Abstract
We aim to systematically review the characteristics of asymptomatic infection in the coronavirus disease 2019 (COVID‐19). PubMed and EMBASE were electronically searched to identify original studies containing the rate of asymptomatic infection in COVID‐19 patients before 20 May 2020. Then mate‐analysis was conducted using R version 3.6.2. A total of 50 155 patients from 41 studies with confirmed COVID‐19 were included. The pooled percentage of asymptomatic infection is 15.6% (95% CI, 10.1%‐23.0%). Ten included studies contain the number of presymptomatic patients, who were asymptomatic at screening point and developed symptoms during follow‐up. The pooled percentage of presymptomatic infection among 180 initially asymptomatic patients is 48.9% (95% CI, 31.6%‐66.2%). The pooled proportion of asymptomatic infection among 1152 COVID‐19 children from 11 studies is 27.7% (95% CI, 16.4%‐42.7%), which is much higher than patients from all aged groups. Abnormal CT features are common in asymptomatic COVID‐19 infection. For 36 patients from 4 studies that CT results were available, 15 (41.7%) patients had bilateral involvement and 14 (38.9%) had unilateral involvement in CT results. Reduced white blood cell count, increased lactate dehydrogenase, and increased C‐reactive protein were also recorded. About 15.6% of confirmed COVID‐19 patients are asymptomatic. Nearly half of the patients with no symptoms at detection time will develop symptoms later. Children are likely to have a higher proportion of asymptomatic infection than adults. Asymptomatic COVID‐19 patients could have abnormal laboratory and radiational manifestations, which can be used as screening strategies to identify asymptomatic infection.
Keywords: asymptomatic infection, children coronavirus disease 2019, Coronavirus disease 2019, COVID‐19, presymptomatic infection, SARS‐CoV‐2
Highlights
By systematically reviewing the proportion and clinical features of asymptomatic infection in COVID‐19, our study provides a useful quantity to understand the true burden of this disease.
1. BACKGROUND
The current COVID‐19 pneumonia pandemic, caused by a novel coronavirus SARS‐CoV‐2 that belongs to the beta‐coronavirus lineage B, is spreading globally at an accelerated rate. First reported in a seafood market in Wuhan province China in December 2019, 1 this disease is now affecting more than 156 countries around the world. As of 5 June 2020, a total number of 4 248 389 laboratory‐confirmed cases have been documented globally, leading to 294 046 deaths, 2 which is far more than two previously identified coronaviruses SARS‐CoV (2003) and MERS‐CoV (2012) that cause Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome (MERS) did.
Clinical manifestation of COVID‐19 is protean. Significant clinical presentations of COVID‐19 include fever, respiratory and gastrointestinal symptoms, pneumonia, 3 and other symptoms such as myasthenia, ageusia, and anosmia. 4 However, patients infected with SARS‐CoV‐2 could also be asymptomatic, confirmed by positive Nucleic acid testing results during the illness. As a potential source of COVID‐19 infection, asymptomatic patients with subclinical manifestation could be missed by detection strategies and put a threat to infection control via person‐to‐person contact. Asymptomatic cases inevitably distorting the COVID‐19 epidemiologic reality. While a variety of studies on asymptomatic infection have been reported, the proportion of asymptomatic patients in confirmed COVID‐19 cases is not well characterized. We conducted this meta‐analysis to better understand the asymptomatic infection of COVID‐19.
2. MATERIALS AND METHOD
2.1. Studies selection
Two databases including PubMed and Embase were searched before 20 May 2020 following the PRISMA guideline. We included the following items:
#1: “COVID‐19” OR “2019 novel coronavirus disease” OR “COVID19” OR”COVID‐19 pandemic” OR “SARS‐CoV‐2 infection” OR “COVID‐19 virus disease” OR “coron121avirus disease‐19” OR “2019 novel coronavirus infection” OR “2019‐nCoV infection” OR “coronavirus disease 2019” OR “2019‐nCoV disease” OR”COVID‐19 virus disease”
#2: “Asymptomatic”
#3: #1 AND #2
We included articles reporting a specific number of asymptomatic infection cases in confirmed COVID‐19 patients. Information describing the epidemiological and clinical features of COVID‐19 asymptomatic infection were extracted from studies to obtain epidemiological and clinical features of asymptomatic infection.
2.2. Selection criteria
Records were identified through database searching. Confirmed COVID‐19 was defined as one that had a throat‐swab or other specimen tested positive for SARS‐CoV2 using real‐time RT‐PCR assay. Asymptomatic infection was defined as patients who developed no symptoms such as fever, cough, or diarrhea during illness. A presymptomatic case was defined as a patient who has no symptoms at diagnosis time but developed symptoms during follow‐up. Patients with no symptoms at screening point were defined as the number of asymptomatic patients plus the number of presymptomatic patients. Two authors (He and Guo) extracted data independently. Disagreements were resolved by discussion until consensus was reached or by consulting a third author. Including criteria included: (a) Study objectives: Patients confirmed infected with SARS‐CoV‐2 (including adult, pediatric patients, and pregnant women). (b) Study types: prospective/retrospective cross‐section cohort studies. There was no language restriction. Original articles reporting asymptomatic infection in confirmed COVID‐19 patients were included for meta‐analysis.
