| At least one case | No cases | At least one case | No cases | |||
| (n = 118) | (n = 223) | P value | (n = 640) | (n = 2,376) | P value | |
| Facility characteristics | ||||||
| Total beds | 134.6 (46.1) | 107.3 (41.5) | <.001 | 133.3 (71) | 97.7 (52.4) | <.001 |
| Admissions per bed per year | 2.8 (2.1) | 2.4 (1.6) | .09 | 2.7 (2.1) | 2.6 (2.8) | .549 |
| Five‐Star rating, overall | 2.9 (1.3) | 2.8 (1.3) | .65 | 3.3 (1.4) | 3.3 (1.4) | 1.00 |
| Five‐Star rating, quality measures | 3.8 (1.2) | 3.6 (1.2) | .11 | 4.2 (1) | 3.9 (1.2) | <.001 |
| Five‐Star rating, overall staffing | 3.3 (.7) | 3 (.9) | .001 | 3.2 (1.1) | 3 (1.1) | .007 |
| Five‐Star rating, RN staffing | 3.5 (.8) | 3.1 (1) | .002 | 3.2 (1.2) | 3 (1.3) | <.001 |
| Five‐Star rating, inspections | 2.3 (1.1) | 2.4 (1.2) | .22 | 2.7 (1.3) | 2.9 (1.3) | .001 |
| Infection control citation, No. (%) | 77 (65) | 152 (68) | .59 | 435 (69) | 1,547 (68) | .803 |
| Resident age, y | 78.5 (5) | 76.5 (6.9) | .007 | 78.6 (7.7) | 78.1 (8.9) | .192 |
| % Black | 17.1 (19.7) | 9.2 (14.5) | <.001 | 13.7 (16.5) | 9.1 (13.3) | <.001 |
| % With dementia | 43.4 (16) | 42.6 (16.2) | .630 | 41.2 (17.5) | 41.3 (20.1) | .983 |
| County characteristics | ||||||
| Population size (1000s) | 1,293.3 (2,447) | 682.8 (1,711.8) | .008 | 2,257.7 (3,394.7) | 1,853.8 (3,096.9) | .004 |
| Population density | 1,612.5 (2,006.9) | 627.8 (1,024.7) | <.001 | 2,116.3 (2,540.5) | 1,111.2 (1,959.1) | <.001 |
| % Black | 13.1 (12.6) | 7.5 (10.2) | <.001 | 11.3 (9.2) | 7.4 (7.9) | <.001 |
| County SARS‐CoV‐2 cases per 100,000 | 463.7 (366.9) | 135.2 (146.3) | <.001 | 544.6 (491.2) | 148.6 (219) | <.001 |
Note: Data are given as mean (standard deviation), unless otherwise indicated. Data were stratified according to whether a facility had at least one resident case of SARS‐CoV‐2 as of April, 21, 2020 (date when collection of state data for non‐Genesis facilities was completed). County cases per population on April 21, 2020 were used. Population density is persons per square land mile.
Abbreviations: RN, registered nurse; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Multivariate analyses (Supplementary Table S1) reveal that SNF demographics and prior infection control deficiency citations were unrelated to the likelihood of SARS‐CoV‐2 outbreak. A 10‐bed difference in facility size was associated with a 0.9 percentage point (95% confidence interval (CI) = 0.6–1.2 percentage point; P < .001) difference in the probability of outbreak. A difference of 1,000 cases per 100,000 in the county (a 1% change) was associated with a 33.6 percentage point (95% CI = 9.6–57.7 percentage point; P = .008) difference in the probability of SNF outbreak. Five‐Star staffing ratings were unrelated to the likelihood of outbreak, whereas the relationship with inspection ratings was inconsistent. Compared with SNFs with a 3 star inspection rating, 4 to 5 star‐rated facilities had a 2.9 percentage point (95% CI = −5.1 to −0.7 percentage point; P = .01) lower probability of outbreak; however, there were no significant differences in the probability of outbreak between SNFs with 1 to 2 versus 3 stars.
For Genesis SNFs, the relationship between county SARS‐CoV‐2 prevalence and SNF cases per 100 beds is shown in Figure 1, indicating a strong and positive association (Spearman's ρ = 0.64). Median case fatality rate was higher for SNFs located in high SARS‐CoV‐2 prevalent counties: 20.0% (interquartile range (IQR) = 13.3%–27.3%) for SNFs in the top 5% of counties versus 14.3% (IQR = 4.3%–18.6%) for SNFs in the bottom 95% of counties (Figure 2).
