Abstract
The emergence of SARS‐CoV‐2 in late 2019 and human responses to the resulting COVID‐19 pandemic in early 2020 have rapidly changed many aspects of human behavior, including our interactions with wildlife. In this commentary, we identify challenges and opportunities at human–primate interfaces in light of COVID‐19, focusing on examples from Asia, and make recommendations for researchers working with wild primates to reduce zoonosis risk and leverage research opportunities. First, we briefly review the evidence for zoonotic origins of SARS‐CoV‐2 and discuss risks of zoonosis at the human–primate interface. We then identify challenges that the pandemic has caused for primates, including reduced nutrition, increased intraspecific competition, and increased poaching risk, as well as challenges facing primatologists, including lost research opportunities. Subsequently, we highlight opportunities arising from pandemic‐related lockdowns and public health messaging, including opportunities to reduce the intensity of problematic human–primate interfaces, opportunities to reduce the risk of zoonosis between humans and primates, opportunities to reduce legal and illegal trade in primates, new opportunities for research on human–primate interfaces, and opportunities for community education. Finally, we recommend specific actions that primatologists should take to reduce contact and aggression between humans and primates, to reduce demand for primates as pets, to reduce risks of zoonosis in the context of field research, and to improve understanding of human–primate interfaces. Reducing the risk of zoonosis and promoting the well‐being of humans and primates at our interfaces will require substantial changes from “business as usual.” We encourage primatologists to help lead the way.
Keywords: biosafety, ethics, human–primate conflict, provisioning, SARS‐CoV‐2, zoonosis
Example of human–primate interface: Highly provisioned Southern pig‐tailed macaques (Macaca nemestrina) at Lumut Mangrove Park, Malaysia receiving daily food handouts from park visitors shortly before the COVID‐19 lockdown in March 2020 (photo by Susana Almagro).

HIGHLIGHTS
In this commentary, we
briefly introduce the problem of zoonosis between humans and nonhuman primates;
discuss challenges that the COVID‐19 pandemic has created for primates and for primate researchers;
identify opportunities created or revealed by the COVID‐19 pandemic to reduce the intensity of problematic human–primate interfaces, reduce the risk of zoonosis, and reduce trade in primates;
identify opportunities for research on human–primate interfaces in light of the COVID‐19 pandemic; and
make specific recommendations for researchers in light of the pandemic and beyond‐pandemic realities.
1. INTRODUCTION
The COVID‐19 pandemic is having catastrophic impacts on human health and livelihoods around the globe. At the time of writing in June 2020, >8.5 million people globally have tested positive for SARS‐CoV‐2, the virus that causes COVID‐19, and >450,000 have died (World Health Organization, 2020), with no end in sight. Human actions to mitigate the impacts of COVID‐19 include border closures and other restrictions on movement and association, closures of government offices, schools, and other public institutions, mandatory and voluntary business closures, stay‐at‐home orders, and voluntary physical distancing. These responses have also negatively impacted human health and welfare, causing severe economic hardship (Fernandes, 2020), increasing marginalization of vulnerable populations (Khalid, 2020; United Nations, 2020; Van Lancker & Parolin, 2020), disruptions to education (Van Lancker & Parolin, 2020; Viner et al., 2020) and health care (Adams & Walls, 2020), and severe psychosocial stress (Rajkumar, 2020).
SARS‐CoV‐2 is zoonotic in origin and appears to have infected humans during a single crossover event (Zhang, Wu, & Zhang, 2020; Zhou et al., 2020). Zoonosis can occur when individuals of different species interact at their interfaces. Human–wildlife interfaces are complex and rapidly changing as a result of increasing human incursions into wildlife habitats (Cunningham, Daszak, & Wood, 2017) and expanding trade in wildlife and wildlife parts (Johnson, Hitchens, Pandit et al., 2020; Mehta, 2020). Better surveillance and understanding of human–wildlife interfaces, particularly those involving taxa closely related to humans (i.e., the nonhuman primates), is crucial to prevent future crossover events.
Human and nonhuman primates (hereafter “primates”) are sympatric across the primate distribution range, and some human–primate interfaces involve frequent and close contact, producing opportunities for zoonosis and other negative outcomes. Our objectives in this commentary are to highlight challenges and opportunities at the human–primate interface in light of the COVID‐19 pandemic and to make specific recommendations for researchers working with wild primates during (and, we hope, after) the pandemic. We begin by briefly reviewing the evidence for zoonosis at the human–primate interface and the risk of human‐to‐primate zoonosis of SARS‐CoV‐2. Next, we identify challenges that have been revealed or exacerbated during the pandemic, with a specific focus on the disruption of long‐standing provisioning relationships. We then discuss research and educational opportunities that the COVID‐19 pandemic has created or revealed, particularly with regard to reducing human–primate conflict and risk of zoonosis. Finally, we make specific recommendations for researchers and conservation managers in light of the pandemic and beyond‐pandemic realities. Although humans have provisioning relationships with wild primates across much of their distribution range (Lee & Priston, 2005; Priston & McLennan, 2013; Sabbatini, Stammatia, Tavares, Giuliani, & Visalberghi, 2006), here we focus on examples from Asia, a region where deeply entrenched cultural practices of primate feeding (Lee & Priston, 2005; Malaivijitnond & Hamada, 2008; Malaivijitnond, Vazquez, & Hamada, 2011) have been disrupted by particularly stringent controls on movement in response to the COVID‐19 pandemic. Asia is also of particular interest as a crucial hotspot for emerging infectious diseases from wildlife (Morse et al., 2012).
As we move through the COVID‐19 pandemic, communities are making consequential decisions about how to balance the need to mitigate the risks of COVID‐19 with other health, social, and economic priorities, and primatologists should be part of these conversations. To reach a “new normal,” some entrenched, and even cherished, practices are changing, because the pandemic has shown that we can, and must, reimagine the ways that we interact with wildlife and each other.
2. THE ZOONOTIC ORIGINS OF SARS‐COV‐2 AND HUMAN–PRIMATE ZOONOSES
The vast majority of pathogenic viruses, protozoa, and helminths, and many bacteria and fungi affecting humans are zoonotic, and most of these (~80%) circulate in other mammals (Morse et al., 2012). Tracing the origins of zoonotic diseases is difficult, as most pathogens circulating in wildlife have not yet been identified or described (Andersen, Rambaut, Lipkin, Holmes, & Garry, 2020; Cunningham et al., 2017; Li et al., 2005). Based on its similarity to viruses detected in wild bats, SARS‐CoV‐2 is likely to have emerged from a bat coronavirus (Andersen et al., 2020; Li et al., 2005; Zhou et al., 2020); however, the means by which the virus was transmitted to humans has not been determined yet (Li et al., 2005; Liu et al., 2020).
Primates, our closest evolutionary relatives, have been implicated in a number of zoonotic disease outbreaks in humans (Morse et al., 2012). Primate‐to‐human zoonotic transmission is implicated in the origins of HIV/AIDS (Peeters et al., 2002), as well as sporadic human infection with several diseases, including simian malaria (Plasmodium knowlesi), Simian Foamy Virus, monkeypox, Herpes B, Kyasanur Forest Disease virus, and other pathogens (Essbauer, Pfeiffer, & Meyer, 2010; Feeroz et al., 2013; Gillespie, Nunn, & Leendertz, 2008; Holbrook, 2012; Ngernna et al., 2019; Sato et al., 2019; Switzer et al., 2004; Tischer & Osterrieder, 2010; Yadav et al., 2020). Primate‐to‐human zoonosis is also suspected in some outbreaks of filoviral hemorrhagic fevers such as Marburg and Ebola (Changula, Kajihara, Mweene, & Takada, 2014; Gillespie et al., 2008).
Primates are also particularly susceptible to human pathogens (Boesch, 2008; Calvignac‐Spencer, Leendertz, Gillespie, & Leendertz, 2012; Gillespie et al., 2008; Patrono et al., 2018). For example, the bacteria that cause tuberculosis, a chronic airborne disease resulting in high morbidity and mortality in both humans and primates, can be bi‐directionally transmitted (Bushmitz et al., 2009; Lécu & Ball, 2011; Mätz‐Rensing et al., 2015). From 2002 to 2004, major Ebola outbreaks in central Africa killed up to 95% of individual western lowland gorillas (Gorilla gorilla gorilla) in affected populations (Genton et al., 2012). While it is unclear whether this occurred due to human‐to‐primate disease transmission or whether both were infected by another animal host, this incident and many others (Boesch, 2008; Holzmann, Agostini, Areta, Beldomenico, & Di Bitetti, 2010; Lappan, Sibarani, Rustiati, & Andayani, 2017; Nunn, 2006) demonstrate the potentially catastrophic consequences of infectious disease epidemics for primates already threatened with extinction. Accordingly, great ape researchers have long emphasized the importance of biosafety practices to reduce the risk of human‐to‐primate zoonosis in research, tourism, and conservation programs, developing and promoting best practice guidelines for great ape health monitoring and disease control (Gilardi et al., 2015) and tourism (Macfie & Williamson, 2010). However, implementation of recommended practices for great apes has been inconsistent (Daud, 2019; Hanes, Kalema‐Zikusoka, Svensson, & Hill, 2018; Singh, 2020), and these measures are rarely discussed or used for other wild primates.
