Abstract
Objective
In the light of increased adverse outcomes for people with diabetes affected by COVID‐19, we have described the clinical course of a cohort of critically ill patients with COVID‐19 and diabetes.
Methods
We retrospectively analysed characteristics, glucometrics and inflammatory markers of patients with diabetes mellitus admitted to intensive care unit (ICU) with COVID‐19.
Results
Eight patients with diabetes were admitted to ICU with COVID‐19. All had type 2 diabetes, with three being newly diagnosed that admission. Mean HbA1c was 9.2%. Glucometric analysis indicated that extremely high insulin doses were required during peak inflammatory response to maintain glycaemic control with a mean peak insulin requirement of 201 units per day (2.2 units/kg/day).
Conclusions
Critically unwell patients with diabetes mellitus and COVID‐19 had high insulin requirements and poorer time in target range at the time of peak inflammatory response, and this improved as their illness resolved.
Keywords: COVID‐19, critical illness, diabetes mellitus, insulin
1. INTRODUCTION
As the world grapples with the crisis of covid‐19, diabetologists are faced with 2 critical questions: what is the nature of the association between covid‐19 and diabetes and what are the direct clinical implications to our patients? Early data raised alarm that diabetes diagnosis and hyperglycaemia are independent predictors for death and morbidity in patients with SARS‐CoV‐2. 1 , 2
2. METHODS
We retrospectively analysed data of patients admitted to intensive care (ICU) in our quaternary referral hospital in Sydney, Australia, with concomitant diagnoses of COVID‐19 and diabetes. Baseline characteristics, glycaemic control, insulin requirements and inflammatory markers were examined.
3. RESULTS
Eight patients with type 2 diabetes were admitted to ICU with COVID‐19 between 20 March and 1 May 2020. Four were male, mean age was 55 years (SD ± 11.9 years) and three were newly diagnosed with diabetes on admission. Of the five patients with pre‐existing diabetes, all were managed with oral agents and one required insulin. Mean HbA1c was 9.2% (SD ± 2.4%; 77 mmol/mol). In those with newly diagnosed diabetes, robust C‐peptide levels and negative anti‐GAD antibodies were found, consistent with type 2 diabetes, and HbA1c ranged from 11.1% to 12.4% (98 mmol/mol to 112 mmol/mol).
All eight patients required mechanical ventilation, one patient required extracorporeal membrane oxygenation and six required vasopressor support. All eight patients had classic radiologic findings of bilateral ground‐glass opacities demonstrated on CT chest. Seven patients required nasogastric feeding. No patients received steroid therapy. At the time of writing, six patients have been successfully discharged to the community, and two remain in hospital. Their baseline characteristics, complications during admission and inflammatory markers are outlined in Table 1.
TABLE 1.
Summary table of baseline characteristics, complications and inflammatory markers during course of admission for patients 1‐6
| Patient number | Age (yrs) | Gender | BMI (g/m2) | New diagnosis of diabetes | HbA1c (%) | Comorbidities | Complications | BGL at start of insulin infusion (mmol/L) | Peak insulin requirement (units/day) | Peak insulin requirement (units/kg/day) | Peak CRP (mg/L) | Peak procalcitonin (µg/L) | Trough lymphocytes (×106/L) | Trough albumin (g/L) | Peak FiO2 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 65 | M | 27.6 | N | 7.2 | HTN, HCHOL, PCKD | Shock, AKI, hepatitis | 7 | 85 | 1.1 | 296 | 0.62 | 0.8 | 18 | 60 |
| 2 | 39 | F | 32.1 | Y | 11.9 | Obesity | Shock, AKI, hepatitis, myocarditis | 16.6 | 108 | 1.4 | 334 | 34.2 | 0.5 | 16 | 55 |
| 3 | 58 | M | 43.4 | Y | 11.1 | Obesity, OSA | 13.5 | 200 | 1.3 | 234 | 0.24 | 0.6 | 23 | 50 | |
| 4 | 59 | M | 35.9 | N | 8 | IHD, HTN, HCHOL, obesity | Shock, hepatitis | 12.8 | 240 | 2.3 | 207 | 0.91 | 0.8 | 20 | 90 |
| 5 | 71 | M | 32.5 | N | 9.9 | HTN, obesity | Shock, AKI | 17.6 | 480 | 4.7 | 214 | 12.03 | 1 | 23 | 80 |