Figure 1.
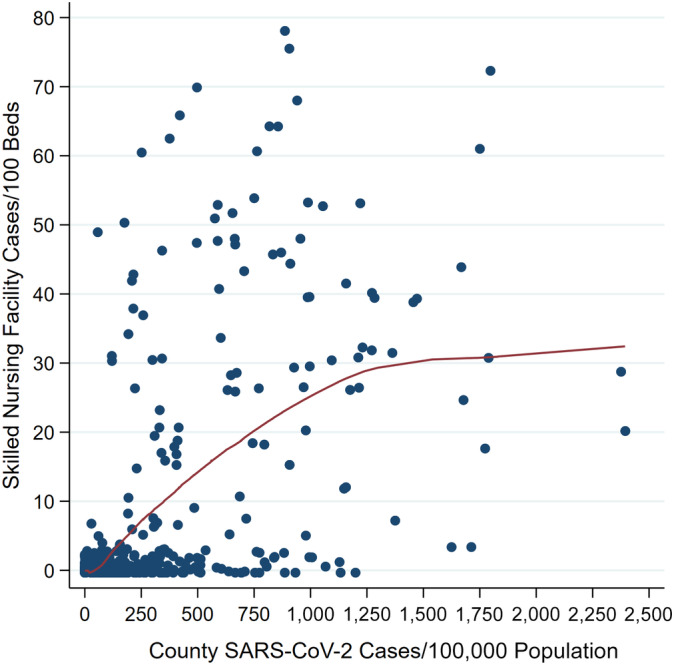
Relationship between skilled nursing facility severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) prevalence and county SARS‐CoV‐2 prevalence among Genesis facilities (n = 341). Note: Skilled nursing facility and county prevalence estimates are based on data as of May 4, 2020. The relationship between the skilled nursing facility and county prevalence is depicted with a loess curve.
Figure 2.
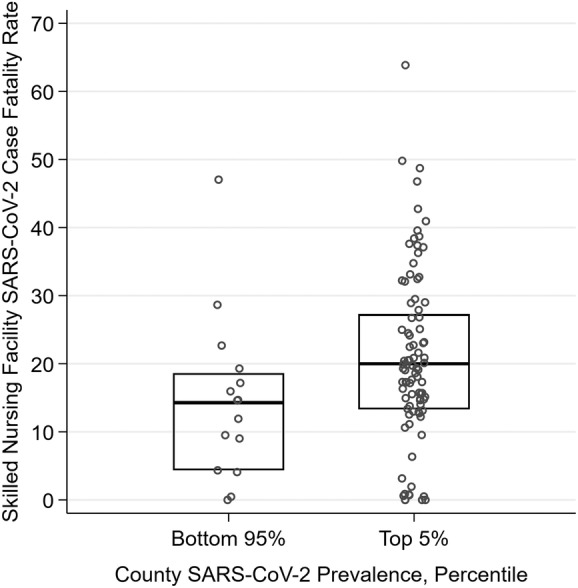
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) case fatality rate, by county SARS‐CoV‐2 prevalence among Genesis skilled nursing facilities (SNFs) with at least five cases (n = 104). Note: Case fatality rates were calculated from data available as of May 4, 2020. The percentile rank of all U.S. counties in terms of COVID‐19 prevalence on this date was calculated and assigned to each facility. The case fatality rate for each SNF is the cumulative number of resident deaths in confirmed SARS‐CoV‐2 cases divided by the cumulative number of confirmed SARS‐CoV‐2 resident cases.
Figure 3 illustrates the distributions in expected case counts for Genesis SNFs conditional on size, admissions per bed, county prevalence, and timing of first county case, stratified by facility quality and demographic characteristics. The expected number of cases is distributed similarly for SNFs with low, medium, and high staffing and inspection Star ratings (Figure 3A,B), and different tertiles representing percentages of Black residents (Figure 3C) and those with dementia (Figure 3D). Supplementary Table S2 provides point estimates of the association between these facility characteristics and the number of cases per SNF. Size was the only facility characteristic associated with case count, and county SARS‐CoV‐2 prevalence was again a significant factor. A difference of 1,000 cases per 100,000 in the county (a 1% change) was associated with a difference of 12.5 SNF cases (95% CI = 4.4–20.8; P = .003).