On March 15, 2020, the International Union for Conservation of Nature (IUCN) released a statement emphasizing the risk that SARS‐CoV‐2 poses for great apes, and stressing the importance of risk mitigation (IUCN, 2020). However, SARS‐CoV‐2 should be considered a potential threat to all wild primates, not just the great apes (Gillespie, 2019; Santos, Guiraldi, & Lucheis, 2020). Rhesus macaques exposed to SARS‐CoV‐2 develop symptoms similar to COVID‐19 (Bao et al., 2020; Shan et al., 2020), and all apes and all African and Asian monkeys examined in a recent study share an identical amino acid sequence with all 12 of the sites on the human angiotensin‐converting enzyme 2 (ACE2) receptor that are critical for SARS‐CoV‐2 binding (Melin, Janiak, Marrone, Arora, & Higham, 2020). These preliminary findings indicate that all catarrhines, as well as some lemurs, may be highly susceptible to infection with SARS‐CoV‐2 (Melin et al., 2020). Infection of wild primates with SARS‐CoV‐2 has the potential to create a new wild reservoir for the virus, which will substantially complicate efforts to prevent future human outbreaks of COVID‐19 and create new opportunities for viral evolution. Close contact between sympatric primate species is common, which means that a sustained outbreak in any wild primate species may result in epizootics in other species as well, potentially resulting in waves of population declines or local extinctions.
3. RISK OF ZOONOSIS AT THE HUMAN–PRIMATE INTERFACE
Human–primate interfaces, including provisioning, research activities, hunting, primate‐related ecotourism, and the keeping of primates as pets, can result in close spatial proximity and may lead to physical contact, thereby creating opportunities for zoonosis (Balasubramaniam, Sueur, Huffman, & MacIntosh, 2020; Filippone et al., 2015; Jones‐Engel, Engel, Schillaci, Babo, & Froehlich, 2001; Muehlenbein & Wallis, 2017). Humans and primates share space across many different habitats spanning the primate distribution range, including forests, open woodlands, agricultural landscapes, roadsides, villages, and urban landscapes (Estrada et al., 2017; Hockings et al., 2015; Radhakrishna, Huffman, & Sinha, 2013). Because humans and primates have broadly similar physiological needs and diets, these sympatric contexts can lead to ecological competition in forests and agricultural landscapes (Hockings, Parathian, Bessa, & Frazão‐Moreira, 2020; Riley, 2007), as well as create opportunities for provisioning and other close encounters between species.
People feed wild primates in parks, beaches, temples, human settlements, and along roadsides (El Alami, Van Lavieren, Rachida, & Chait, 2012; Hsu, Kao, & Agoramoorthy, 2009; Koirala et al., 2017; Malaivijitnond et al., 2011; Sabbatini et al., 2006), with many negative consequences for both humans and primates (Maréchal, Semple, Majolo, & MacLarnon, 2016; Morrow, Glanz, Ngakah, & Riley, 2019). Most deliberate feeding involves terrestrial or semi‐terrestrial primates, but in fragmented habitats, even strictly arboreal species such as siamangs (Symphalangus syndactylus; T. Q. Bartlett, personal communication, July 1, 2017), Javan gibbons (Hylobates moloch; R. Oktaviani, personal communication, February 19, 2020), agile gibbons (H. agilis; M. Iqbal, personal communication, May 13, 2018), dusky langurs (Trachypithecus obscurus; S. Malaivijitnond, personal obs. May 17, 2019), and silvery langurs (T. selangorensis; TripAdvisor, 2014) are sometimes fed. Primates also forage on garbage around human settlements, sometimes entering homes and public facilities (Md‐Zain, Ruslin, & Idris, 2014; Naher, Khan, & Ahmed, 2017), which may not always require close physical proximity to humans but can result in disease transmission through the handling or ingestion of fomites (Sapolsky & Share, 2004).
In many parts of the primate distribution range, the practice of keeping primates as pets is common (e.g., Amazonia: Cormier, 2003; Indonesia: Jones‐Engel et al., 2001; Jones‐Engel, Schillaci, Engel, Paputungan, & Froehlich, 2005); posing risks for zoonotic disease transmission. For example, in Sulawesi, Indonesia, Jones‐Engel et al. (2001) found evidence of exposure of pet Sulawesi macaques to endemic human pathogens, including measles, influenza A, and parainfluenza 1, 2, and 3: all respiratory viruses that are easily transmitted and highly contagious. It is believed that the pet macaques likely acquired the pathogens as a result of close interaction with their owners and other members of the village. The fact that the same human pathogens were also detected in wild populations suggests that the pet macaques may act as a sort of “vector” (from human to wild populations) since wild macaques are often attracted to pet ones (e.g., when females are in estrus; Jones‐Engel et al., 2005). The pet trade may also create opportunities for primate‐to‐human zoonosis of emerging infectious diseases. One example is the trade of Asian apes. There is some evidence that Asian apes may often be captured opportunistically for pets as land is deforested and converted to agriculture (Freund, Rahman, & Knott, 2017; Nijman, Spaan, Rode‐Margono, & Nekaris, 2017). The concern here is that wildlife in recently deforested areas may be particularly vulnerable to zoonosis, and therefore may have higher pathogen loads, because of compression of multiple species into smaller spaces (Borremans, Faust, Manlove, Sokolow, & Lloyd‐Smith, 2019; Cunningham et al., 2017; Mehta, 2020).
4. COVID‐19 AND DISRUPTIONS TO THE HUMAN–PRIMATE INTERFACE: MAJOR CHALLENGES AND OPPORTUNITIES
4.1. Challenges created or exacerbated by the COVID‐19 pandemic
4.1.1. Reduced nutrition for primates
Human provisioning can offer substantial nutritional benefits to wild primates, although those benefits may not be experienced by all group members (El Alami et al., 2012; Marty et al., 2019; Sinha & Mukhopadhyay, 2013). While some primate populations that forage in temples, villages, and towns also have access to wild foods in adjacent forests, other populations that live in urban landscapes where wild foods are scarce may be highly nutritionally dependent on anthropogenic food. However, throughout much of Asia, changes in human behavior in response to the COVID‐19 pandemic have disrupted human–primate feeding interactions. For example, nationwide movement controls in Bangladesh, India, Malaysia, Thailand, and Vietnam and more localized controls in China, Indonesia, and the Philippines initiated in mid‐to‐late March 2020 prohibited most people from leaving their homes for purposes other than essential work, medical care, or to obtain necessities such as food and medicine. In addition, border and park closures have also sharply restricted the number of tourists visiting recreational areas such as parks, beaches, and temples where primates are routinely fed (Bisht, 2020; Phull, 2020; Pulitzer, 2020). As a consequence, many of these primate populations that have been nutritionally dependent on human handouts for generations are now experiencing an abrupt loss of this food source (Bangkok Post, 2020; Kieu, 2020).
4.1.2. Increased intraspecific competition for resources
For primate populations that are nutritionally dependent on provisioned foods, intraspecific competition is inevitable when rates of provisioning are reduced. For example, in Lopburi, a small city in Thailand that celebrates its large urban monkey population with an annual Monkey Festival (Kieu, 2020), local residents have reported observing intense aggression between two large groups of long‐tailed macaques that previously foraged in separate areas (Bangkok Post, 2020; Kieu, 2020). One group depended on handouts from tourists visiting the Prang Sam Yod temple, and the other relied on tourists visiting the Phra Kan Shrine, closer to the city center (Thaitrakulpanich, 2020). Witnesses reported that reduced provisioning of the Prang Sam Yod group caused them to move into the range of the city group, resulting in intense conflict involving hundreds of monkeys (Kieu, 2020; Thaitrakulpanich, 2020). To mitigate the conflict, local officials have started provisioning the monkeys at both temple sites (Johnson & Tun, 2020; Thaitrakulpanich, 2020).
4.1.3. Increased poaching
Despite laws against hunting of protected species, poaching has become a greater problem during the COVID‐19 lockdowns likely due to a combination of increased economic and nutritional stress in marginalized communities and reduced intensity of law enforcement (WCS, 2020). For example, in Sabah, Malaysia, even though wildlife conservation is listed as an “essential” activity, rangers were not allowed to patrol during the country's movement control order unless there was actual evidence of poaching, and as a result, illegal hunting activities have increased (Lee, 2020). Many forest areas across India have also seen an increase in wildlife poaching and illegal wildlife trade, ostensibly due to the administrative emergency situation and rising unemployment created by the nationwide lockdown to contain the spread of the virus (Bhardwaj, 2020; Sen, 2020).
