Abstract
In patients with severe SARS‐CoV‐2 infection, the development of cytokine storm induces extensive lung damage, and monocytes play a role in this pathological process. Non‐classical (NC) and intermediate (INT) monocytes are known to be involved during viral and bacterial infections. In this study, 30 patients with different manifestations of acute SARS‐CoV‐2 infection were investigated with a flow cytometric study of NC, INT, and classical (CL) monocytes. Significantly reduced NC and INT monocytes and a downregulated HLA‐DR were found in acute patients with severe SARS‐CoV‐2 symptoms. Conversely in patients with moderate symptoms NC and INT monocytes and CD11b expression were increased. © 2020 International Society for Advancement of Cytometry
Keywords: monocytes, classical monocytes, non‐classical monocytes, intermediate monocytes, multicolor flow cytometry, SARS‐CoV2, COVID‐19
Patients with severe COVID‐19 symptoms show extensive lung damage due to the development of a cytokine storm involving IL2, IL6, and TNF‐α release (1). In this process, a crucial role is played by monocytes as effectors. Circulating monocytes are a heterogeneous and plastic population including three different subsets: classical (CL), intermediate (INT), and non‐classical (NC) cells (2). The three monocyte subsets are identified by the differential expression of CD14 and CD16. Monocyte subsets are differently involved in innate and adaptive immune response, in various phases of inflammation and in the pathogenesis of inflammatory diseases. CD14++ CD16dim CL monocytes and CD14++ CD16+ INT monocytes account for 90–95% of peripheral blood monocytes and are known to respond to bacterial‐associated signals. Conversely, CD14dim CD16+ NC monocytes account for the remaining 5–10% and are more responsive to virus‐associated stimuli (2, 3). In this study, patients with acute SARS‐CoV‐2 infection were studied and NC, INT, and CL monocytes subsets were evaluated by flow cytometry at the first clinical presentation. The cytometric data were correlated to patients' status and outcome.
Methods
Patients and Control Group
Thirty acute COVID‐19 patients (8 females and 22 men) with a median age of 68 years were studied at their first presentation to the emergency department for SARS‐CoV‐2‐related symptoms. Full blood count, D‐dimer, and C‐reactive protein (CRP) were measured along with the evaluation of blood gases, chest X‐rays, and CAT scans. In all patients with fever, rapidly evolving respiratory symptoms, oxygen desaturation (<90%), and typical chest imaging findings, SARS‐CoV‐2 infection was confirmed by SARS‐CoV‐2 nucleic acid RT‐PCR of nasal‐throat swab specimens (Gene Finder™ COVID‐19 Plus Real KIT, OSANG Healthcare, Korea). Twenty patients (67%) with severe COVID‐19‐related respiratory distress required intubation and ventilatory support. The other 10 patients with no need of ventilatory support were included in the moderate SARS‐CoV‐2 infection group. All testings were performed on blood samples taken at admission, before any medical treatment was given. In addition, 20 healthy donors were included as the normal control group. The approval for this prospective and observational study was obtained from the local scientific committee. Conscious patients were submitted an informed consent form to agree on experimental testings and personal data interexchange. However, only patients with no need of ventilatory support were able to sign these forms (example in Supplementary Material).
Flow Cytometric Analysis of NC, INT, and CL Monocyte Subsets
Briefly, 50 μl of EDTA whole blood were stained with a combination of conjugated monoclonal antibodies: anti‐CD16‐FITC (clone 3G8), anti‐CD64‐PE (clone 10.1), anti‐CD11b‐APC (clone ICRF44), anti‐CD14‐APC‐H7 (clone MΦP9), and anti‐HLADR‐V450 (clone L243) (Becton Dickinson Biosciences, Milan, Italy) for 20 min in the dark at room temperature. After the staining the samples were processed with a lyse‐no‐wash technique using ammonium chloride. Flow cytometric analysis was performed on a FACS Lyric (Becton Dickinson). Data from at least 100,000 total white cell events were acquired. Monocytes were identified and gated by their scatter features and CD64 expression. CL, INT, and NCL monocytes were identified according to their respective expression of CD14 and CD16 (Fig. 1). CD11b and HLA‐DR expression on the whole monocyte population were also evaluated and expressed in geometric means of calibrated MFI units. The medians of CD11b and HLA‐DR expression intensities in the three subject groups were also compared. Details of the gating strategy are illustrated in Supplementary Figure S1.