Figure 3.
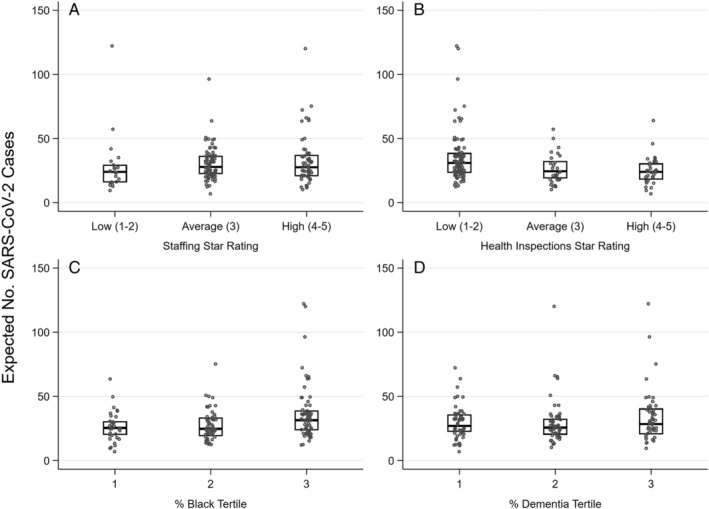
Distribution of skilled nursing facilities' expected count of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) resident cases by facility characteristics, for Genesis facilities with one or more cases (n = 154). Note: Expected count of SARS‐CoV‐2 cases is adjusted for facility size, county SARS‐CoV‐2 prevalence, date of first county case, and whether or not the facility underwent universal testing. Estimates were obtained from a robust Poisson regression model (see Methods for details).
Figure 4 illustrates the distribution of the percentage of residents with SARS‐CoV‐2 in 64 Genesis SNFs that underwent universal testing as of May 4, 2020, stratified by county SARS‐CoV‐2 prevalence and whether the SNF already had at least one known case before testing. Figure 4A includes the 19 SNFs that had at least one case before testing. After testing, the median percentage of residents with SARS‐CoV‐2 in these SNFs was 19.5% (range = 1.7%–91.7%), and 17 SNFs (89.5%) identified new cases. The median number of new cases detected with testing was 16 (range = 1–74). SNFs located in counties with high SARS‐CoV‐2 prevalence (top 5th percentile) had a median of 28.6% of residents with SARS‐CoV‐2, compared with a much lower median of 5.8% in SNFs located in the bottom 95th percentile of counties. Figure 4B includes the 45 SNFs that had no cases at time of testing. Of these, seven (15.6%) identified new cases (range = 1–4 cases). Thus, after testing, the median percentage of residents with SARS‐CoV‐2 was 0% (range = 0%–10.7%).
Figure 4.
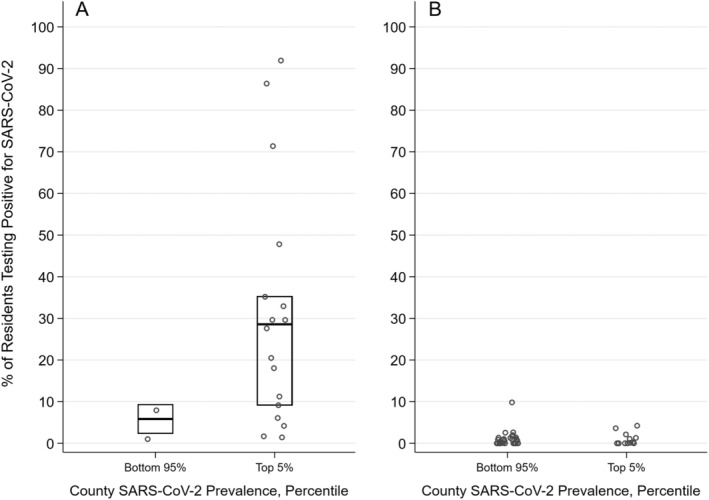
Percentage of residents testing positive for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in universally tested Genesis skilled nursing facilities (N = 64) with at least one confirmed resident case the day before testing (n = 19) (A) and no confirmed resident cases before testing (n = 45) (B). Note: For each day between April 1, 2020, and May, 4, 2020, we calculated the percentile rank of all U.S. counties in terms of SARS‐CoV‐2 prevalence and assigned each facility the percentile rank on the date of universal testing.