4.1.4. Loss of opportunities for primate research
Restrictions on travel and movement have resulted in the postponement or cancellation of new research projects (Kimbrough, 2020; Pope, 2020), and interrupted data collection for long‐established research programs. In some cases, this has meant leaving habituated primates unattended, which may result in loss of habituation and increased vulnerability to poaching (Williamson & Feistner, 2011). Delays or interruptions in project implementation may result in lost opportunities for students and trainees, and delays or interruptions of even a few months may result in the loss of time‐sensitive data from specific seasons, individuals, or life stages. For long‐term projects dependent on volunteers, students, or field assistants from outside of the local area, shuttering projects, even temporarily, can lead to loss of institutional knowledge and loss of project memory, as international project personnel that were forced to abruptly depart are unlikely to return to train incoming staff when and if the projects are able to resume.
4.2. Opportunities to improve the human–primate interface during and post COVID‐19
4.2.1. Reduced intensity of human–primate interfaces
Global responses to the COVID‐19 pandemic from January to June 2020 have resulted in some shifts to problematic human–primate interfaces as a result of substantial changes in human behavior. As noted above, in many areas, the direct provisioning of primates has been dramatically reduced, and, as a result, wild primates should shift to foraging on other foods to the extent possible, just as they adjust their foraging in response to seasonal and episodic periods of low availability of other preferred foods (Catenaacci, Pessoa, Nogueira‐Filho, & De Vleescchouwer, 2016; Hongo, Nakashima, Akomo‐Okoue, & Mindonga‐Nguelet, 2018; Ning, Guan, Huang, Fan, & Jiang, 2019; Tang et al., 2015). Unfortunately, in many locations, opportunities for researchers to observe these transitions have been limited due to international and domestic travel restrictions, stay‐at‐home orders, and park closures. Nonetheless, there is anecdotal evidence that primates change their behavior when human provisioning ceases. For example, long‐tailed macaques and southern pig‐tailed macaques (Macaca nemestrina) in Lumut in Perak, Malaysia, which normally rely heavily on handouts from local park visitors, started to forage in nearby mangroves for natural foods, such as molluscs, crabs, and plant foods when the park was closed (N. Ruppert, personal obs. April 2020). The complexity of the problem of habituation to human food, though, is illustrated by the fact that some individuals at this site started crossing the usually busy, but now quiet, road into a nearby housing area to forage in garbage dumps (N. Ruppert, personal obs. April 2020). Similarly, long‐tailed macaques in some public parks in Singapore started to forage more frequently on wild fruiting trees as the closure of car parks reduced visitor presence in the parks (S. Jabbar, personal communication, June 4, 2020). In Shimla in northern India, usually a hotspot for human–macaque conflict, the number of rhesus macaques in the town substantially decreased, presumably because macaque groups moved toward forest areas on the periphery (Bisht, 2020; Phull, 2020). Rhesus macaques and bonnet macaques also disappeared from some temple and tourist sites in India when the pandemic‐fueled lockdown began (Bisht, 2020; Sudhish, 2020).
Primates habituated to humans and human food can become aggressive toward humans (Hsu et al., 2009; Radhakrishna & Sinha, 2011; Zhou & Deng, 1992). In the COVID‐19 era, reduced reliance on provisioning means that human–primate encounters, and the likelihood of human‐directed aggression and potential risk of zoonotic exchange from these encounters, are also reduced. While these may constitute more positive outcomes of the pandemic, it is likely that the resulting changes to the intensity of human–primate interfaces will only be temporary. Unless governments and local communities take action to promote more permanent changes, most of these positive outcomes may quickly be reversed. For example, when the Penang Botanic Garden in Malaysia was reopened on June 6, 2020, there were no signs of anthropogenic food or packaging and all macaques visible from the main trails were foraging on plant foods. However, by the next day, macaques were waiting in the car park, handling food packaging and eating anthropogenic food (S. Lappan, personal obs. June 6, 2020), indicating a return to pre‐lockdown conditions. A rapid return to pre‐lockdown behavior by humans and macaques was also observed in Lumut, Malaysia when the mangrove park reopened (N. Ruppert, personal obs. June 2020), and in Shimla, India when lockdown measures were eased (Sharma, 2020).
4.2.2. Reduced risk of zoonosis due to changes in human risk perception
The pandemic has also affected human perceptions of risk among governments, organizations, and community members in ways that may result in enduring behavior change. The probable zoonotic origins of the SARS‐CoV‐2 virus (Zhang et al., 2020; Zhou et al., 2020), and the hypothesized source of the outbreak at a wet market selling wildlife (Zhou et al., 2020) have been widely reported in the media, which has focused public attention on the dangers that close interactions between humans and wildlife can pose (Walzer, 2020). As a result, there is now broad public support for action to reduce the risk of zoonosis (WWF, 2020), which has led to policy changes such as a temporary ban on the sale of wildlife for human consumption in China (Wescott & Deng, 2020) and the temporary closure of several African National Parks to human visitors to prevent human‐to‐ape transmission of SARS‐CoV‐2 (Bennett, 2020; Vyawahare, 2020b). The government of Gibraltar has also banned all physical contact between humans and Barbary macaques (Macaca sylvanus), as well as “interference with a natural behavior” (which presumably includes feeding), with the stated goal of preventing human‐to‐primate transmission of SARS‐CoV‐2, but also primate‐to‐human zoonosis of other pathogens (HM Government of Gibraltar, 2020).
Such governmental policies, however, may be ineffective in reducing the risk of zoonosis if they are not matched by behavior change by individuals and communities living in close contact with wild primates. Even communities that are motivated to reduce zoonosis risk may not be adequately informed about the risks involved in different types of human–wildlife interactions, underestimating the risks involved in some activities, such as feeding wild monkeys in a public park or temple, while overestimating the risks from other wildlife encounters. For example, large numbers of bats have been killed in India, Cuba, Rwanda, and Indonesia as a result of community fears about the potential for bat‐to‐human SARS‐CoV‐2 zoonosis (Bittel, 2020), despite the fact that SARS‐CoV‐2 has already become a human virus (Mallapaty, 2020). Increased public awareness about the risks of zoonoses in primate range countries can create negative perceptions about primates among communities living adjacent to wild primates. For example, rhesus and long‐tailed macaques, two primate species that closely interface with humans in many range countries, are the animals of choice for preclinical testing of candidate COVID‐19 vaccines because their lungs express similar ACE2 proteins to humans and their immune responses are also homologous (Bao et al., 2020; Rockx et al., 2020). Local people in Prachuap Khiri Khan, Thailand, who have learned about the susceptibility of these primates to COVID‐19, have started to panic about the risk of zoonotic transmission from monkeys to humans (Siamrath, 2020). In response, the local governor has recommended biosafety protocols for locals that include keeping a distance from monkeys and chasing monkeys away when they invade houses. Primatologists can play a role in these conversations by working closely with local organizations to create accurate messaging about strategies for minimizing the risk of zoonosis.
4.2.3. Opportunities to reduce trade in wildlife
The COVID‐19 pandemic, by focusing public attention on the problem of zoonosis, has changed conversations about biodiversity and human–wildlife interactions in ways that may benefit wild primates and primate habitats. For example, in addition to the temporary ban on trade in wildlife for consumption (Wescott & Deng, 2020), China is reviewing relevant laws (the Wildlife Protection Law of China, the Biosecurity Law, and the Animal Epidemic Prevention Law) to enshrine more permanent legislation to reduce the risk of zoonosis by banning trade in wildlife for human consumption (Vyawahare, 2020a) and reducing the list of animals that can be traded for Traditional Chinese Medicine (Leng & Wan, 2020). While these laws focus on trade in wildlife for human consumption and medicinal use, these changes and similar movements by other governments may also create greater motivation on the part of wildlife authorities to enforce existing laws against trade‐in and keeping of primates as pets.
4.2.4. Research and conservation opportunities
The disruption to “business as usual” due to COVID‐19 has created unprecedented opportunities to better understand, and potentially change, interactions between humans and primates for the better. “Removal experiments,” where an organism is removed from an ecosystem to better understand how it affects other variables in the system, are the gold standard for determining the relationships between ecological variables, but it is generally impossible (and ethically problematic) for researchers to remove humans as an ecological factor to better understand our effects on other animals. Therefore, the dramatic change in human use of these landscapes during government‐mandated closures during, and likely after, the COVID‐19 pandemic represents a potentially fruitful opportunity for researchers interested in understanding the ecological and conservation implications of the human–primate interface.
Researchers may not be able to predict when the closed parks, beaches, temples, and other spaces where primates are routinely fed by humans will re‐open, and the conditions under which this reopening will occur. Nonetheless, it is reasonable to assume that the re‐entry of visitors to some of these spaces may be gradual. Governments may continue to restrict crowd sizes for some time after stay‐at‐home orders are relaxed. Local people may visit parks after movement restrictions are lifted, but many are likely to be preoccupied with other matters during this tumultuous time. Tourism in primate habitat countries is unlikely to return to the pre‐pandemic baseline any time soon, and ongoing physical distancing practices and urgent economic concerns may also reduce the rates of visitation to some of these spaces by local visitors. Therefore, there is likely to be a period of time after formal restrictions are lifted when relatively few people are frequenting these spaces. As noted in the specific recommendations below, given the risk of human‐to‐primate transmission of COVID‐19, researchers engaging in new projects must follow appropriate safety protocols both to reduce transmission risks and to model appropriate behaviors for bystanders.