| 6 | 36 | F | 29.6 | Y | 12.4 | Obesity | Shock, hepatitis | 15 | 266 | 3.3 | 302 | 0.71 | 1 | 22 | 60 |
| 7 | 57 | F | 35.8 | N | 6 | HTN, HCHOL, obesity | AKI | 13 | 88 | 0.89 | 203 | 2.1 | 0.6 | 22 | 50 |
| 8 | 55 | F | 30.9 | N | 7.7 | Obesity, COPD | Shock, AKI, hepatitis | 14 | 145 | 2.3 | 440 | 42.5 | 0.2 | 22 | 100 |
Abbreviations: AKI, acute kidney injury; BGL, blood glucose level; BMI, body mass index; CRP, C‐reactive protein; FiO2, fraction of inspired oxygen; HCHOL, hypercholesterolaemia; HTN, hypertension; IHD, ischaemic heart disease; OSA, obstructive sleep apnoea; PCKD, polycystic kidney disease.
Figure 1 depicts the insulin requirements alongside C‐reactive protein (CRP) throughout the admission for the eight patients. Mean peak insulin requirement was 201 unit per day (2.2 units/kg/day). Median glucose level on insulin infusion was 9.5 mmol/L, with 54.0% of glucose levels in target (4‐9.9 mmol/L). Once off insulin infusion, seven of the eight patients required subcutaneous insulin. Median glucose level was 8.0 mmol/L off infusion, with 72.1% of glucose levels in target. Of the six patients discharged, all required oral hypoglycaemic agents on discharge and four required insulin (mean total daily dose 18 ± 10.9 units/day).
4. DISCUSSION
Within the limits of our small cohort size, there are several points of interest. We have demonstrated at a granular level the stark contrast between extremely high insulin requirements at the peak of COVID‐19 illness mirrored by CRP levels, in comparison with relatively minimal insulin requirements by time of discharge.
The peak insulin requirements were considerably higher than that administered in trials of tight glyacemic control in ICU though these studies included non‐diabetic patients. 3 In one cohort of 1548 patients, the control group required 33 units/day of insulin in comparison with 71 units/day in the intensive control group, acknowledging that only 13% of this cohort had diabetes. 3 Similarly, in the NICE‐SUGAR study of 6104 patients in intensive care, 16.9 units/day was required in the control group compared with 50.2 units/day in the intensive glucose control group. 4 In a study of 415 intensive care patients who received insulin infusion aiming for a target glucose of 6‐10 mmol/L, 87% of whom had type 2 diabetes, the mean daily insulin dose was only 34 units. 5 It remains to be determined whether this significant insulin requirement seen in our cohort is purely due to profound insulin resistance from systemic inflammation and critical illness or whether islet inflammation and beta‐cell stunning may contribute.
As SARS‐CoV‐2 utilizes ACE2 receptors for cell entry and that ACE2 receptors are expressed in the pancreas, 6 there is the attractive hypothesis that SARS‐CoV‐2 can lead to direct islet injury and insulin deficiency. The poorer glucose time in target noted when on insulin infusion versus off infusion may reflect the challenges of acute and rapid changes in insulin resistance driven by inflammation at early stages of admission and the use of nasogastric feeding. It is unknown as to whether poor glycaemic control is a key cause of increased morbidity and mortality as suggested by some authors, 2 or whether the suboptimal control is principally a reflection of severe systemic illness.
A third of our patients had a new diagnosis of diabetes at presentation based on HbA1c. It is established that hyperglycaemia and diabetes predispose to infection and in turn COVID‐19 infection has led to the diagnosis of previously unrecognized diabetes. A previous study of 39 patients with SARS‐CoV‐2 who had new‐onset diabetes found only two still with diabetes at three years, raising the possibility of virus‐induced transient islet injury‐causing diabetes. 7 Our patients had robust C‐peptide levels, and with the presence of elevated HbA1c at diagnosis, it is unlikely that SARS‐CoV‐2 predated onset of diabetes or led to a significant beta‐cell toxicity.
