Abstract
Since its first appearance in Wuhan, China, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has rapidly spread throughout the world and has become a global pandemic. Several medical comorbidities have been identified as risk factors for coronavirus disease 2019 (COVID‐19). However, it remains unclear whether people living with human immunodefeciency virus (PLWH) are at an increased risk of COVID‐19 and severe disease manifestation, with controversial suggestion that HIV‐infected individuals could be protected from severe COVID‐19 by means of antiretroviral therapy or HIV‐related immunosuppression. Several cases of coinfection with HIV and SARS‐CoV‐2 have been reported from different parts of the globe. This review seeks to provide a holistic overview of SARS‐CoV‐2 infection in PLWH.
Keywords: antiretroviral therapy/coinfection, COVID‐19, HIV, immunodeficiency
Highlights
Coronavirus disease 2019 has fast spread around the world to become a global pandemic.
Despite the potentially poor prognosis, data on HIV/SARS‐CoV‐2 co‐infection is still scarce.
HIV‐related immunosuppression may predispose them to severe forms of COVID‐19.
Analysis of available data did not show excess morbidity and mortality among PLWH.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a potentially fatal respiratory illness caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), a newly identified coronavirus, which was first recognized in December 2019 in Wuhan, Hubei Province, China and has since rapidly spread to over 200 countries/territories/areas and have been declared a global pandemic by the World Health Organization (WHO). 1 As at the time of writing (3 June 2020), the total confirmed cases were 6 383 805 with 2 732 976 recoveries and a staggering 380 384 deaths have been officially reported globally. 2 The clinical spectrum of COVID‐19 is broad, and while most people with COVID‐19 develop only mild or uncomplicated illness, especially in the early phase of illness, 14% to 26% of infected persons develop severe disease that requires hospitalization and oxygen support, with some even requiring admission to an intensive care unit. 3 , 4 Organ dysfunction, particularly progressive respiratory failure, heart and kidney injuries, is associated with the highest rates of mortality. 5 , 6 Clinical evidence has shown that disease severity and mortality are associated with older age and underlying comorbidities, such as diabetes, hypertension, and cardiovascular disease (CVD). 4 , 6 , 7 In line with this, one of the puzzling questions in the wake of this pandemic is “Does HIV infection increases the risk of getting and disease severity of COVID‐19?.” This is important, especially in Africa, as the region accounts for more than 70% of the global burden of human immunodefeciency virus (HIV) infection. 8 Generally, people living with HIV (PLWH) are perceived to be at high risk of contracting SARS‐CoV‐2, even though currently no specific information about the risk of COVID‐19 in people with HIV is available. At the end of 2018, it was estimated that 37.9 million people globally were living with HIV with 20.6 million (54%) in the eastern and southern Africa region, 5.9 million in Asia and the Pacific region, 5.0 million in western and central Africa, and 2.2 million in western and central Europe and north America region. 9 In an attempt to halt the spread of COVID‐19, governments across the globe are shutting cities down, restricting movements and encouraging residents to stay indoors. Beyond the unprecedented disruption of lives, the COVID‐19 pandemic has severely interrupted HIV care delivery among several other health care services globally as attention, resources and personnel have been diverted to the fight against COVID‐19. 10 , 11 , 12 It is estimated that about 19% of HIV‐infected patients were unable to get antiretroviral medications or therapy (ART) refills due to the pandemic. In addition, there have been reports that several HIV/AIDS prevention and control centers globally have been converted to COVID‐19 treatment centers, which denies HIV patients of their ART. 13 , 14 , 15 This has left a greater proportion of the HIV community in a venerable state, considering that they require regular medication to maintain good health. Despite the potentially poor prognosis for most patients within this category when infected with SARS‐CoV‐2, data on HIV/SARS‐CoV‐2 co‐infection is still scarce. Herein we summarize the global instances of SARS‐CoV‐2/HIV coinfections.