The economic impacts of the COVID‐19 pandemic may reduce the availability of funding for research and conservation in the upcoming months and years, but the pandemic may also shift the priorities of funding agencies toward supporting disease surveillance, epidemiology, and prevention of zoonosis at the human–primate interface, creating new opportunities for research and conservation activities in these areas. Many granting organizations have created new funding opportunities for research related to COVID‐19, including some specifically focusing on wildlife.
4.2.5. Enhanced public receptiveness to education
The pandemic may also create opportunities to use public education and outreach campaigns to change behaviors. As discussed above, the public is increasingly aware of the potential risk of zoonoses, which may facilitate behavior change around feeding interactions. In places where feeding interactions have already been disrupted, and the resident primate communities have survived by foraging on other, non‐anthropogenic, food sources, people motivated by compassion for primates that they perceive as hungry may be more open to the argument that provisioning is not necessary, and can be harmful. In particular, enhanced awareness of the risk of zoonosis may have made communities more receptive to messaging about the parallel risk of human‐to‐primate disease transmission and the risks that feeding creates for primate communities. In addition, during and beyond the pandemic, effective messaging about the risks of zoonosis to pet owners and the community may reduce the appeal of primates to potential buyers and to local residents who are considering capturing a primate to keep as a pet.
5. SPECIFIC RECOMMENDATIONS
To address the challenges and take advantage of the opportunities described above, we enumerate recommendations for primatologists and other interested parties below.
5.1. Recommendations for immediate implementation
-
1.
Adopt stringent biosafety protocols to reduce the risk of zoonoses between humans and primates, and especially human‐to‐primate SARS‐CoV‐2 zoonosis, in field and captive settings. Best practice guides from the IUCN (Gilardi et al., 2015; Macfie & Williamson, 2010) provide protocols for reducing the risk of zoonosis between humans and wild great apes. We recommend that researchers working with all primate species adhere to these guidelines, with a modification in the context of SARS‐CoV‐2. The best practice guide recommends a 7‐day quarantine after the resolution of symptoms of infectious disease or after travel from outside of the area (Gilardi et al., 2015). However, given the long incubation period and high probability of asymptomatic infection with SARS‐CoV‐2, we recommend a 14‐day quarantine, following the recommendations of the World Health Organization (WHO, 2020) and the USA Center for Disease Control (CDC, 2020). Individuals infected with SARS‐CoV‐2 and their close contacts should not visit sites with primates until their negative infection status has been verified.
-
2.
Model safe and appropriate practices with primates in field settings, outreach, and social media materials. Primatologists and conservationists must follow safe distance and masking protocols when being observed or photographed. They should not be photographed holding primates (even in captive care settings) and should avoid sharing images showing close human–primate spacing in outreach materials, on social media accounts, or in public presentations. Such images may create public perceptions that primates are appealing and tame, increasing the risks of inappropriate behavior toward wild primates, and increasing demand for primates as pets (Ross, Vreeman, & Lonsdorf, 2011).
-
3.
Collect time‐sensitive data on human–primate interfaces as movement controls in range countries are lifted. Researchers who are positioned to visit areas where wild primates are routinely fed should act quickly to record the responses of primates to human re‐entry into these spaces when restrictions on movement are lifted, especially at sites where baseline data from earlier time periods are available. Researchers holding research permissions or funding and those positioned to conduct observational research during the opening period should collaborate to facilitate the rapid initiation of field projects. Specific topics of interest include primate feeding ecology, demography, population dynamics, ranging, and health, as well as human behavior as shared spaces reopen. Studies comparing sites where provisioning continued throughout the lockdown and those where it did not may be particularly fruitful, so collaboration across research groups using standardized research protocols should be considered.
-
4.
Minimize provisioning or capture of free‐ranging primates. Non‐essential provisioning and capture should be avoided during the pandemic. In cases where provisioning or capture is essential (e.g., rehabilitant animals cannot be allowed to starve, and animals may need to be captured for veterinary care or disease surveillance), contact should be minimized and appropriate biosafety protocols should be followed. Where animals are fed, particularly stringent protocols should be put into place to minimize habituation to human observers and the risk of zoonosis.
-
5.
Funding agencies (including governmental and nongovernmental organizations) should prioritize funding for research on human–primate interfaces in the pandemic and post‐pandemic period.
5.2. Recommendations for actions to be initiated in the near term
-
1.
Collaborate with local stakeholders, including governmental and nongovernmental organizations, to develop and implement education programs to reduce provisioning, the keeping of primates as pets, and other practices that create risks of zoonosis between humans and primates. Communities around the globe have already been primed with messaging about the importance of hand and respiratory hygiene, and physical distancing in human social interactions. Conservationists can build on and extend local public health messaging during this crucial period. Specific topics for education programs could include the negative consequences of provisioning, harms caused by the primate pet trade, and the ecological importance of primates and forest habitats.
-
2.
Establish health monitoring protocols for all habituated primate groups in collaboration with veterinarians, epidemiologists, and public health authorities. The IUCN (Gilardi et al., 2015) provides guidelines for monitoring primate health.
-
3.
Partner with protected area (PA) and park management to develop educational materials for PA visitors to educate them about appropriate biosafety protocols during visits to parks with resident primate populations.
-
4.
Provide accurate and accessible information about primates to communities at the human–primate interface to prevent the development of negative attitudes toward primates and other wildlife.
6. CONCLUSION
The COVID‐19 pandemic has caused tremendous suffering and continues to pose a grave threat to humans, primates, and other animals. At the same time, it has created opportunities for interventions to address long‐standing problems at human–primate interfaces. In this commentary, we have identified concrete steps that primatologists can take to support local communities at human–primate interfaces in their efforts to reduce contact, aggression, and the risk of zoonosis between humans and primates. We have also identified opportunities that primatologists should seize to improve our understanding of human–primate interfaces in light of the pandemic, as well as steps that primatologists should take to reduce potential bidirectional exposures to pathogens in a research context (Figure 1). Many widespread practices that increase our exposure to emerging pathogens, such as human encroachment into forested landscapes (Cunningham et al., 2017; Johnson et al., 2020) and legal and illegal trade in animals (Karesh, Cook, Bennett, & Newcomb, 2005; Morse et al., 2012) also threaten wild primate populations and create human–primate conflict. Ultimately, reducing the risk of zoonotic emerging infectious diseases and promoting the well‐being of humans and primates at our interfaces will require additional substantial changes from “business as usual.” The onus is on primatologists to help lead the way.
Figure 1.
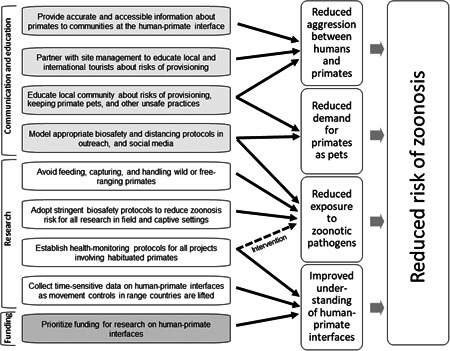
Flow diagram showing the relationships between recommended actions and intended outcomes. Actions involving working with local partners to effect change through communication and education in communities at human–primate interfaces are lightly shaded. Actions affecting the practice of field research are unshaded. Actions by funders are dark shaded
ACKNOWLEDGMENTS
SL and NR thank the Department of Wildlife and National Parks of Peninsular Malaysia (PERHILITAN) and Forestry Department of Peninsular Malaysia for permission to conduct primatological research in Malaysia. We especially thank Malaysian Primatological Society for extensive logistical and technical support and Aini Hasanah, Priscillia Miard, Joleen Yap, and Zaki Zainol for many helpful conversations about how to reduce human provisioning of primates in Malaysia. NR and SL thank USM (RU/PBIOLOGI/8011063) and Disney Conservation Fund (awarded through Malaysian Primatological Society) for funding research on and conservation of Malaysian primates. SL thanks the USA Fulbright Scholar Program and Appalachian State University for funding support to conduct research in Malaysia. NR thanks the Ministry of Higher Education (FRGS/PBIOLOGI/6711649), the Rufford Foundation, and The Habitat Foundation for funding support to study macaques. SM thanks the Thailand Research Fund Senior Scholar Grant RTA 6280010 and the Thailand Research Fund‐Chinese Academy of Science (TRF‐CAS) Grant DBG60 for funding supports. SR thanks the Department of Science and Technology, Government of India (Grant Order SERB/F/I0032/2016‐17) for funding support and the Forest Department of Himachal Pradesh for logistical support. ER thanks the Indonesian Ministry of Research and Technology (RISTEK) for permission to conduct research in Indonesia, Hasanuddin University for sponsoring her research, staff, and administration at Bantimurung Bulusaraung National Park for field assistance and logistical support, and the American Institute for Indonesian Studies, Wenner Gren Foundation, and San Diego State University for funding. Thanks to Rahayu Oktaviani, Muhammad Iqbal, Sabrina Jabbar, and Thad Q. Bartlett for sharing their observations of primate provisioning. We thank Brenda McCowan and her team, namely Bidisha Chakraborty, Emily Dura, Josephine Hubbard, Krishna Balasubramaniam, Nalina Aiempichitkijkarn and Stefano Kaburu, for many helpful comments that substantially improved the manuscript.