The methodological quality of the studies included in meta‐analysis was assessed using an 11‐item checklist which was recommended by Agency for Healthcare Research and Quality (AHRQ). If an item was answered “NO” or “UNCLEAR” it would be scored “0” and if it was answered “YES,” then the item scored “1.” Article quality was assessed as follows: low quality = 0 to 3; moderate quality = 4 to 7; high quality = 8 to 11.
2.3. Data extraction
After removing the duplicates, the abstract review was conducted through titles and abstracts. The following data were extracted: author, date of publication, site of study, study group, total number of people included in the study, age, sex, the number of asymptomatic infections, and the number of presymptomatic infection in patients if available. For detail information of asymptomatic patients, information containing age, sex, conversion time of illness (the time between the first day with a positive reverse transcription‐polymerase chain reaction [RT‐PCR] result and the day of a second negative RT‐PCR result), laboratory analysis results and CT examination results were extracted if available.
2.4. Data analysis
All statistical analysis was performed using R version 3.6.2 (R Foundation for Statistical Computing) statistical software and Rstudio. Packages “meta,” “metafor,” and “weightr” were used. The proportion of asymptomatic infection was transformed using the logit transformation to make it conform to the normal distribution. A random effects model was applied to calculate the effect size and its 95% confidence interval (95% CI) by the method of moments (the Dorsmanin and Laird method) and as presented by Forest plot. The tau2 and I 2 statistic was used to estimate the proportion of the observed heterogeneity. Studies containing the number of presymptomatic patients were extracted to analyze the proportion of presymptomatic infection in patients with no symptoms at screening point. Untransformed proportions and a random effects model by the method of moments (the Dorsmanin and Laird method) were applied to calculate the effect size and its 95% confidence interval (95% CI) and as presented by Forest plot. Leave‐one‐out diagnostics and regression diagnostics were used to identify influential studies that pronouncedly contribute to heterogeneity in meta‐analytic data. Meta‐analysis via linear was conducted to find the factor attributing to the overall heterogeneity, which was described in the article published by Wang. 5 Subgroup summary proportion analysis were conducted to explain the factor contributing to heterogeneity. Then subgroups forest plot was created by different study group: all, children, pregnant women or elderly people, and different place: China or outside of China. Publication bias was detected with funnel plot and Egger's regression test.
3. RESULTS
3.1. Study characteristic of meta‐analysis
Study process is depicted in Figure S1. Of the 470 studies identified, 40 studies 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 and 1 additional study 46 including 50155 patients were included in the meta‐analysis. Place where the study was conducted, age, sex, and reported proportion of asymptomatic infection, number of presymptomatic infection, and quality scores were abstracted (Table S1). There were 25 (59.5%) studies from China and 16 from other countries (South Korea: 4, United States of America: 3, Europe region: 3, UK: 2, Brunei: 1, Iraqi Kurdistan: 1, Thailand: 1, and Japan: 1). All studies were of high (27) or moderate (14) quality. There were no articles with low quality rating.
3.2. Results of meta‐analysis
3.2.1. Pooled proportion of asymptomatic infection
A total of 50 155 patients with confirmed COVID‐19 were included. The pooled percentage of all asymptomatic infection is 15.6% (95% CI, 10.1%‐23.0%) with significant heterogeneity noted among studies (P < .01; Q, 1653.8; tau2, 2.34; I 2, 97.6%) (Figure 1).
Figure 1.
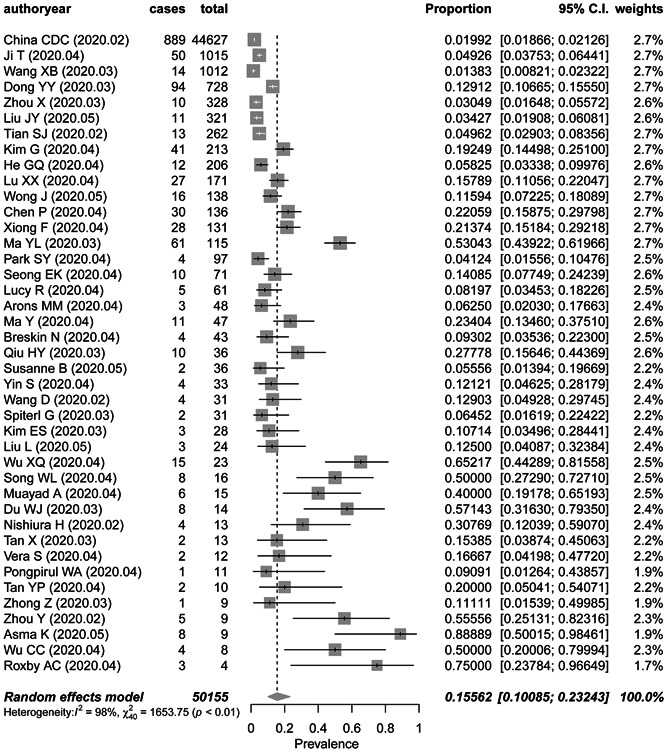
Frost plot of the proportion of asymptomatic infection in COVID‐19 patients
3.2.2. Proportion of presymptomatic infection
There was a total of 10 studies containing the number of patients who were identified as silent COVID‐19 patients but developed symptoms during follow‐up. A total of 180 initial no‐symptoms COVID‐19 patients were included. The pooled percentage of presymptomatic infection among patients with no symptoms at screening point is 48.9% (95% CI, 31.6‐66.2%) with heterogeneity noted among studies (P < .01; I 2, 85%) (Figure 2).