DISCUSSION
In this empirical analysis of multistate SNF and county SARS‐CoV‐2 data, we found the most significant and consistent predictors of SNF outbreak, case count, and case fatality rate to be larger bed size and higher SARS‐CoV‐2 prevalence in the county where the SNF is located. We did not find consistent relationships between SNF quality and either the probability or severity of outbreak. The associations we observed regarding the predictors of outbreak were similar for Genesis SNFs, for which we had detailed prevalence data, and non‐Genesis SNFs, for which we relied on less precise state data, thus supporting the generalizability of our findings.
Larger facilities accept more admissions and have more employees, thus encountering more movement of persons to and from the community. If that community has high SARS‐CoV‐2 prevalence, there is a greater risk of new residents or staff serving as infection sources. Even with regular employee symptom screening, the use of face masks and other personal protective equipment, and other infection control measures, staff‐to‐resident transmission can occur while infected staff are asymptomatic or afebrile with mild symptoms that they fail to report. 5 , 6 , 10 Direct care staff also often work across multiple facilities, increasing the risk of cross‐contamination. Many SNFs have implemented policies to limit staff working in more than one facility, but these policies could be further augmented by states offering financial support for hazard pay, paid sick leave, and other benefits to incentivize staff to limit movement outside their workplace.
Median case fatality rate was higher among SNFs located in high prevalence counties. This may be a function of timing, with both higher county prevalence and case fatality within SNFs likely signifying earlier community exposure to SARS‐CoV‐2. Because there is often a significant lag between SARS‐CoV‐2 infection and death, residents of SNFs exposed to the virus earlier may be further along their disease trajectory. Furthermore, numerous reports suggest that SNFs with earlier outbreaks were more affected by testing limitations and shortages of personal protective equipment. 11 , 12
We did not find consistent relationships between Nursing Home Compare Five‐Star ratings, past infection control deficiency citations, and either the probability or the severity of outbreak. In bivariate analyses, SNFs with outbreaks had slightly higher staffing ratings than SNFs without outbreaks, but in multivariate analyses, we found no significant association between staffing rating and the probability of outbreak or the count of cases. SNFs with high (4–5 Star) ratings for health inspections had a 2.9 percentage point lower probability of outbreak than SNFs with average (3 Star) ratings, but we found no difference between SNFs with poor (1–2 Star) versus average ratings. We also found no relationship between case count and health inspection rating. Whether the SNF had a past infection control deficiency was unrelated to probability or severity of outbreak. Future research may reveal the impact of specific infection control procedures, but systematic data on these factors are not currently available.
Our findings regarding SNF quality and SARS‐CoV‐2 outbreaks are similar to those of two recent multistate analyses that also found inconsistent relationships. 13 , 14 By contrast, single‐state studies of SNFs in California and Connecticut found that higher Five‐Star ratings were associated with lower case counts. 15 , 16 However, the California study did not account for SNF location or county SARS‐CoV‐2 prevalence, which we found to be the strongest predictor of outbreaks in our models. Although the Connecticut study did control for county prevalence, Connecticut only has eight counties that are heterogeneous in terms of population density, which may blunt the county prevalence effect. Unadjusted analyses of other states, like New Jersey and Colorado, have actually found SNFs with higher Five‐Star ratings to be slightly more likely to experience outbreaks. 13 Overall, this emerging literature shows that evidence linking SNF Five‐Star rating to the probability and severity of SARS‐CoV‐2 outbreak is inconsistent. Our data suggest that SNF location is a far stronger predictor, and that both high‐ and low‐quality SNFs are vulnerable when the virus is highly prevalent in the surrounding community.
In bivariate analyses, SNFs with outbreaks on average had a higher proportion of Black residents and were more frequently located in counties with larger Black populations; however, this relationship was not significant in multivariate analyses adjusted for county prevalence and other facility characteristics. Geographical location has been identified as an important factor in institutional racial segregation across SNFs, with SNF populations typically reflecting the racial composition of the communities in which they are located. 17 Our findings suggest that the racial disparity we see is likely related to SNFs with a higher proportion of Black residents being located in counties with higher SARS‐CoV‐2 prevalence. Further work should explore disparities in resident outcomes within SNFs, as well as disparities in risk of infection for SNF staff who often live in the surrounding communities.