Lappan S, Malaivijitnond S, Radhakrishna S, Riley EP, Ruppert N. The human–primate interface in the New Normal: Challenges and opportunities for primatologists in the COVID‐19 era and beyond. Am J Primatol. 2020;82:e23176 10.1002/ajp.23176
REFERENCES
- Adams, J. G. , & Walls, R. M. (2020). Supporting the health care workforce during the COVID‐19 global epidemic. Journal of the American Medical Association, 323, 1439–1440. 10.1001/jama.2020.3972 [DOI] [PubMed] [Google Scholar]
- Andersen, K. G. , Rambaut, A. , Lipkin, W. I. , Holmes, E. C. , & Garry, R. F. (2020). The proximal origin of SARS‐CoV‐2. Nature Medicine, 26, 450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam, K. N. , Sueur, C. , Huffman, M. A. , & MacIntosh, A. J. J. (2020). Primate infectious disease ecology: Insights and future directions at the human–macaque interface In Li J.‐H., Sun L. & Kappeler P. (Eds.), The behavioral ecology of the Tibetan macaque (pp. 249–284). Cham: Springer. [Google Scholar]
- Bangkok Post . (2020). Monkey brawl in Lop Buri shocks humans March 11, 2020. Retrieved from https://www.bangkokpost.com/thailand/general/1876489/monkey-brawl-in-lop-buri-shocks-humans
- Bao, L. , Deng, W. , Gao, H. , Xiao, C. , Liu, J. , Xue, J. , … Qin, C. (2020). Reinfection could not occur in SARS‐Cov‐2 infected rhesus macaques. Preprint at bioRxiv, 10.1101/2020.1103.1113.990226 [DOI]
- Bennett, E. L. (2020). Quarantining also means caring for our great ape relatives. Crossroads Blog: Open Letters to IUCN Members. April 15, 2020. Retrieved from https://www.iucn.org/crossroads-blog/202004/quarantining-also-means-caring-our-great-ape-relatives [Google Scholar]
- Bhardwaj, M. (2020). COVID‐19 lockdown effect: Dogs, people killing Karnataka wildlife for food. The New Indian Express, April 29, 2020. Retrieved from https://www.newindianexpress.com/states/karnataka/2020/apr/2025/covid-2019-lockdown-effect-dogs-people-killing-karnataka-wildlife-for-food-2135043.html [Google Scholar]
- Bisht, G. (2020). Amid lockdown, Shimla gets respite from monkey menace. Hindustan Times, April 29, 2020. Retrieved from https://www.hindustantimes.com/india-news/amid-lockdown-shimla-gets-respite-from-monkey-menace/story-gHcLt3EnxcshwJhobqaF3O.html [Google Scholar]
- Bittel, J. (2020). Experts urge people all over the world to stop killing bats out of fears of coronavirus. NRDC Science Spotlight, June 2, 2020. Retrieved from https://www.nrdc.org/stories/experts-urge-people-all-over-world-stop-killing-bats-out-fears-coronavirus [Google Scholar]
- Boesch, C. (2008). Why do chimpanzees die in the forest? The challenges of understanding and controlling for wild ape health. American Journal of Primatology, 70, 722–726. 10.1002/ajp.20571 [DOI] [PubMed] [Google Scholar]
- Borremans, B. , Faust, C. , Manlove, K. R. , Sokolow, S. H. , & Lloyd‐Smith, J. O. (2019). Cross‐species pathogen spillover across ecosystem boundaries: Mechanisms and theory. Philosophical Transactions of the Royal Society, B: Biological Sciences, 374, 20180344 10.1098/rstb.2018.0344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushmitz, M. , Lecu, A. , Verreck, F. , Preussing, E. , Rensing, S. , & Mätz‐Rensing, K. (2009). Guidelines for the prevention and control of tuberculosis in non‐human primates: Recommendations of the European Primate Veterinary Association Working Group on Tuberculosis. Journal of Medical Primatology, 38, 59–69. [DOI] [PubMed] [Google Scholar]
- Calvignac‐Spencer, S. , Leendertz, S. A. J. , Gillespie, T. R. , & Leendertz, F. H. (2012). Wild great apes as sentinels and sources of infectious disease. Clinical Microbiology and Infection, 18, 521–527. [DOI] [PubMed] [Google Scholar]
- Catenaacci, L. S. , Pessoa, M. S. , Nogueira‐Filho, S. L. G. , & De Vleescchouwer, K. M. (2016). Diet and feeding behavior of Leontopithecus chrysomelas (Callitrichidae) in degraded areas of the Atlantic forest of South‐Bahia, Brazil. International Journal of Primatology, 37, 136–157. 10.1007/s10764-016-9889-x [DOI] [Google Scholar]
- CDC . (2020). Quarantine and isolation. Coronavirus Disease 2019 (COVID‐19), May 6, 2020. Retrieved from https://www.cdc.gov/coronavirus/2019-ncov/if-you-are-sick/quarantine-isolation.html [Google Scholar]
- Changula, K. , Kajihara, M. , Mweene, A. , & Takada, A. (2014). Ebola and Marburg virus diseases in Africa: Increased risk of outbreaks in previously unaffected areas? Microbiology and Immunology, 58, 483–491. 10.1111/1348-0421.12181 [DOI] [PubMed] [Google Scholar]
- Cormier, L. A. (2003). Kinship with monkeys: The Guaja Foragers of Eastern Amazonia. New York: Columbia University Press. [Google Scholar]
- Cunningham, A. A. , Daszak, P. , & Wood, J. L. N. (2017). One Health, emerging infectious diseases and wildlife: Two decades of progress? Philosophical Transactions of the Royal Society, B: Biological Sciences, 372(1725), 20160167 10.1098/rstb.2016.0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daud, Z. (2019). Sepilok centre must heed the rules. New Straits Times. December 7, 2019. Retrieved from https://www.nst.com.my/opinion/letters/2019/2012/545536/sepilok-centre-must-heed-rules [Google Scholar]
- El Alami, A. , Van Lavieren, E. , Rachida, A. , & Chait, A. (2012). Differences in activity budgets and diet Between semiprovisioned and wild‐feeding groups of the endangered Barbary macaque (Macaca sylvanus) in the central High Atlas Mountains, Morocco. American Journal of Primatology, 74, 210–216. 10.1002/ajp.21989 [DOI] [PubMed] [Google Scholar]
- Essbauer, S. , Pfeiffer, M. , & Meyer, H. (2010). Zoonotic poxviruses. Veterinary Microbiology, 140, 229–236. 10.1016/j.vetmic.2009.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada, A. , Garber, P. A. , Rylands, A. B. , Roos, C. , Fernandez‐Duque, E. , Di Fiore, A. , … Li, B. (2017). Impending extinction crisis of the world's primates: Why primates matter. Science Advances, 31(1), e1600946 10.1126/sciadv.1600946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeroz, M. M. , Soliven, K. , Small, C. T. , Engel, G. A. , Pacheco, M. A. , Yee, J. L. , … Jones‐Engel, L. (2013). Population dynamics of rhesus macaques and associated foamy virus in Bangladesh. Emerging Microbes and Infections, 2(5), e29 10.1038/emi.2013.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes, N. (2020). Economic effects of coronavirus outbreak (COVID‐19) on the World Economy, March 22, 2020. Available at SSRN, 10.2139/ssrn.3557504 [DOI]
- Filippone, C. , Betsem, E. , Tortevoye, P. , Cassar, O. , Bassot, S. , Froment, A. , … Gessain, A. (2015). A severe bite from a nonhuman primate Is a major risk factor for HTLV‐1 infection in hunters From Central Africa. Clinical Infectious Diseases, 60, 1667–1676. [DOI] [PubMed] [Google Scholar]
- Freund, C. , Rahman, E. , & Knott, C. (2017). Ten years of orangutan‐related wildlife crime investigation in West Kalimantan, Indonesia. American Journal of Primatology, 79, e22620 10.1002/ajp.22620 [DOI] [PubMed] [Google Scholar]
- Genton, C. , Cristescu, R. , Gatti, S. , Levréro, F. , Bigot, E. , Caillaud, D. , … Ménard, N. (2012). Recovery potential of a western lowland gorilla population following a major Ebola outbreak: Results from a ten year study. PLOS One, 7(5), e37106 10.1371/journal.pone.0037106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilardi, K. V. , Gillespie, T. R. , Leendertz, F. H. , Macfie, E. J. , Travis, D. A. , Whittier, C. A. , & Williamson, E. A. (2015). Best practice guidelines for health monitoring and disease control in great ape populations. Gland, Switzerland: IUCN/SSC Primate Specialist Group. [Google Scholar]
- Gillespie, T. R. (2019). Guest editorial: Protecting wild primates during the novel coronavirus pandemic and beyond. Asian Primates, 8 Retrieved from http://static1.1.sqspcdn.com/static/f/1200343/28272165/1584507472433/1584507472020March1584507472416_Vol_1584507472438_1584507472431.pdf?token=1584507472430gVjrTZ1584507472434A1584507472437yqATta1584507472433OXjTZfd [Google Scholar]