Figure 2.

Frost plot of the proportion of presymptomatic infection in initial no‐symptom COVID‐19 patient
3.2.3. Subgroup meta‐analysis
Study group
There were 24 studies of 48 868 people in study cohorts from all age groups, 11 studies of 1152 children, 3 studies of 75 elderly people, and 4 studies of 83 pregnant women. The pooled prevalence of asymptomatic infection was 9.0% (95% CI, 5.5%‐14.6%), 27.7% (95% CI, 16.4%‐42.7%), 28.3% (95% CI, 0.94%‐94.2%), and 49.9% (95% CI, 14.9%‐84.9%) in studies from all aged group, children, the elderly, and pregnant women respectively (Figure 3). There was a significant subgroup difference between the studies (P = .0041).
Figure 3.
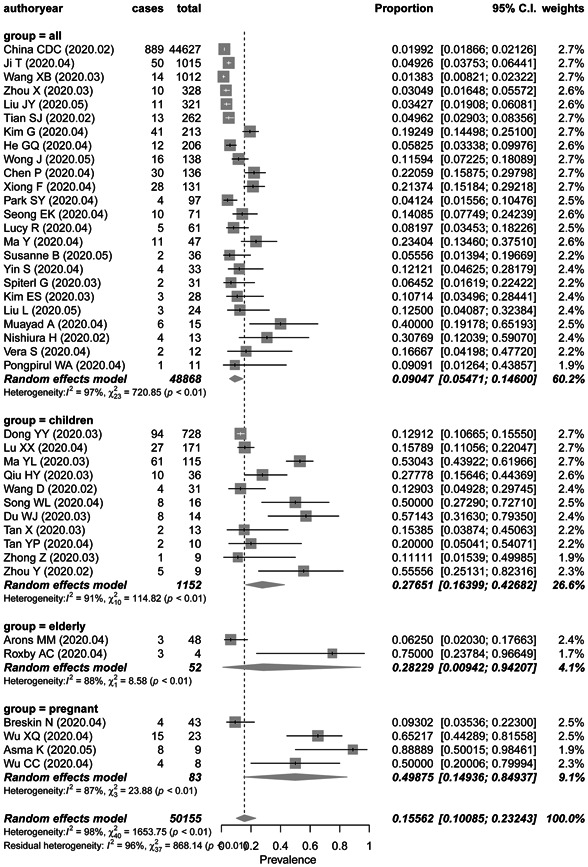
Frost plot of the proportion of asymptomatic infection in COVID‐19 patients by study group
Study place
The pooled prevalence of asymptomatic infection was 15.5% (95% CI, 8.8%‐25.7%) and 14.5% (95% CI, 9.8%‐21.1%) in studies from China and other countries respectively (Figure 4). The P value between these two groups is .8313 with no significance. There was significant heterogeneity among the studies conducted in China (P < .01; I 2 = 98.3%) and fewer heterogeneity studies from other countries (P < .01; I 2 = 70.1%).
Figure 4.