1.1. General impact of COVID‐19 on patients with HIV
The COVID‐19 pandemic, as well as measures taken by governments across the world, to minimize its spread has triggered unintended consequences in terms of HIV testing and care. 16 The Euroguidelines in Central and Eastern Europe Network Group recently found that 60% of HIV physicians simultaneously are involved in work related to patients with COVID‐19. In the worst scenarios, they abandon their original duties entirely and engage fully in COVID‐19 cases. 17 Moreover, to compensate for the increasing numbers of recorded cases (patients), HIV treatment facilities have been designated as COVID 19 care centers. According to Kowalska and coworkers, about 70% of HIV treatment facilities in central and Eastern Europe were not functioning due to COVID‐19. 17 This together with the fear of contracting COVID‐19 have kept many patient with HIV from accessing their usual ART. The Lancet HIV recently reported that HIV patients in Egypt are petrified to go for their ART refills because the only HIV treatment Center, have been converted to COVID‐19 quarantine and treatment facility. 18 In the Hubei province of China, about 64.15% of HIV patients could not have access to their ART due to the measures imitated to curb the spread of the virus. 19 As Gokengin et al reported, antiretrovirals are purchased and distributed via designated clinics 20 thus, patients living outside the perimeter could not have access to their ART. The situation is further aggravated by shortage of medication as medical consignments are stuck in procurement systems in other countries with no further supplies able to come in. 21 The pandemic has as well compromised the psychological and emotional wellbeing of PLWH. Shiau et al 22 reported that many HIV patient being managed via telephone have indicated that they are extremely stressed, anxious, and unable to sleep. A recent survey in China, 19 revealed that 28.93% of the respondents hoped they had some social and psychological support. Therefore, these psychosocial issues have to be addressed to avoid exacerbating adverse medical consequence among PLWH. 22
2. METHODS
SCOPUS, Web of Science, PubMed and Google Scholar were searched for relevant peer‐reviewed publications from December 2019 to 3 June 2020, using the following combination of terms: (“HIV” AND “COVID‐19”), (“Immunodeficiency” AND “COVID‐19”) and (“HIV” AND “Coronavirus”). The search was limited to only publications in English. Publications with information on HIV/SARS‐CoV‐2 co‐infection were manually sorted out and included in this study. Websites of relevant organizations including WHO, CDC, and USAIDS were also reviewed for additional information. Data on coinfection cases were extracted and entered into Microsoft Excel. Statistical analyses were carried out using IBM SPSS Statistics version 25.
3. RESULTS AND DISCUSSION
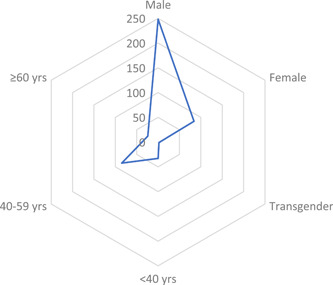
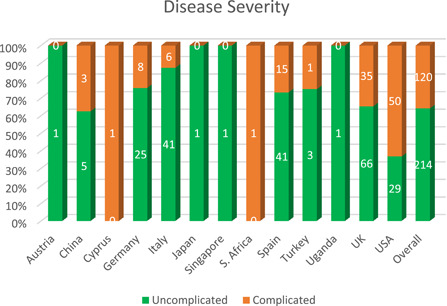
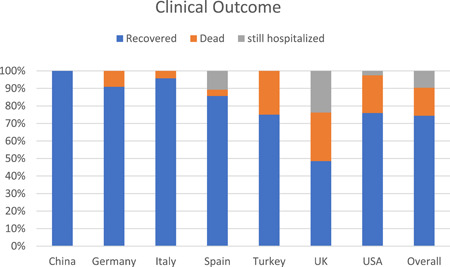
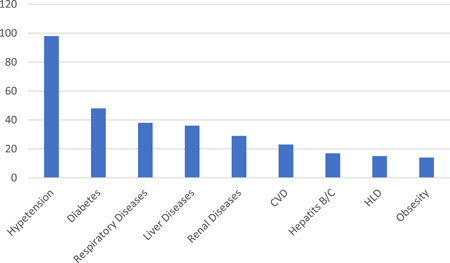
The first case of HIV/SARS‐CoV‐2 coinfection was reported in Wuhan, China, the terminus a quo of the pandemic. Subsequent cases of coinfection have been reported in UK, USA, Spain, Italy, Germany and other countries (Table 1). Interestingly, only two cases of coinfection have been reported in the whole of the Africa continent, notwithstanding the fact that South Africa which is at present the epicenter of the COVID‐19 pandemic in Africa 1 and home to over close to 8 million PLWH, the largest HIV epidemic in the world. 23 The situation of Africa is probably not because less PLWH have actually contracted COVID‐19, but more likely due to unpublished data, taking into account the uncoordinated and poor data collection and management in hospitals and health centers, lack of enthusiasm and ability of most health professionals to conduct research and prepare manuscripts for publication as well as poor collaboration between researchers and health professionals. The region currently accounts for just a little of 1% of global health publications 24 with a significant part being championed by researchers from high income countries. 