- Gillespie, T. R. , Nunn, C. L. , & Leendertz, F. H. (2008). Integrative approaches to the study of primate infectious disease: Implications for biodiversity conservation and global health. Yearbook of Physical Anthropology, 51, 58–69. [DOI] [PubMed] [Google Scholar]
- Hanes, A. C. , Kalema‐Zikusoka, G. , Svensson, M. S. , & Hill, C. M. (2018). Assessment of health risks posed by tourists visiting mountain gorillas in Bwindi Impenetrable National Park, Uganda. Primate Conservation, 32, 123–132. [Google Scholar]
- HM Government of Gibraltar . (2020). 370/2020—Touching macaques to be an offence. May 29, 2020 Press Release, 370/2020. Retrieved from https://www.gibraltar.gov.gi/press-releases/3702020-touching-macaques-to-be-an-offence-3705938
- Hockings, K. J. , McLennan, M. R. , Carvalho, S. , Ancrenaz, M. , Bobe, R. , Byrne, R. W. , … Hill, C. M. (2015). Apes in the Anthropocene: Flexibility and survival. Trends in Ecology and Evolution, 30(4), 215–222. 10.1016/j.tree.2015.02.002 [DOI] [PubMed] [Google Scholar]
- Hockings, K. J. , Parathian, H. , Bessa, J. , & Frazão‐Moreira, A. (2020). Extensive overlap in the selection of wild fruits by chimpanzees and humans: Implications for the management of complex social–ecological systems. Frontiers in Ecology and Evolution, 19 10.3389/fevo.2020.00123 [DOI] [Google Scholar]
- Holbrook, M. R. (2012). Kyasanur forest disease. Antiviral Research, 96(3), 353–362. 10.1016/j.antiviral.2012.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzmann, I. , Agostini, I. , Areta, J. I. , Beldomenico, P. , & Di Bitetti, M. S. (2010). Impact of yellow fever outbreaks on two howler monkey species (Alouatta guariba clamitans and A. caraya) in Misiones, Argentina. American Journal of Primatology, 72, 475–480. 10.1002/ajp.20796 [DOI] [PubMed] [Google Scholar]
- Hongo, S. , Nakashima, Y. , Akomo‐Okoue, E. F. , & Mindonga‐Nguelet, F. L. (2018). Seasonal change in diet and habitat use in wild mandrills (Mandrillus sphinx). International Journal of Primatology, 39, 27–48. 10.1007/s10764-017-0007-5 [DOI] [Google Scholar]
- Hsu, M. J. , Kao, C. , & Agoramoorthy, G. (2009). Interactions between visitors and Formosan macaques (Macaca cyclopis) at Shou‐Shan Nature Park, Taiwan. American Journal of Physical Anthropology, 71, 214–222. [DOI] [PubMed] [Google Scholar]
- International Union for the Conservation of Nature (IUCN) . (2020). Great apes, COVID‐19 and the SARS CoV‐2. Joint Statement of the IUCN SSC Wildlife Health Specialist Group and the Primate Specialist Group, Section on Great Apes, March 15, 2020. Gland, Switzerland: International Union for the Conservation of Nature.
- Johnson, C. K. , Hitchens, P. L. , Pandit, P. S. , Rushmore, J. , Evans, T. S. , Young, C. C. W. , & Doyle, M. M. (2020). Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proceedings of the Royal Society Series B: Biological Sciences, 287, 2019.2736 10.1098/rspb.2019.2736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, K. , & Tun, S. Z. (2020). Brawling monkeys fed by Thai villagers as tourism dries up. Reuters, March 20, 2020. Retrieved from https://www.reuters.com/article/us-health-coronavirus-thailand-monkeys/brawling-monkeys-fed-by-thai-villagers-as-tourism-dries-up-idUSKBN217103 [Google Scholar]
- Jones‐Engel, L. , Engel, G. A. , Schillaci, M. A. , Babo, R. , & Froehlich, J. (2001). Detection of antibodies to selected human pathogens among wild and pet macaques (Macaca tonkeana) in Sulawesi, Indonesia. American Journal of Primatology, 54, 171–178. 10.1002/ajp.1021 [DOI] [PubMed] [Google Scholar]
- Jones‐Engel, L. , Schillaci, M. A. , Engel, G. A. , Paputungan, U. , & Froehlich, J. (2005). Characterizing primate pet ownership in Sulawesi: Implications for disease transmission In Patterson J. D. & Wallis J. (Eds.), Commensalism and conflict: The human–primate interface (pp. 196–221). Norman, Oklahoma: American Society of Primatologists. [Google Scholar]
- Karesh, W. B. , Cook, R. A. , Bennett, E. L. , & Newcomb, J. (2005). Wildlife trade and global disease emergence. Emerging Infectious Diseases, 11, 1000–1002. 10.3201/eid1107.050194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid, H. (2020). COVID‐19 and the most vulnerable among us. UNDP Malaysia, Singapore, and Brunei Darussalam, May 28, 2020. Retrieved from https://www.my.undp.org/content/malaysia/en/home/blog/2020/covid-2019-and-the-most-vulnerable-among-us.html [Google Scholar]
- Kieu, A. (2020). Monkeys in Thailand are revolting because of COVID‐19: 10 things to know. The Travel, March 20, 2020. Retrieved from https://www.thetravel.com/monkeys-thailand-revolting-covid-19/ [Google Scholar]
- Kimbrough, L. (2020). Field research, interrupted: How the COVID‐19 crisis is stalling science. Mongabay, 17 April 2020. Retrieved from https://news.mongabay.com/2020/2004/field-research-interrupted-how-the-covid-2019-crisis-is-stalling-science/ [Google Scholar]
- Koirala, S. , Chalise, M. K. , Katurwal, H. B. , Gaire, R. , Pandey, B. , & Ogawa, H. (2017). Diet and activity of Macaca assamensis in wild and semi‐provisioned groups in Shivapuri Nagarjun National Park, Nepal. Folia Primatologica, 88, 57–74. [DOI] [PubMed] [Google Scholar]
- Lappan, S. , Sibarani, M. , Rustiati, E. L. , & Andayani, M. (2017). Abrupt decline in a protected population of siamangs (Symphalangus syndactylus) in southern Sumatra. Folia Primatologica, 88, 255–266. 10.1159/000478776 [DOI] [PubMed] [Google Scholar]
- Lee, P. C. , & Priston, N. E. C. (2005). Human attitudes to primates: Perception of pests, conflict, and consequences for primate conservation, Commensalism and Conflict, the human–primate interface (pp. 1–23). Norman, OK: American Society of Primatologists. [Google Scholar]
- Lee, S. (2020). Rangers' movement restricted under MCO, illegal hunters taking advantage. The Star, April 29, 2020 Retrieved from https://www.thestar.com.my/news/nation/2020/2004/2024/rangers-movement-restricted-under-mco-illegal-hunters-taking-advantage [Google Scholar]
- Leng, S. , & Wan, L. (2020). Pangolin officially removed from TCM list. Global Times, June 9, 2020. Retrieved from https://www.globaltimes.cn/content/1191044.shtml?fbclid=IwAR1191040E1191824S1191041z1191047lXaAozZqnyD1191047Q1191047YxihseCbe1191046ftoluNUdVLA_NnFrnQiktPX1191048 [Google Scholar]
- Li, W. , Shi, Z. , Yu, M. , Ren, W. , Smith, C. , Epstein, J. H. , … Crameri, G. (2005). Bats are natural reservoirs of SARS‐like coronaviruses. Science, 310, 676–679. 10.1126/science.1118391 [DOI] [PubMed] [Google Scholar]
- Liu, P. , Jiang, J.‐Z. , Wan, X.‐F. , Hua, Y. , Li, L. , Zhou, J. , … Chen, J. (2020). Are pangolins the intermediate host of the 2019 novel coronavirus (SARS‐CoV‐2)? PLOS Pathogens, 16(5), e1008421 10.1371/journal.ppat.1008421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lécu, A. , & Ball, R. (2011). Mycobacterial infections in zoo animals: Relevance, diagnosis, and management. International Zoo Yearbook, 45, 183–202. [Google Scholar]
- Macfie, E. J. , & Williamson, E. A. (2010). Best practice guidelines for great ape tourism. Gland, Switzerland: IUCN/SSC Primate Specialist Group (PSG). [Google Scholar]
- Malaivijitnond, S. , & Hamada, Y. (2008). Current situation and status of long‐tailed macaques (Macaca fascicularis) in Thailand. The Natural History Journal of Chulalongkorn University, 8, 185–204. [Google Scholar]
- Malaivijitnond, S. , Vazquez, Y. , & Hamada, Y. (2011). Human impact on long‐tailed macaques in Thailand In Jones‐Engel L., Gumert M. D. & Fuentes A. (Eds.), Monkeys on the edge: Ecology and management of long‐tailed macaques and their interface with humans (pp. 118–158). Cambridge: Cambridge University Press. [Google Scholar]