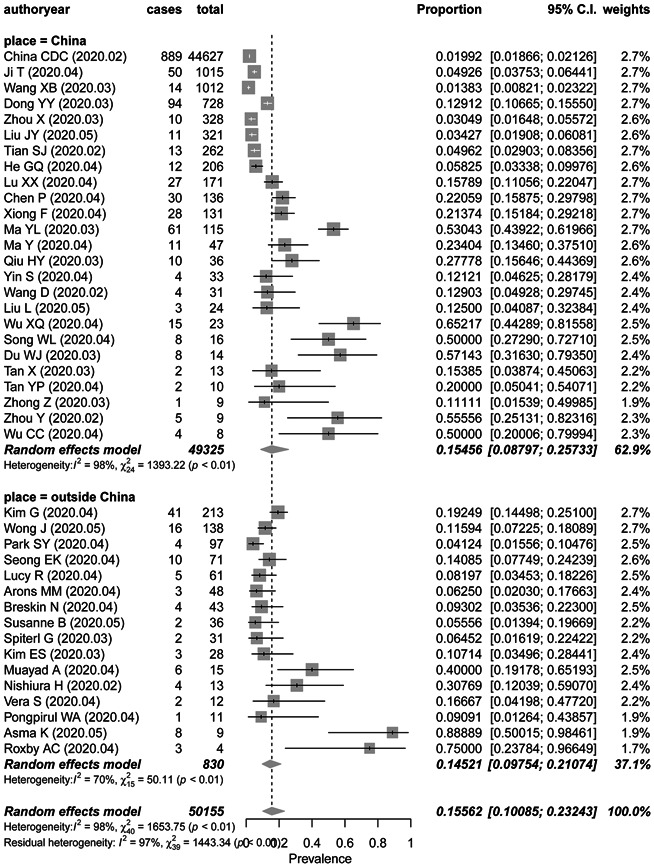
Frost plot of the proportion of asymptomatic infection in COVID‐19 patients by study place
3.2.4. Sensitivity analysis and publication bias
Leave‐one‐out diagnostics (Figure S2) and regression diagnostics (Figure S3) showed that no specific study has a pronounced impact on the original summary proportion. Meta‐analysis via linear was conducted to explaining heterogeneity by different independent variables: study place, study group, quality scores, and the number of confirmed cases. The P values were .96, .0005, .06, and .0028 differently for them. Different study group and sample size may account for the high heterogeneity among studies. The Funnel plot (Figure 5) and Egger's regression test indicate that there may be publication bias (t = 5.65; P < .0001).
Figure 5.
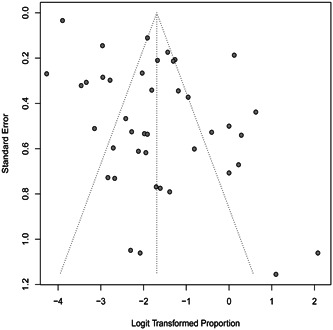
Funnel plot based on the proportion of asymptomatic infection for evaluation of publication bias
3.3. Clinical features of asymptomatic infection
Fifty‐nine patients from four studies included in the meta‐analysis 9 , 26 , 27 , 28 and one additional case series study 47 were included. A summary of the characterizes of asymptomatic COVID‐19 infection is shown in Table 1. Illness duration ranged from 3 to 34 days. CT imaging results could be normal and abnormal. For 36 patients from 4 studies, 15 (41.7%) had bilateral involvement and 14 (38.9%) had unilateral involvement in CT results. Some patients may have abnormal laboratory results. Detail information was available from two studies respectively conducted by Ma et al 26 and Xu et al. 47 In those two studies, 27.3% (3/26) of asymptomatic patients had reduced white blood cell count, 42.3% (11/26) of patients showed increased lactate dehydrogenase, and 11.5% (3/26) of patients recorded increased C‐reactive protein. Increased creatine kinase‐MB, both decreased lymphocyte count and increased lymphocyte count were also recorded in those two studies.
Table 1.
Characteristics of the studies included for clinical characteristics of asymptomatic infection
| (ID) Study | (20) Ma Y | (13) Xiong F | (9) He GQ | (32) Du WJ | Xu TM |
|---|---|---|---|---|---|
| Number of asymptomatic patients | 11 | 28 | 12 | 8 | 15 |
| Age (range) | 23 (1‐60) | 62.1 | 31 (24‐51) | NA | 27 |
| Male (percentage) | 6 (54.5%) | 15 (53.6%) | 6 (50%) | 5 (62.5%) | 10 (66.7%) |
| Conversion time, d | 10 (3‐34) | NA | NA | 5.43 | NA |
| Hospital stay, d | 14 (10‐30) | NA | NA | NA | NA |
| Abnormal CT result | 7 (63.6%) | NA | NA | 5 (62.5%) | NA |
| Bilateral involvement (CT) | 4 | NA | 4 | 3 | 4 |
| Unilateral involvement (CT) | 3 | NA | 5 | 2 | 4 |
| White blood cell count (109/L) | NA | 5.3 (3.5‐7.9) | 6.1 (4.6‐6.9) | 7.11 | 6.3 (4.8‐8.1) |
| Decreased | 3 (27.3%) | NA | NA | NA | 0 |
| Lymphocyte count (109/L) | NA | 1.0 (0.8‐1.3) | 1.9 (1.5‐2.1) | 4.64 | 2.3 (1.7‐3.4) |
| Increased | 4 (36.4%) | NA | NA | NA | NA |
| Decreased | 0 (0.0%) | NA | NA | NA | 1 (6.7%) |
| Neutrophils count (109/L) | NA | 4.0 (2.6‐6.4) | 3.4 (2.6‐5.0) | 1.93 | 3.0 (2.7‐4.6) |
| Hemoglobin, g/L | NA | 110.0 (91.0‐121.0) | 136.0 (121.0‐144.0) | 131.63 | 138.0 (131.0‐162.0) |
| PLT (109/L) | NA | 151.5 (108.9‐191.0) | 272.0 (210.0‐311.0) | 260.88 | 214.0 (142.0‐277.0) |
| C‐reaction protein, mg/L | NA | NA | 0.80 (0.46‐1.10) | 0.65 | NA |
| Increased | 1 (9.1%) | NA | NA | NA | 2 (2/14, 14.3%) |
| Lactate dehydrogenase, U/L | NA | NA | 154.0 (134.0‐182.0) | 333.03 | 195.0 (166.0‐388.0) |
| Increased | 5 (45.5%) | NA | NA | NA | 6 (40%) |
| Creatine kinase, U/L | NA | NA | 65.1 (36.0‐73.9) | 116.85 | NA |
| Increased | 6 (54.5%) | NA | NA | NA | NA |
| Level of D‐dimer, µg/mL | NA | NA | NA | 0.49 | 0.2 (0.1‐ 0.3) |
| ESR, mg/L | NA | NA | 14 (10‐15) | NA | NA |
4. DISCUSSION
This study conducted a meta‐analysis studying the epidemiological and clinical characteristics of asymptomatic COVID19 patients. 50155 confirmed COVID‐19 patients from 41 studies were included and the pooled proportion of asymptomatic infection is 15.6%. Meanwhile nearly half of the patients who were asymptomatic at screening time may develop symptoms during follow‐up. Our results also show that there is no significant difference in the percentage of asymptomatic infection between studies conducted in China or other countries. Meantime, 11 studies whose research objects are children, got a pooled asymptomatic proportion of 27.7% among confirmed cases. This result is much higher than the result obtained from all aged groups.