Among a subset of 64 Genesis SNFs that had universal testing, the crucial factor related to SARS‐CoV‐2 prevalence was whether there was already a confirmed resident case at the time of testing. County prevalence was also related to case identification. These findings support a strategy for allocating testing resources to the many areas of the country where testing capacity is still limited. Higher priority should be given to universally testing SNFs in which SARS‐CoV‐2 is already confirmed, as well as SNFs in high prevalent areas.
The Centers for Medicare and Medicaid Services issued guidelines on May 18, 2020, recommending weekly testing of all staff in SNFs, but allowing state and local leaders to adjust this requirement based on local conditions. 18 Our findings suggest that testing policies adapted to local conditions may make more efficient use of limited testing resources than would statewide mandates that do not account for geographical variation in risk. Once testing resources become readily available, universal staff testing could be used as an early warning surveillance system to identify and furlough staff with SARS‐CoV‐2, limiting exposure to residents. However, widespread testing must be coupled with state and federal efforts to mitigate testing costs for SNFs and coordinate strategic staffing reserves to supplement staff who must be out of work.
Limitations
SARS‐CoV‐2 case counts for both counties and SNFs that have not been universally tested may be measured with error due to geographical variation in testing capacity. 19 We would expect that testing capacity limits would similarly bias the count of both SNF and non‐SNF cases in the same county. Deaths should be less sensitive to testing capacity, but case fatality rates may be biased downward in areas with more aggressive testing as the number of cases in the denominator would likely be higher. To account for these issues, we supplemented our analyses with data from a subset of Genesis facilities that had been universally tested, limiting bias introduced by low testing capacity. We found similar patterns among these facilities.
CONCLUSIONS
The COVID‐19 pandemic presents an urgent threat to the lives of long‐term care residents and staff. Geographical location in a county of high SARS‐CoV‐2 prevalence and larger facility size seem to be major factors influencing the probability and severity of SARS‐CoV‐2 outbreak for SNFs. We did not find consistent relationships between overall SNF quality and either the probability or the severity of outbreak. Universal testing is an essential epidemiological surveillance tool, both to detect the introduction of SARS‐CoV‐2 into an SNF and to help SNF leadership implement mitigation strategies once cases are confirmed. Our data suggest that larger SNFs and SNFs in areas of high SARS‐CoV‐2 prevalence are at high risk for outbreaks and must have access to universal testing to detect cases, implement mitigation strategies, and prevent further potentially avoidable cases and related complications.
Supporting information
Supplementary Table S1: Association of Skilled Nursing Facility Characteristics and County SARS‐CoV‐2 Prevalence with the Probability of a Facility Having at Least One Resident with SARS‐CoV‐19 (n = 3,357).
Supplementary Table S2: Association Between Skilled Nursing Facility Characteristics and Count of SARS‐CoV‐2 Resident Cases Among Genesis Facilities with an Outbreak (n = 154).
ACKNOWLEDGMENTS
We thank Rich Castor, Cliff Boyd, and Joe Montgomery of Genesis HealthCare and Jeffrey Hiris and Christopher Santostefano from Brown University for their extensive technical support.
Financial Disclosure
This research was supported by the National Institute on Aging (3P01AG027296‐11S1; principal investigator: Vincent Mor).
Conflict of Interest
Vincent Mor is Chair of the Scientific Advisory Board at NaviHealth, Inc, former Chair of the Independent Quality Committee at HCR ManorCare, and former Director of PointRight, Inc, where he holds less than 1% equity. The other authors have no conflicts of interest to disclose.
Author Contributions
Study design: E.M.W., C.M.K., and V.M. Data acquisition: E.M.W., C.M.K., R.A.F., C.B., and V.M. Data analysis: E.M.W., C.M.K., and V.M. Data interpretation: E.M.W., C.M.K., R.A.F., C.B., S.G., J.O., and V.M. Writing and critical revision of the manuscript: E.M.W., C.M.K., R.A.F., C.B., S.G., J.O., and V.M.
Sponsor's Role
The National Institute on Aging had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
See related Special Article by Ouslander et al. in this issue.
Elizabeth M. White and Cyrus M. Kosar contributed equally to this article.
Preliminary study findings were presented at the IMPACT Collaboratory Grand Rounds on May 7 and June 11, 2020.
REFERENCES
- 1. Kaiser Family Foundation . State reports of long‐term care facility cases and deaths related to COVID‐19. 2020. https://www.kff.org/health-costs/issue-brief/state-data-and-policy-actions-to-address-coronavirus/. Accessed July 3, 2020.