- Mallapaty, S. (2020). Animal source of the coronavirus continues to elude scientists. Nature, May 18, 2020. 10.1038/d41586-41020-01449-41588 [DOI] [PubMed] [Google Scholar]
- Marty, P. R. , Balasubramaniam, K. N. , Kaburu, S. S. , Hubbard, J. , Beisner, B. , Bliss‐Moreau, E. , … McCowan, B. (2019). Individuals in urban dwelling primate species face unequal benefits associated with living in an anthropogenic environment. Primates, 61, 249–255. [DOI] [PubMed] [Google Scholar]
- Maréchal, L. , Semple, S. , Majolo, B. , & MacLarnon, A. (2016). Assessing the effects of tourist provisioning on the health of wild barbary macaques in Morocco. PLOS One, 11, e0155920 10.1371/journal.pone.0155920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Md‐Zain, B. M. , Ruslin, F. , & Idris, W. M. R. (2014). Human‐macaque conflict at the main campus of Universiti Kebangsaan Malaysia. Tropical Agricultural Science, 37, 73–85. [Google Scholar]
- Mehta, A. (2020). To prevent future pandemics 'no company should source from recently deforested land’. Ethical Corporation. May 2, 2020. Retrieved from http://www.ethicalcorp.com/prevent-future-pandemics-no-company-should-source-recently-deforested-land [Google Scholar]
- Melin, A. D. , Janiak, M. C. , Marrone, F. , Arora, P. S. , & Higham, J. P. (2020). Comparative ACE2 variation and primate COVID‐19 risk. Preprint in bioXriv 10.1101/2020.04.09.034967 [DOI] [PMC free article] [PubMed]
- Morrow, K. S. , Glanz, H. , Ngakah, P. O. , & Riley, E. P. (2019). Interactions with humans are jointly influenced by life history stage and social network factors and reduce group cohesion in moor macaques (Macaca maura). Scientific Reports, 9, 20162 10.1038/s41598-019-56288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse, S. S. , Mazet, J. A. K. , Woolhouse, M. , Parrish, C. R. , Carroll, D. , Karesh, W. B. , … Daszak, P. (2012). Prediction and prevention of the next pandemic zoonosis. The Lancet, 380, 1956–1965. 10.1016/S0140-6736(12)61684-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenbein, M. P. , & Wallis, J. (2017). Considering risks of pathogen transmission associated with primate‐based tourism In Russon A. & Wallis J. (Eds.), Primate tourism: A tool for conservation? (pp. 278–291). Cambridge: Cambridge University Press. [Google Scholar]
- Mätz‐Rensing, K. , Hartmann, T. , Wendel, G. M. , Frick, J. S. , Homolka, S. , Richter, E. , … Kaup, F.‐J. (2015). Outbreak of tuberculosis in a colony of rhesus monkeys (Macaca mulatta) after possible indirect contact with a human TB patient. Journal of Comparative Pathology, 153, 81–91. 10.1016/j.jcpa.2015.05.006 [DOI] [PubMed] [Google Scholar]
- Naher, H. , Khan, S. I. , & Ahmed, T. (2017). Threats and conservation problems of non‐human primates in moist deciduous forest of Bangladesh. Journal of the Asiatic Society of Bangladesh, Sciences, 43, 11–22. [Google Scholar]
- Ngernna, S. , Rachaphaew, N. , Thammapalo, S. , Prikchoo, P. , Kaewnah, O. , Manopwisedjaroen, K. , … Nguitragool, W. (2019). Case report: Case series of human Plasmodium knowlesi infection on the southern border of Thailand. American Journal of Tropical Medicine and Hygiene, 101, 1397–1401. 10.4269/ajtmh.19-0063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijman, V. , Spaan, D. , Rode‐Margono, E. J. , & Nekaris, K. A. I. (2017). Changes in the primate trade in Indonesian wildlife markets over a 25‐year period: Fewer apes and langurs, more macaques, and slow lorises. American Journal of Primatology, 79(11), e22517 10.1002/ajp.22517 [DOI] [PubMed] [Google Scholar]
- Ning, W.‐H. , Guan, Z.‐H. , Huang, B. , Fan, P.‐F. , & Jiang, X.‐L. (2019). Influence of food availability and climate on behavior patterns of western black crested gibbons (Nomascus concolor) at Mt. Wuliang, Yunnan, China. American Journal of Primatology, 81, e23068 10.1002/ajp.23068 [DOI] [PubMed] [Google Scholar]
- Nunn, C. L. (2006). Infectious Diseases in Primates: Behavior, Ecology, and Evolution. Oxford: Oxford University Press. [Google Scholar]
- Patrono, L. V. , Samuni, L. , Corman, V. M. , Nourifar, L. , Röthemeier, C. , Wittig, R. M. , … Leendertz, F. H. (2018). Human coronavirus OC43 outbreak in wild chimpanzees, Côte d'Ivoire. Emerging Microbes & Infections, 7, 118 10.1038/s41426-41018-40121-41422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters, M. , Courgnaud, V. , Abela, B. , Auzel, P. , Pourrut, X. , Bibollet‐Ruche, F. , … Delaporte, E. (2002). Risk to human health from a plethora of Simian Immunodeficiency Viruses in primate bushmeat. Emerging Infectious Diseases, 8(5), 451–457. 10.3201/eid0805.01-0522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phull, A. (2020). Covid‐19 ‘lockdown’: Monkeys desert Shimla, back to jungle for food! The Statesman, April 29, 2020. Retrieved from https://www.thestatesman.com/cities/shimla/covid-19-lockdown-monkeys-desert-shimla-back-jungle-food-1502872805.html [Google Scholar]
- Pope, K. (2020). How scientists are keeping irreplaceable research going during the COVID‐19 pandemic. Smithsonian, March 20, 2020. Retrieved from https://www.smithsonianmag.com/science-nature/science-canceled-coronavirus-180974449/?fbclid=IwAR180974440S180974440SMSCo180974440av180974499vUCdJY180974447wvAG180974475R180974443C180974444m180974445U180974445mF180974441U180974443RVe180974446qblzVgQLcCEZMk [Google Scholar]
- Priston, N. E. C. , & McLennan, M. R. (2013). Managing humans, managing macaques: Human–macaque conflict in Asia and Africa In Radhakrishna S., Huffman M. A. & Sinha A. (Eds.), The macaque connection (pp. 225–250). New York: Springer. [Google Scholar]
- Pulitzer, G. (2020). Monkeys plunder town hall building in South, apparently searching for food. The Thaiger, March 14, 2020. Retrieved from https://thethaiger.com/coronavirus/monkeys-plunder-town-hall-building-in-south-apparently-searching-for-food [Google Scholar]
- Radhakrishna, S. , Huffman, M. A. , & Sinha, A. (2013). The macaque connection: Cooperation and conflict between humans and macaques. New York: Springer. [Google Scholar]
- Radhakrishna, S. , & Sinha, A. (2011). Dr. Jekyll & Mr. Hyde: The strange case of human‐macaque interactions in India. Current Conservation, 4, 39–40. [Google Scholar]
- Rajkumar, R. P. (2020). COVID‐19 and mental health: A review of the existing literature. Asian Journal of Psychiatry, 52, 102066 10.1016/j.ajp.2020.102066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley, E. P. (2007). The human‐macaque interface: Conservation implications of current and future overlap and conflict in Lore Lindu National park, Sulawesi, Indonesia. American Anthropologist, 109, 473–484. [Google Scholar]
- Rockx, B. , Kuiken, T. , Herfst, S. , Bestebroer, T. , Lamers, M. M. , Munnick, B. B. O. , … Haagmans, B. L. (2020). Comparative pathogenesis of COVID‐19, MERS, and SARS in a nonhuman primate model. Science, 368(6494), 1012–1015. 10.1126/science.abb7314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, S. R. , Vreeman, V. M. , & Lonsdorf, E. V. (2011). Specific image characteristics influence attitudes about chimpanzee conservation and use as pets. PLOS One, 6, e22050 10.1371/journal.pone.0022050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbatini, G. , Stammatia, M. , Tavares, M. C. H. , Giuliani, M. V. , & Visalberghi, E. (2006). Interactions between humans and capuchin monkeys (Cebus libidinosus) in the Parque Nacional de Brasília, Brazil. Applied Animal Behaviour Science, 97, 272–283. [Google Scholar]