Many viral infections are associated with asymptomatic, subclinical, or very mild symptoms. Influenza was estimated to be 5.2% to 35.5%. 48 Asymptomatic infections were also reported during SARS and MERS. Our result of asymptomatic proportion is lower than in many independent studies. In the study conducted by Nishiura et.al estimating the asymptomatic ratio of COVID‐19 by using the information on Japanese chartered flights evacuated from Wuhan, China, this number was calculated to be 30.8%. 19 This could be explained by the relatively small observation sample size. Presymptomatic is common in patients who had no symptoms at diagnosis. Those patients developed symptoms later during follow‐up and are easy to be mistakenly classified as asymptomatic patients if the observation time is not long enough, which disturbs figuring out the true burden of asymptomatic infection. In 10 studies containing the number of presymptomatic infection, the pooled proportion of presymptomatic infection of COVID‐19 among no‐symptoms patients at screening time is 48.8%. This result indicates that nearly half of the patients who were diagnoses with COVID‐19 asymptomatically at screening time may be in their incubation period and develop symptoms later in the nature course of the disease. In a study reporting 55 asymptomatic cases, even admitted asymptomatically, 39 of them developed symptoms and two of them even developed severe COVID‐19 during hospitalization. 49 In one extreme case, an asymptomatic patient did not show any symptoms of COVID‐19 until her sudden death due to arterial and venous thromboembolic events of COVID‐19. 50
Studies showed that most children's cases were less severe than adults. The main reason why the majority of children had a benign course of illness with mild respiratory symptoms is still unknown. This may be explained by host factors. Angiotensin‐converting enzyme 2 (ACE2), the main means of pathogenesis, is significantly lower in children than in adults. Moreover, children's immune function is less mature than adults with a blunt immune response in SARS‐CoV2 infection. This may highlight a possible likelihood of underestimation of children COVID‐19 patients, owing to the not so ideal performance of current screening strategy relying hugely on clinical symptoms to detect COVID‐9. Some studies also showed that the median age of asymptomatic patients is younger than the symptomatic patients. 26 The elderly and pregnant women also showed higher proportions of asymptomatic infection in our subgroup meta‐analysis. Reasons may be explained by the relatively small sample size of these two types of patients. Special immune system states may also be a possible reason for this phenomenon.
Clinical manifestations of asymptomatic patients show that most asymptomatic patients were moderate in their clinical manifestations and stay asymptomatic until their RNA testing turned negative. Some transient symptoms were recorded in some studies. In the study conducted by Lee et al, acute anosmia or ageusia was observed in 15.7% (367/2342) patients with asymptomatic‐to‐mild disease severity owing to damage to the olfactory nerve during invasion and multiplication of SARS‐CoV‐2. 4 An asymptomatic patient can also have a transient high temperature 51 or a slightly dry cough during illness. 52 COVID‐19 patients may show varying degrees of laboratory abnormalities, for example, leukopenia, increased lactate dehydrogenase, lymphocytosis, lymphopenia, etc. We still don't know whether there are differences in laboratory test results between asymptomatic patients and symptomatic patients. Though imaging examination could be a potential approach to identify asymptomatic COVID‐19 patients. Even without clinical features, some asymptomatic patients do have abnormal CT features indicating pulmonary involvement, 53 which is mainly patchy shadowing and GGOs, demonstrating that chest CT method could be helpful to screen asymptomatic COVID‐19 patients. IgG and IgM levels of the patients showed a gradually increasing trend during COVID‐19. Noticeably, one study from Wuhan showed that 98/1021(9.6%) nucleic acid testing negative patients had lgG positive results, suggesting possible recovery from asymptomatic SARS‐CoV‐2 infection. 54 A study from Germany also demonstrates the importance of serological tests in COVID‐19. In 5/316(1.6%) healthcare workers SARS‐CoV‐2‐IgG antibodies could be detected. Four of the five subjects were tested negative for SARS‐CoV‐2 via PCR. One subject was not tested via PCR since he was asymptomatic. 55 All those results suggest that asymptomatic patients could use serological tests to detect COVID‐19 infection.