On the basis of these findings, it is now established practice at our centre to screen all patients with COVID‐19 for diabetes with HbA1c and plasma glucose. Tight glycaemic control is sought, using insulin infusions when critically unwell and transitioning to subcutaneous insulin when off enteral nutrition. Oral hypoglycaemic agents with low‐risk profile are gradually introduced as oral intake stabilizes. Follow‐up will elucidate whether these patients have an ongoing need for insulin which might suggest long‐term islet cell damage.
5.
FIGURE 1.
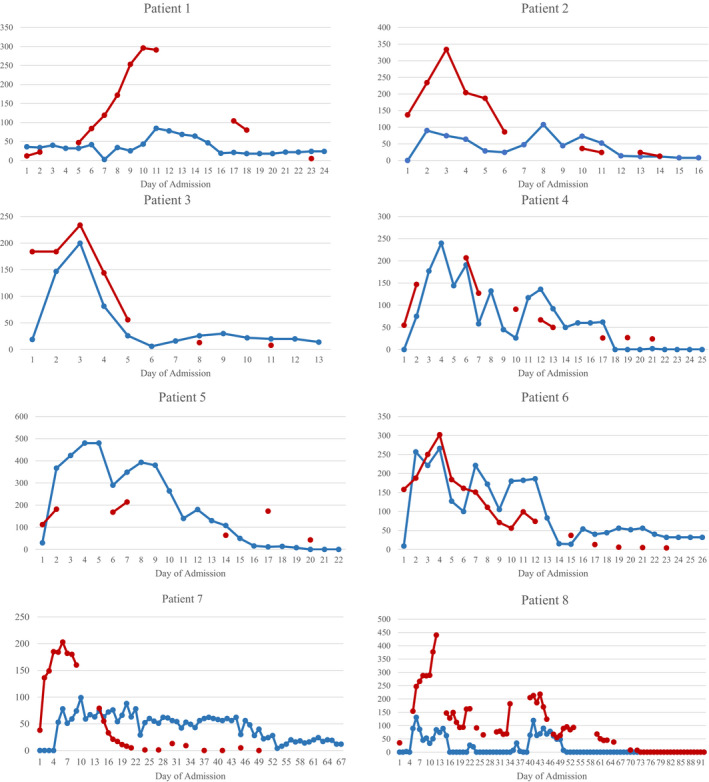
Insulin requirements and inflammatory markers depicted for the eight patients throughout the course of admission. Total daily dose (units) is depicted in blue, and CRP (mg/L) is depicted in red. Patients 2, 3 and 6 were newly diagnosed with diabetes
Wu L, Girgis CM, Cheung NW. COVID‐19 and diabetes: Insulin requirements parallel illness severity in critically unwell patients. Clin Endocrinol (Oxf). 2020;93:390–393. 10.1111/cen.14288
REFERENCES
- 1. Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID‐19 patients hospitalised in the United States. J Diabetes Sci Technol. 2020;14(4):813‐821. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu L, She Z, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID‐19 and pre‐existing type 2 diabetes. Cell Metab. 2020;31(6):1068‐1077.e3. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. New Eng J Med. 2001;345:1359‐1367. [DOI] [PubMed] [Google Scholar]
- 4. Investigators N‐SS, Finfer S, Chittock DR, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360(13):1283‐1297. [DOI] [PubMed] [Google Scholar]
- 5. Egi M, Bellomo R, Stachowski E, et al. The interaction of chronic and acute hyperglycemia with mortality in critically ill patients with diabetes. Crit Care Med. 2011;39(1):105‐111. [DOI] [PubMed] [Google Scholar]
- 6. Hoffman M, Kleine‐Weber H, Schroeder S, et al. SARS‐COV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinical proven protease inhibitor. Cell. 2020;181:271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang J‐K, Lin S‐S, Ji X‐J, et al. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]