- Santos, W. J. , Guiraldi, L. M. , & Lucheis, S. B. (2020). Should we be concerned about COVID‐19 with nonhuman primates? American Journal of Primatology, early online, e23158 10.1002/ajp.23158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky, R. M. , & Share, L. J. (2004). A pacific culture among wild baboons: Its emergence and transmission. PLOS Biology, 2, 0534–0541. 10.1371/journal.pbio.0020106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, S. , Tojo, B. , Hoshi, T. , Minsong, L. I. f , Kugan, O. K. , Giloi, N. , … Kita, K. (2019). Recent incidence of human malaria caused by Plasmodium knowlesi in the villages in Kudat Peninsula, Sabah, Malaysia: Mapping of the infection risk using remote sensing data. International Journal of Environmental Research and Public Health, 16, 10.3390/ijerph16162954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, N. (2020). COVID‐19: Surge in illegal wildlife trading amid nationwide lockdown. DownToEarth, April 14, 2020. Retrieved from https://www.downtoearth.org.in/news/wildlife-biodiversity/covid-19-surge-in-illegal-wildlife-trading-amid-nationwide-lockdown-70434
- Shan, C. , Yao, Y.‐F. , Yang, X. , Zhou, Y.‐W. , Wu, J. , Gao, G. , … Yuan, Z. (2020). Infection with novel coronavirus (SARS‐CoV‐2) causes pneumonia in the rhesus macaques. Preprint with ResearchSquare, 10.21203/rs.2.25200/v1 [DOI] [PMC free article] [PubMed]
- Sharma, A. (2020). With lockdown easing, monkeys are back to trouble Shimla. Rediff News, May 15, 2020. Retrieved from https://www.rediff.com/news/report/shimla-sees-return-of-monkeys-as-coronavirus-lockdown-eases/20200515.htm [Google Scholar]
- Siamrath (2020). Local people are afraid that long‐tailed macaques at Khao Chongkrachok could be carriers for COVID‐19, and (the governor) suggests the locals keep a distance from monkeys (in Thai). Siamrath online, March 26, 2020. Retrieved from https://siamrath.co.th/n/142170 [Google Scholar]
- Singh, U. (2020). Covid‐19: Time for change at Sepilok orangutan centre. Malay Mail. 29 April 2020 https://www.malaymail.com/news/what-you-think/2020/2004/2029/covid-2019-time-for-change-at-sepilok-orangutan-centre-upreshpal-singh/1861291 [Google Scholar]
- Sinha, A. , & Mukhopadhyay, K. (2013). The monkey in the town's commons, revisited: An anthropogenic history of the Indian bonnet macaque In Radhakrishna S., Huffman M. A. & Sinha A. (Eds.), The macaque connection (pp. 187–208). New York: Springer. [Google Scholar]
- Sudhish, N. (2020). Residents relieved as monkeys go back to woods. The Hindu, April 18, 2020. Retrieved from https://www.thehindu.com/news/national/kerala/residents-relieved-as-monkeys-go-back-to-woods/article31378050.ece [Google Scholar]
- Switzer, W. M. , Bhullar, V. , Shanmugam, V. , Cong, M. , Parekh, B. , Lerche, N. W. , … Heneine, W. (2004). Frequent Simian Foamy Virus infection in persons occupationally exposed to nonhuman primates. Journal of Virology, 78, 2780–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, C. , Huang, L. , Huang, Z. , Krzton, A. , Lu, C. , & Zhou, Q. (2015). Forest seasonality shapes diet of limestone‐living rhesus macaques at Nonggang, China. Primates, 57, 83–92. 10.1007/s10329-015-0498-7 [DOI] [PubMed] [Google Scholar]
- Thaitrakulpanich, A. (2020). Lopburi's monkeys food war blamed on plunge in tourism. Khaosod English, March 12, 2020. Retrieved from https://www.khaosodenglish.com/news/2020/2003/2012/lopburis-monkeys-food-war-blamed-on-plunge-in-tourism/ [Google Scholar]
- Tischer, B. K. , & Osterrieder, N. (2010). Herpesviruses—A zoonotic threat? Veterinay Microbiology, 140, 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TripAdvisor . (2014). Kuala Selangor Nature Park (Taman Alam Kuala Selangor). TripAdvisor, April 25, 2020. Retrieved from https://www.tripadvisor.com.my/ShowUserReviews-g1777471-d1971956-r226199267-Kuala_Selangor_Nature_Park_Taman_Alam_Kuala_Selangor-Kuala_Selangor_Selangor.html [Google Scholar]
- United Nations . (2020). Coronavirus impact on world's indigenous, goes well beyond health threat. UN News, May 18, 2020. Retrieved from https://news.un.org/en/story/2020/2005/1064322. [Google Scholar]
- Van Lancker, W. , & Parolin, Z. (2020). COVID‐19, school closures, and child poverty: A social crisis in the making. The Lancet, 5, E243–E244. 10.1016/S2468-2667(20)30084-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viner, R. M. , Russell, S. J. , Croker, H. , Packer, J. , Ward, J. , Stansfield, C. , … Booy, R. (2020). School closure and management practices during coronavirus outbreaks including COVID‐19: A rapid systematic review. The Lancet Child and Adolescent Health, 4, 397–404. 10.1016/S2352-4642(20)30095-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyawahare, M. (2020a). Chinese ban on eating wild animals likely to become law: Q&A with WCS's Aili Kang. Mongabay, April 9, 2020. Retrieved from https://news.mongabay.com/2020/2004/chinese-ban-on-eating-wild-animals-likely-to-become-law-qa-with-wcss-aili-kang/ [Google Scholar]
- Vyawahare, M. (2020b). National parks in Africa shutter over COVID‐19 threat to great apes. Mongabay, March 26, 2020. Retrieved from https://news.mongabay.com/2020/2003/national-parks-in-africa-shutter-over-covid-2019-threat-to-great-apes/ [Google Scholar]
- Walzer, C. (2020). The COVID‐19 pandemic has introduced us to a new word: Zoonosis. LiveScience, April 29, 2020. Retrieved from https://www.livescience.com/dangers-of-zoonoses-pandemics.html [Google Scholar]
- WCS . (2020). Three critically endangered giant Ibis—Cambodia's national bird—killed in protected area. Wildlife Conservation Society, April 29, 2020. Retrieved from https://newsroom.wcs.org/News-Releases/articleType/ArticleView/articleId/14039/COVID-14019-FUELING-AN-UPTICK-IN-POACHING-Three-Critically-Endangered-Giant-Ibis-Cambodias-National-Bird-killed-in-Protected-Area.aspx [Google Scholar]
- Wescott, B. , & Deng, S. (2020). China has made eating wild animals illegal after the coronavirus outbreak. But ending the trade won't be easy. CNN Online, March 6, 2020. Retrieved from https://edition.cnn.com/2020/2003/2005/asia/china-coronavirus-wildlife-consumption-ban-intl-hnk/index.html [Google Scholar]
- WHO . (2020). Considerations for quarantine of individuals in the context of containment for coronavirus disease (COVID‐19): Imterim guidance, March 19, 2020. WHO/2019‐nCoV/IHR_Quarantine/2020.2012.
- Williamson, E. A. , & Feistner, A. T. C. (2011). Habituating primates: Processes, techniques, variables and ethics In Setchell J. M. & Curtis D. J. (Eds.), Field and laboratory methods in primatology: A practical guide (pp. 33–50). Cambridge: Cambridge University Press. [Google Scholar]
- World Health Organization . (2020). Coronavirus disease (COVID‐19). World Health Organization Situation Report, 152 Retrieved from https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200620-covid-20200619-sitrep-20200152.pdf?sfvrsn=20200683aff20200628ee_20200622 [Google Scholar]
- WWF . (2020). Opinion survey on COVID‐19 and wildlife trade in five Asian markets, April 6, 2020. Retrieved from https://www.worldwildlife.org/publications/opinion-survey-on-covid-19-and-wildlife-trade-in-five-asian-markets
- Yadav, P. D. , Patil, S. , Jadhav, S. M. , Nyayanit, D. A. , Kumar, V. , Jain, S. , … Cherian, S. S. (2020). Phylogeography of Kyasanur Forest Disease virus in India (1957–2017) reveals evolution and spread in the Western Ghats region. Scientific Reports, 10, 1966 Retrieved from https://www.nature.com/articles/s41598-41020-58242-w.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T. , Wu, Q. , & Zhang, Z. (2020). Probable pangolin origin of SARS‐CoV‐2 associated with the COVID‐19 Outbreak. Current Biology, 30, 1346–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X.‐L. , Wang, X.‐G. , Hu, B. , Zhang, L. , Zhang, W. , … Shi, Z.‐L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579, 270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Q. , & Deng, Z. (1992). Dramatic consequences of food handouts to Macaca thibetana at Mount Emei, China. Folia Primatologica, 58, 24–31. [Google Scholar]