Asymptomatic infection was believed to be less contagious as a consequence of a decreased virulence throughout the successive transmission, like SARS‐CoV. In the study conducted by Schwierzeck et al, 36 the viral load of six asymptomatic patients is lower than six symptomatic cases. This result was supported by a mass screening by Rivett et al of health care workers as well as their contacts in the UK. Viral loads were significantly lower for 31 asymptomatic health care workers screening group than in those 30 individuals tested positive due to the presence of symptoms. 42 However, it's still too early to conclude that asymptomatic patients are less likely to transmit the virus. Relatively high viral load was also detected in asymptomatic patients 51 , 56 and the stool sample was tested positive in a well infant of COVID‐19, 51 a man in his 20s 57 and a 10 years old boy in Zhejiang, China. 58 The role of asymptomatic patients in potential transmission of infection to close contact is still a concern. The study conducted by Hu et al certificated that asymptomatic carriers can result in person‐to‐person transmission and should be considered a source of COVID‐19. Case 13 in that study transmitted the virus to his cohabiting family members and one of the infected individuals developed severe COVID‐19 pneumonia. 59 In a family cluster report, the index patient is asymptomatic during hospitalization. 60 A familial cluster of five patients with COVID‐19 pneumonia in Anyang, China, had contact with an asymptomatic family member before their symptom onset. 61 Presymptomatic patient can also transmit the virus by close contact. 62 Laboratory screening tests should be regarded as part of active case monitoring and contact investigations. When asymptomatic patients are identified, it is better to put the patients under monitor.
There are highlights in our studies. First, by using logit transversion of the original data, the pooled proportion of asymptomatic infection was more accurately estimated. Besides, the pooled percentage of presymptomatic infection in patients without symptoms at screening point was also analyzed in the 10 studies containing the detailed information. Meanwhile, there are limitations to our study. First of all, some including studies' observation time were not long enough or did not record negative PCR results of patients. This may lead to some presymptomatic cases to be mistaken for asymptomatic patients. Second, recalling bias may exist, some studies recorded symptoms of patients mainly basing on self‐reporting. Some asymptomatic patients may have symptoms before screening and thus symptomatic person might have failed to report mild or subclinical symptoms after symptoms resolved. Third, serum viral conversion time and treatments are not available in many articles, making it's hard to conclude whether asymptomatic patients are more likely to clear virus shedding in organs and whether those patients with slightly clinical manifestations should undergo routine treatment or should only take quarantine till recovery. More studies are needed to get a comprehensive understanding of asymptomatic infection of COVID‐19 to guide the prevention measures employed in the real‐world.
5. CONCLUSION
Probing into asymptomatic infection proportion is a useful quantity to understand the true burden of disease transmission. In our meta‐analysis, asymptomatic infection is estimated to be 15.6% of all confirmed cases. Nearly half of the patients who have no symptoms at the screening point can develop symptoms during follow‐up. Children are likely to have a higher proportion of asymptomatic infection of COVID‐19 than adults. One‐third of confirmed children with COVID‐19 are asymptomatic. A comprehensive analysis of a possible patient's epidemical history, nucleic acid tests, serological tests, and imaging test results are required to identify asymptomatic infections of COVID‐19 to intercept the transmission of this virus.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Data curation, methodology, and writing‐original draft: JH and YG. Funding acquisition, writing‐review, and editing, and supervision: JZ and RM. Software: JH.
Supporting information
Supporting information
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
The present study was supported by the Shanghai Pujiang Pujiang Program (17PJD005) and the National Natural Science Foundation of China (81670528, 81672009, and 81871640).
He J, Guo Y, Mao R, Zhang J. Proportion of asymptomatic coronavirus disease 2019: A systematic review and meta‐analysis. J Med Virol. 2021;93:820–830. 10.1002/jmv.26326
Contributor Information
Jingjing He, Email: jingjinghe19@fudan.edu.cn.
Richeng Mao, Email: jmzhang@fudan.edu.cn, Email: richengmao@gmail.com.
Jiming Zhang, Email: jmzhang@fudan.edu.cn, Email: richengmao@gmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Coronavirus Disease (COVID‐19) Dashboard. https://covid19.who.int/
- 3. Del Rio C, Malani PN. COVID‐19‐new insights on a rapidly changing epidemic. JAMA. 2020;323(14):1339‐1340. [DOI] [PubMed] [Google Scholar]
- 4. Lee Y, Min P, Lee S, Kim SW. Prevalence and duration of acute loss of smell or taste in COVID‐19 patients. J Korean Med Sci. 2020;35(18):e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang N How to conduct a meta‐analysis of proportions in R: a comprehensive tutorial. 2018.