Figure 1.
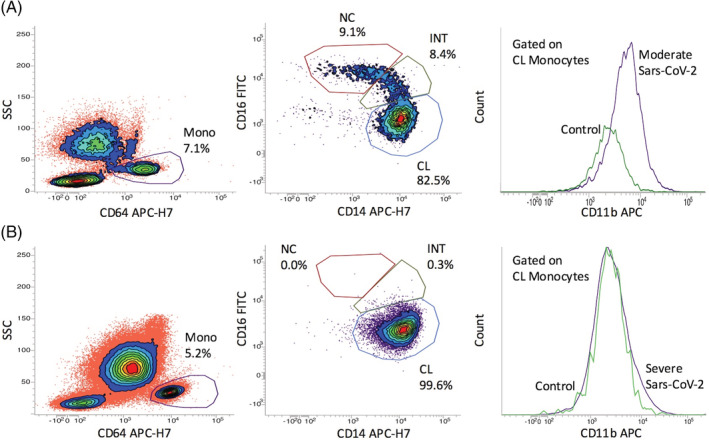
(A) Monocyte subsets and monocyte CD11b expression in a COVID‐19 patient with moderate clinical manifestations. Monocytes are gated with CD64 and monocyte subsets are dissected with a CD14/CD16 plot. (B) The same analysis in a severely infected patient. The profound decrease of the intermediate (INT) and non‐classical (NC) monocyte subsets and the lack of CD11b up‐regulation is evident in the severely infected subject. [Color figure can be viewed at wileyonlinelibrary.com]
The continuous analytical data were not normally distributed, as assessed with the Kolmogorov–Smirnov test. The Mann–Whitney test was therefore used for comparisons between patient groups and controls. Statistically significant differences were established by a P value <0.05.
Results
Thirty acutely infected patients were included in this study. Twenty patients, 16 males and 4 females, with a median age of 66 years showed a severe acute clinical picture. Five patients were younger than 60 years. Six patients were admitted to the intensive care unit for respiratory failure and required intubation. In 14 severe cases, non‐invasive ventilation (CPAP) was required, whereas the 10 cases with moderate symptoms did not require any ventilatory support. No significant difference was found between the two groups with respect to age. Among the 10 patients with moderate symptoms, half were females. At admission to the emergency unit, the most frequent symptoms in both groups were fever and cough. Two patients with severe acute infection died (Supplementary Table S1 for demographic and clinical data and Supplementary Table 2 for laboratory parameters).
Flow cytometry analyses results are summarized in Figure 2. The patients and control group continuous variables were compared by the Mann–Whitney test.
Figure 2.

Flow cytometric analysis of monocytes in patients and controls. (A) INT, NC, and CL monocyte subset frequencies, expressed as percentage of total monocytes. (B) Quantitative expression of monocyte CD11b‐APC and HLA‐DR‐V450 (geometric means in MFI units). Red asterisks indicate outliers. P values not significant unless otherwise specified. [Color figure can be viewed at wileyonlinelibrary.com]
No differences were recorded in monocyte absolute counts. Absolute lymphocyte counts were only slightly lower in both patient groups as compared to controls (Supplementary Table 2).
Significantly different proportions of CL, INT, and CL monocytes subsets were observed between the two patient groups and as compared with normal controls. Namely, in the severe infection group monocytes were 95% of the CL type, and NC monocytes were significantly lower than in controls. In the moderate infection group, a higher percentage of INT cells was observed, as compared with controls. Interestingly, the minimum levels of INT monocytes (<0.4%) were found in the two patients with severe infection who died.
Moderately symptomatic patients had an overall upregulated CD11b expression, whereas severely affected patients expressed CD11b like healthy controls. A significant difference in HLA‐DR expression was also observed between the two patient groups, with a marked downregulation of monocyte HLA‐DR expression in severely symptomatic patients (Fig. 2).
