Dear Editors,
COVID‐19 is a disease which can range from paucisymptomatic cases to interstitial pneumonia and systemic involvement with abnormal immune responses and inflammation which may lead to multiorgan failure.
A 59‐year‐old male smoker with COPD presented to the emergency room with dyspnoea, fever and cough. Vital signs were pulse 102 bpm, body temperature 37.5°C, oxygen saturation 97% and respiratory rate 20 breaths/minute.
Laboratory tests revealed lymphopenia, elevated D‐dimer (899 ng/mL) and 4.2 mg/dL of C reactive protein. The platelet count and coagulation parameters were normal. SARS‐CoV‐2 was detected by PCR on a naso‐pharyngeal swab specimen, and bilateral interstitial pneumonia was evident at chest CT scan.
He was admitted to the hospital and treatment with azithromycin and hydroxychloroquine was initiated.
Erythematous lesions were identified on his limbs and oxygen supplementation by nasal cannula (2 l/min) was performed for 24 hours owing to the development of hypoxemia (55 mmHg).
Because of persistent hypoxemia and high D‐dimer levels, he was admitted in the intensive care unit. Concomitantly, a necrotic lesion was identified on the foot of the patient (Fig. 1).
Figure 1.
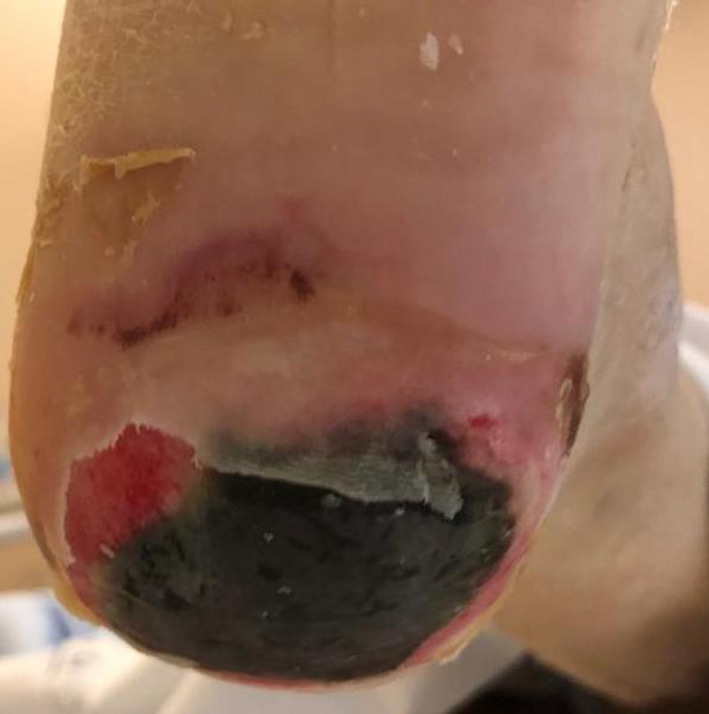
Necrotic lesion on the left sole of the patient. Ischemic and erosive necrotic plaque extending to the dermis on the left heel of the patient which is surrounded by an erythematous area and skin desquamation. Above and on the left of the main lesion, two other less defined necrotic foci are visible.
Following the administration of tocilizumab as a single dose, no new cutaneous lesions were identified on physical examination.
A Pseudomonas aeruginosa superinfection diagnosed by blood culture lead to death.
There are several hypotheses concerning skin involvement in COVID‐19: SARS‐CoV‐2 induces immune complexes formation with inflammation and vasculitis. Skin biopsies of patients deceased due to COVID‐19 showed small vessel thrombosis with no viral skin identification, 1 suggesting that tissue abnormalities are due to systemic toxicity triggered by a disproportionate immune response, rather than to direct viral spread.
Another hypothesis is that the virus itself causes vascular damage, binding to angiotensin‐converting enzyme 2 receptor, which is widely expressed in endothelial cells. 2 Moreover, severe infections result in disseminated intravascular coagulopathy (DIC), with further skin damage.
A recent overview revealed that among the known cutaneous COVID‐19 manifestations maculopapular exanthems are the most frequently reported. 3
In a recent study that included 375 COVID‐19 patients, acral ischemic lesions were classified into two groups: ‘pseudo‐chilblain’ and ‘livedo or necrosis’. 4 Livedo or necrosis frequently involved the acral regions and were related to older age and severe disease with up to 10% mortality. 4 Acral cyanosis, blistering and gangrene were reported in seven critical COVID‐19 subjects, with altered coagulation leading to DIC and death in 5 cases, suggesting that acral ischemia resulted from vascular occlusion due to endothelial damage rather than primary viral lesions.
Our report suggests that acral lesions are favoured by hypoxemia and tissue hypoxia due to smoke‐induced COPD and pneumonia, hence smoking cessation may be beneficial in the improvement of the prognosis. 5
In conclusion, the early identification of cutaneous lesions may help to rapidly start treatment, since their worsening may be related to a severe systemic involvement.
Funding sources: None.
Conflicts of interest: None.
Patient Consent to publication: Yes
References
- 1. Yao XH, Li TY, He ZC et al. A Pathological Report of Three COVID‐19 Cases by Minimally Invasive Autopsies. Zhonghua Bing Li Xue Za Zhi. 2020; 49: E009. [DOI] [PubMed] [Google Scholar]
- 2. Hamming I, Timens W, Bulthuis ML et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004; 203: 631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tammaro A, Adebanjo GAR, Parisella FR et al. Cutaneous manifestations in COVID‐19: the experiences of Barcelona and Rome [published online ahead of print, 2020 Apr 24]. J. Eur. Acad. Dermatol. Venereol. 2020; 10.1111/jdv.16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Galván Casas C, Català A, Carretero Hernández G et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. [published online ahead of print, 2020 Apr 29]. Br. J. Dermatol. 2020; 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pezzuto A, Carico E. Effectiveness of smoking cessation in smokers with COPD and nocturnal oxygen desaturation: Functional analysis. Clin. Respir. J. 2020; 14: 29–34. 10.1111/crj.13096. [DOI] [PubMed] [Google Scholar]