- 6. Tian S, Hu N, Lou J, et al. Characteristics of COVID‐19 infection in Beijing. J Infect. 2020;80(4):401‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Ju XL, Xie F, et al. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua er ke za zhi. 2020;58(4):269‐274. [DOI] [PubMed] [Google Scholar]
- 8. Qiu H, Wu J, Hong L, Luo Y, Song Q, Chen D. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID‐19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689‐696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Du W, Yu J, Wang H, et al. Clinical characteristics of COVID‐19 in children compared with adults in Shandong Province, China. Infection. 2020;48:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang X, Fang J, Zhu Y, et al. Clinical characteristics of non‐critically ill patients with novel coronavirus infection (COVID‐19) in a Fangcang Hospital. Clin Microbiol Infect. 2020;26:1063‐1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pongpirul WA, Mott JA, Woodring JV, et al. Clinical characteristics of patients hospitalized with coronavirus disease, Thailand. Emerging Infect Dis. 2020;26:7‐1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim ES, Chin BS, Kang CK, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean Cohort Study on COVID‐19. J Korean Med Sci. 2020;35(13):e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou Y, Yang GD, Feng K, et al. Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(3):215‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Am J Obstet Gynecol MFMBreslin N, Baptiste C, Gyamfi‐Bannerman C, et al. COVID‐19 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020:100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roxby AC, Greninger AL, Hatfield KM, et al. Detection of SARS‐CoV‐2 among residents and staff members of an independent and assisted living community for older adults‐Seattle, Washington, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):416‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan YP, Tan BY, Pan J, Wu J, Zeng SZ, Wei HY. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol. 2020;127:104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi. 2020;41(2):145‐151. [DOI] [PubMed]
- 18. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145. [DOI] [PubMed] [Google Scholar]
- 19. Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID‐19). Int J Infect dis. 2020;94:154‐155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spiteri G, Fielding J, Diercke M, et al. First cases of coronavirus disease 2019 (COVID‐19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25(9):200178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou X, Li Y, Li T, Zhang W. Follow‐up of asymptomatic patients with SARS‐CoV‐2 infection. Clin Microbiol Infect. 2020;26:957‐959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ji T, Chen HL, Xu J, et al. Lockdown contained the spread of 2019 novel coronavirus disease in Huangshi city, China: early epidemiological findings. Clin Infect Dis. 2020:ciaa390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu X, Sun R, Chen J, Xie Y, Zhang S, Wang X. Radiological findings and clinical characteristics of pregnant women with COVID‐19 pneumonia. Int J Gynaecol Obstet. 2020;150:58‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim G, Kim MJ, Ra SH, et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID‐19. Clin Microbiol Infect. 2020;26:948.e1‐948.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu JY, Chen TJ, Hwang SJ. Analysis of imported cases of COVID‐19 in Taiwan: a nationwide study. Int J Environ Res Public Health. 2020;17:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma Y, Xu Q, Wang F, et al. Characteristics of asymptomatic patients with SARS‐CoV‐2 infection in Jinan, China. Microb Infect. 2020;22:212‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xiong F, Tang H, Liu L, et al. Clinical characteristics of and medical interventions for COVID‐19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020;31:1387‐1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cai X, Xu Y, Yu K, et al. The clinical feature of silent infections of novel coronavirus infection (COVID‐19) in Wenzhou. J Med Virol. 2020;84:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tan X, Huang J, Zhao F, Zhou Y, Li JQ, Wang XY. Clinical features of children with SARS‐CoV‐2 infection: an analysis of 13 cases from Changsha, China. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(4):294‐298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma YL, Xia SY, Wang M, Zhang SM, Du WH, Chen Q. Clinical features of children with SARS‐CoV‐2 infection: an analysis of 115 cases. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22(4):290‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song W, Li J, Zou N, Guan W, Pan J, Xu W. Clinical features of pediatric patients with coronavirus disease (COVID‐19). J Clin Virol. 2020;127:104377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu C, Yang W, Wu X, et al. Clinical manifestation and laboratory characteristics of SARS‐CoV‐2 infection in pregnant women. Virol Sin. 2020;35:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Park SY, Kim YM, Yi S, et al. Coronavirus disease outbreak in call center, South Korea. Emerging Infect Dis. 2020;26:8‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Merza MA, Haleem Al, Mezori AA, Mohammed HM, Abdulah DM. COVID‐19 outbreak in Iraqi Kurdistan: the first report characterizing epidemiological, clinical, laboratory, and radiological findings of the disease. Diabetes Metab Syndr. 2020;14(4):547‐554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen P, Zhang Y, Wen Y, et al. Epidemiological and clinical characteristics of 136 cases of COVID‐19 in main district of Chongqing. J Formosan Med Assoc. 