Discussion
During the monitoring of patients infected by SARS‐CoV‐2, a reproducible decrease of peripheral INT and NC monocytes was found in subjects with the most severe clinical status at admission. Conversely, patients with moderate symptoms not requiring ventilatory support displayed increased proportions of INT and NC monocytes. Moderately symptomatic patients had an upregulated expression of monocyte surface CD11b, whereas the monocyte HLA‐DR expression was remarkably downregulated in patients with severe symptoms, as a surrogate indicator of infection‐induced immunosuppression. These findings can be related to SARS‐CoV‐2 pathogenesis and to the underlying lung damage. The decrease of INT and NC monocytes, along with the downregulation of monocyte HLA‐DR can be taken as an early indicator of an ongoing severe inflammatory lung damage, and may have prognostic significance.
The clinical manifestations of COVID‐19 are heterogeneous. It is now well established that SARS‐CoV‐2 can cause different degrees of pulmonary involvement, requiring ventilatory support in many cases, often with a fatal outcome (1, 4). The activation of the innate immune system in the early phase of SARS‐CoV infection seems to have an important role in the control of viral replication (5).
The severe cases are frequently reported with lymphopenia, higher levels of D‐dimer and CRP, as compared to clinically moderate SARS‐CoV‐2 infections. In addition, high levels of IL‐2, IL‐6, and TNF‐α have been described in patients admitted to intensive care units (1). We found significant leucocytosis in severely infected patients, whereas both patient groups displayed mild to marked lymphopenia, increased levels of D‐dimer and CRP, but without statistically significant differences.
The expansion of CD16+ monocytes has been observed in infectious or inflammatory conditions (6). The flow cytometric findings described here were not conditioned by medical treatment since samples were obtained at presentation.
In our study, COVID‐19 patients with moderate symptoms had a higher level of INT monocytes than the control subjects. We have also reproducibly found decreased NC monocytes in patients with the most severe infection, and the minimum values of INT monocytes were recorded in the two patients who died. Our findings are in agreement with a large recent study that also included intracellular cytokine measurements (7), in which the fall of peripheral NC and INT monocytes was related to the recruitment by the inflamed lung tissue. On the contrary, another study (8) found a higher percentage of CD14+CD16+ inflammatory monocytes in severely SARS‐CoV‐2 infected patients.
Channappanavar et al have shown that a robust viral replication promotes the accumulation of pathogenic monocyte–macrophages in lungs, resulting in elevated local cytokine/chemokine secretion and suboptimal T‐cell responses, both in the experimental setting and in patients. Increased numbers of macrophages in the lungs were found in patients with severe SARS‐CoV‐2 infection (9). The decrease of NC and INT monocytes observed in severe SARS‐CoV infection seems therefore related to the concentration of monocyte–macrophages in the lungs.
In our study, we have also found a stronger expression of CD11b in moderately symptomatic patients than in severe cases, whereas monocytes from severely infected subjects had a downregulated HLA‐DR expression. In acute lung injury CD11b+ macrophages are recruited in airspaces and play a key role in promoting anti‐inflammatory function. However, a depletion of CD11b+ macrophages leads to a hyperactive immune response, thereby impairing the physiological tissue repair process (10).
This study has some limitations: intracellular cytokine measurements were not performed, which could have corroborated the phenotypic findings, but no patients under study were treated with tocilizumab at presentation. Moreover, bronchoalveolar lavage analysis was not carried out in our series, which could have allowed the evaluation of CD11b expression on alveolar macrophages.
Our findings suggest that the decrease of NC and ITM monocytes and the downregulation of HLA‐DR may be considered prognostic indicators that may help in the early identification of patients with the worst and most rapid unfavorable outcome. In this study, only 30 acutely infected patients have been included, therefore additional studies are warranted to further corroborate our findings.
Conflict of interest
The authors declare no conflict of interest.
Author Contributions
Antonino Mazzone: Investigation; supervision. Arianna Gatti: Conceptualization; formal analysis; methodology; supervision; writing‐review and editing. Bruno Brando: Conceptualization; data curation; methodology; supervision; writing‐original draft; writing‐review and editing. Danilo Radrizzani: Investigation; supervision. Paolo Viganò: Investigation; supervision.