- 2. Comas‐Herrera A, Zalakaín J, Litwin C, Hsu AT, Lane N, Fernández JL. Mortality associated with COVID‐19 outbreaks in care homes: early international evidence. 2020. https://ltccovid.org/wp-content/uploads/2020/05/Mortality-associated-with-COVID-3-May-final-5.pdf. Accessed May 7, 2020.
- 3. Centers for Disease Control and Prevention . Groups at higher risk for severe illness. 2020. https://www.cdc.gov/coronavirus/2019‐ncov/need‐extra‐precautions/groups‐at‐higher‐risk.html. Accessed May 10, 2020.
- 4. McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid‐19 in a long‐term care facility in King County, Washington. N Engl J Med. 2020;382:2005‐2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID‐19. Nat Med. 2020;26:672‐675. [DOI] [PubMed] [Google Scholar]
- 6. Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS‐CoV‐2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Medicare & Medicaid Services . Guidance for infection control and prevention of coronavirus disease 2019 (COVID‐19) in nursing homes (revised). 2020. QSO‐20‐14‐NH. https://www.cms.gov/files/document/3-13-2020-nursing-home-guidance-covid-19.pdf. Accessed May 10, 2020.
- 8. Brown University School of Public Health . LTCfocus: long‐term care: facts on care in the US. 2018. http://ltcfocus.org/. Accessed May 23, 2020.
- 9. Johns Hopkins University . Coronavirus resource center. 2020. https://coronavirus.jhu.edu/us-map. Accessed May 23, 2020.
- 10. Chow EJ, Schwartz NG, Tobolowsky FA, et al. Symptom screening at illness onset of health care personnel with SARS‐CoV‐2 infection in King County, Washington. JAMA. 2020;323:2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berklan JM. McKnight's COVID survey reveals vast PPE, staffing shortages. McKnight's Long‐Term Care News. March 30, 2020.
- 12. Condon B, Sedensky M, Mustian J. Nursing homes plead for more coronavirus tests. PBS News Hour. April 23, 2020. https://www.pbs.org/newshour/health/nursing-homes-plead-for-more-coronavirus-tests. Accessed May 10, 2020.
- 13. Testimony of R . Tamara Konetzka, PhD: caring for seniors amid the COVID‐19 crisis. Special Committee on Aging, U.S. Senate. 116th Congress, 2nd Session. 2020, May 21. https://www.aging.senate.gov/imo/media/doc/SCA_Konetzka_05_21_20.pdf. Accessed May 10, 2020.
- 14. Abrams HR, Loomer L, Gandhi A, Grabowski DC. Characteristics of U.S. nursing homes with COVID‐19 cases. J Am Geriatr Soc. 2020;68(8). [Epub ahead of print]. https://onlinelibrary.wiley.com/doi/10.1111/jgs.16661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He M, Li Y, Fang F. Is there a link between nursing home reported quality and COVID‐19 cases? evidence from California skilled nursing facilities. JAMDA. 2020;21(7):905‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li Y, Temkin‐Greener H, Gao S, Cai X. COVID‐19 infections and deaths among Connecticut nursing home residents: facility correlates. J Am Geriatr Soc. 2020;68(9). https://onlinelibrary.wiley.com/doi/full/10.1111/jgs.16689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mack DS, Jesdale BM, Ulbricht CM, Forrester SN, Michener PS, Lapane KL. Racial segregation across U.S. nursing homes: a systematic review of measurement and outcomes. Gerontologist. 2020;60(3):e218‐e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Medicare & Medicaid Services Center for Clinical Standards and Quality/Quality Safety & Oversight Group . Nursing home reopening recommendations for state and local officials. Baltimore, MD: Centers for Medicare & Medicaid Services; 2020. QSO‐20‐30‐NH. [Google Scholar]
- 19. Basu A. Estimating the infection fatality rate among symptomatic COVID‐19 cases in the United States. Health Aff (Millwood). 2020;39(7):1229‐1236. 10.1377/hlthaff.2020.00455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Association of Skilled Nursing Facility Characteristics and County SARS‐CoV‐2 Prevalence with the Probability of a Facility Having at Least One Resident with SARS‐CoV‐19 (n = 3,357).
Supplementary Table S2: Association Between Skilled Nursing Facility Characteristics and Count of SARS‐CoV‐2 Resident Cases Among Genesis Facilities with an Outbreak (n = 154).