2020;119:1180‐1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwierzeck V, König JC, Kühn J, et al. First reported nosocomial outbreak of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in a pediatric dialysis unit. Clin Infect Dis. 2020: ciaa491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wong J, Abdul Aziz ABZ, Chaw L, et al. High proportion of asymptomatic and presymptomatic COVID‐19 infections in travelers and returning residents to Brunei. J Travel Med. 2020:taaa066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yin S, Peng Y, Ren Y, et al. The implications of preliminary screening and diagnosis: clinical characteristics of 33 mild patients with SARS‐CoV‐2 infection in Hunan, China. J Clin Virol. 2020;128:104397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS‐CoV‐2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081‐2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Khalil A, Hill R, Ladhani S, Pattisson K, O'Brien P. SARS‐CoV‐2 in pregnancy: symptomatic pregnant women are only the tip of the iceberg. Am J Obstet Gynecol. 2020;223(2):296‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lu X, Zhang L, Du H, et al. SARS‐CoV‐2 infection in children. N Engl J Med. 2020;382(17):1663‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rivett L, Sridhar S, Sparkes D, et al. Screening of healthcare workers for SARS‐CoV‐2 highlights the role of asymptomatic carriage in COVID‐19 transmission. eLife. 2020;9:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu L, Hong X, Su X, et al. sOptimizing screening strategies for coronavirus disease 2019: a study from Middle China. J Infect Public Health. 2020;13:868‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brandstetter S, Roth S, Harner S, et al. Symptoms and immunoglobulin development in hospital staff exposed to a SARS‐CoV‐2 outbreak. Pediatr Allergy Immunol. 2020:1‐6. [DOI] [PubMed] [Google Scholar]
- 45. Kim SE, Jeong HS, Yu Y, et al. Viral kinetics of SARS‐CoV‐2 in asymptomatic carriers and presymptomatic patients. Int J Infect Dis. 2020;95:441‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhong Z, Xie X, Huang W, Zhao W, Yu Q, Liu J. Chest CT findings and clinical features of coronavirus disease 2019 in children. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020;45(3):236‐242. [DOI] [PubMed] [Google Scholar]
- 47. Xu T, Huang R, Zhu L, et al. Epidemiological and clinical features of asymptomatic patients with SARS‐CoV‐2 infection. J Med Virol. 2020:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Furuya‐Kanamori L, Cox M, Milinovich GJ, Magalhaes RJ, Mackay IM, Yakob L. Heterogeneous and dynamic prevalence of asymptomatic influenza virus infections. Emerging Infect Dis. 2016:22(6):1052‐1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wang Y, Liu Y, Liu L, Wang X, Luo N, Li L. Clinical outcomes in 55 patients with severe acute respiratory syndrome coronavirus 2 who were asymptomatic at hospital admission in Shenzhen, China. J Infect Dis. 2020;221(11):1770‐1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Polat V, Bostancı Gİ. Sudden death due to acute pulmonary embolism in a young woman with COVID‐19. J Thromb Thrombolysis. 2020;50:239‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kam KQ, Yung CF, Cui L, et al. A well infant with coronavirus disease 2019 (COVID‐19) with high viral load. Clin Infect Dis. 2020;71(15):847‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lu S, Lin J, Zhang Z, et al. Alert for non‐respiratory symptoms of Coronavirus Disease 2019 (COVID‐19) patients in epidemic period: a case report of familial cluster with three asymptomatic COVID‐19 patients. J Med Virol. 2020. [DOI] [PubMed] [Google Scholar]
- 53. Lin C, Ding Y, Xie B, et al. Asymptomatic novel coronavirus pneumonia patient outside Wuhan: the value of CT images in the course of the disease. Clin Imaging. 2020;63:7‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wu X, Fu B, Chen L, Feng Y. Serological tests facilitate identification of asymptomatic SARS‐CoV‐2 infection in Wuhan, China. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Korth J, Wilde B, Dolff S, et al. SARS‐CoV‐2‐specific antibody detection in healthcare workers in Germany with direct contact to COVID‐19 patients. J Clin Virol. 2020;128:104437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Scott SE, Zabel K, Collins J, et al. First Mildly Ill, non‐hospitalized case of coronavirus disease 2019 (COVID‐19) without viral transmission in the United States‐Maricopa County, Arizona, 2020. Clin Infect Dis. 2020;70(15):807‐812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nicastri E, D'Abramo A, Faggioni G, et al. Coronavirus disease (COVID‐19) in a paucisymptomatic patient: epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020. Euro Surveill. 2020;25:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tang A, Tong ZD, Wang HL, et al. Detection of novel coronavirus by RT‐PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis. 2020;26:6‐1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hu Z, Song C, Xu C, et al. Clinical characteristics of 24 asymptomatic infections with COVID‐19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63(5):706‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang J, Tian S, Lou J, Chen Y. Familial cluster of COVID‐19 infection from an asymptomatic. Crit Care. 2020;24(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. JAMA. 2020;323(14):1406‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tong ZD, Tang A, Li KF, et al. Potential presymptomatic transmission of SARS‐CoV‐2, Zhejiang Province, China, 2020. Emerg Infect Dis. 2020;26(5):1052‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information
Supporting information
Supporting information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