Supporting information
Appendix S1. Author Checklist: MIFlowCyt‐Compliant Items
Supplementary Figure S1 Details of the sequential gating strategy. Upper row, from left to right: P1: inclusion of singlets with FSC‐A vs FSC‐H, P2: exclusion of debris with FSC/SSC, P3: gating of monocytes with CD64 vs SSC. Dissection of monocyte subpopulations gated in P3 according to CD14 vs CD16 expression. P4: Classical monocytes (CD14++ CD16dim); P5: Intermediate monocytes (CD14+ CD16+); P6: Non‐Classical monocytes (CD14dim CD16++). The quantitative expression of CD11b (P7 histogram) and HLA‐DR (P8 histogram) by the whole monocyte population in P3 is further evaluated.
Supplementary Table S1 Demographic and main clinical data of the two groups of patients.
Supplementary Table S2. Main laboratory data of the two groups of patients. CRP = C‐Reactive Protein.
Literature Cited
- 1. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020;130(5):2620–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG, Metes DM. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One 2017;12(4):e0176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ong SM, Teng K, Newell E, Chen H, Chen J, Loy T, Yeo TW, Fink K, Wong SC. A novel, five‐marker alternative CD16‐CD14 gating to identify the three human monocyte substes. Front Immunol 2019;10:1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, Li P, Zhou Y, Lin Y, Duan Q, et al. Clinical characteristics of coronavirus disease 2019 (COVID‐19) in China: A systematic review and meta‐analysis. J Infect 2020;80(6):656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen J, Lau YF, Lamirande EW, Paddock CD, Bartlett JH, Zaki SR, Subbarao K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS‐CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS‐CoV infection. J Virol 2010;84(3):1289–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Greco M, Mazzei A, Palumbo C, Verri T, Lobreglio G. Flow cytometric analysis of monocytes polarization and reprogramming from inflammatory to immunosuppressive phase during sepsis. eJIFCC 2019;30(4):371–384. [PMC free article] [PubMed] [Google Scholar]
- 7. Sanchez‐Cerrillo I, Landete P, Aldave B, Sanchez‐Alonso S, Sanchez‐Azofra A, Marcos‐Jimenez A, Avalos E, Alcaraz‐Serna A, de los Santos I, Mateu‐Albero T, et al. Differential redistribution of activated monocyte and dendritic cell subsets to the lung associates with severity of COVID‐19. medRxiv 2020. 10.1101/2020.05.13.20100925. [DOI] [Google Scholar]
- 8. Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi Y, Sun R, Tian Z, Xu X, Wei H. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID‐19 patients. Natl Sci Rev 2020;7(6):998–1002. 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Channappanavar R, Fehr AR, Vijay R, Mack M, Zhao J, Meyerholz DK, Perlman S. Dysregulated type I interferon and inflammatory monocyte‐macrophage responses cause lethal pneumonia in SARS‐CoV‐infected mice. Cell Host Microbe 2016;19(2):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Joshi JC, Joshi B, Rochford I, Rayees S, Zahid Akhter M, Baweja S, Rao Chava K, Tauseef M, Abdelkarim H, Natarajan V, et al. SPHK2‐generated S1P in CD11b+ macrophages blocks STING to suppress the inflammatory function of alveolar macrophages. Cell Rep 2020;30:4096–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Author Checklist: MIFlowCyt‐Compliant Items
Supplementary Figure S1 Details of the sequential gating strategy. Upper row, from left to right: P1: inclusion of singlets with FSC‐A vs FSC‐H, P2: exclusion of debris with FSC/SSC, P3: gating of monocytes with CD64 vs SSC. Dissection of monocyte subpopulations gated in P3 according to CD14 vs CD16 expression. P4: Classical monocytes (CD14++ CD16dim); P5: Intermediate monocytes (CD14+ CD16+); P6: Non‐Classical monocytes (CD14dim CD16++). The quantitative expression of CD11b (P7 histogram) and HLA‐DR (P8 histogram) by the whole monocyte population in P3 is further evaluated.
Supplementary Table S1 Demographic and main clinical data of the two groups of patients.
Supplementary Table S2. Main laboratory data of the two groups of patients. CRP = C‐Reactive Protein.