25 Nonetheless, 378 HIV/SARS‐CoV‐2 coinfection cases have so far been reported globally with a majority originating from UK (101 cases) and USA (122 cases). The high number of coinfection cases from these countries however does not particularly suggest any increased risk of COVID‐19 among PLWH in them. It is worth noting that studies characterizing a larger population of patients with COVID‐19 originated from these countries, and hence the high reported cases of coinfection. Similar to that observed in the general population, majority (72.3% of 334) of the COVID‐19 cases in PLWH were males. While WHO earlier reported 51% of cases in China are males, studies from UK 26 and USA 27 indicated males represent up 60% of the COVID‐19 cases. Also, among a total of 142 coinfection cases where information on age is available, 59.9% were in the range of 40 to 59 years (Figure 1) with 23.2% being under 40 years. Taking into consideration studies from Italy, 28 Spain, 29 Turkey 30 and UK 31 that characterizes COVID‐19 in HIV cohorts (n = 12 296), the prevalence of HIV/SARS‐CoV‐2 coinfection is 0.98%. On the other hand, considering reports from China, 32 , 33 , 34 Japan, 35 Spain, 36 UK, 26 and USA, 27 , 37 PLWH account for only 0.64% of the general COVID‐19 population (n = 26, 049; see supplementary data) as compared with 16.9% to 56.6% for CVD (including hypertension), 8.2% to 33.8% for diabetes and 1.5% to 17.7% for respiratory diseases. 26 , 27 , 38 This correlate to several studies among large populations which observed that PLWH made up approximately less than 1% of COVID‐19 cases. 26 , 27 The low proportion of PLWH among patients with COVID‐19 should, however, be interpreted with caution as it could be as a result unyielding commitment to safety precautions (including wearing of nose masks, hand hygiene, social distancing, etc.) by individuals with HIV to limit their exposure to the SARS‐CoV‐2, bearing in mind their compromised immune system and the fact that even before COVID‐19 they were at risk of a broad range of infections, including respiratory tract infections, 23 rather than protection afforded by HIV or ART. It could also be that less of the HIV population is being screened for COVID‐19, looking at the fact some are not even enthused to visit treatment centers and clinics for their ART refills. 18 In general, 214 of a total 334 coinfection cases were uncomplicated (ie, mild and moderate) cases while the remaining 35.9% were classified as either severe or critical (complicated), requiring oxygen therapy and/or admission to intensive care unit (Figure 2). Among subjects with known outcomes (closed cases; n = 300) 82.3% had recovered while the remaining (53 patients) died, thus giving an overall case‐fatality rate of 14% among PLWH, which is more than 2 times higher than the current rate among the global population. 1 However, just like the country‐specific case‐fatality ratio of COVID‐19 in the general population, the case‐fatality rate among PLWH differ from one country to another, ranging from as high as 27.7% in UK to 0% in China (Figure 3). Similar to UK, the results of the analysis indicated USA also has a high case‐fatality ratio (13.9%) among its HIV population which is 2.4 time higher than that of the general population (5.8%). 39 Among the top five countries in terms of number of coinfection cases reported, the case‐fatality ratio among PLWH in Spain (3.6%) and Italy (4.3%) are lower than that recorded for the general population, 11.3% and 14.4% respectively. 39 Compared with that of the general population of the country, the case‐fatality rate among the HIV population in UK and Germany are almost two times higher. Differences in mortality numbers of COVID‐19 among countries have been attributed to several factors including differences in number of people tested, demographic characteristics, characteristics of the healthcare system, among others. 39 Nonetheless, it should be noted that majority of the cases included in this study (particularly from UK and USA) were hospitalized patients and as such the high case‐fatality rates recorded might not be particularly peculiar to HIV/SARS‐CoV‐2 coinfection since as much as 26% (in UK) 26 and 21% (in USA) 27 of hospitalized patients with COVID‐19 were reported dead. Earlier reports on HIV/SARS‐CoV‐2 coinfection suggested that PLWH do not have an increased risk of COVID‐19 and that HIV patients who are compliant to ART and have achieved viral suppression are even less likely to have severe/complicated COVID. 28 , 30 , 37 , 40 From our analysis (Table 1), 228 out of 244 were on ART before being diagnosed with COVID‐19. About 76.8% of them were taking nucleoside reverse transcriptase inhibitors (mostly tenofovir and emtricitabine), 89.7% were on integrase strand transfer inhibitors (chiefly, bictegravir and dolutegravir), 77.4% on non‐nucleoside reverse transcriptase inhibitors (particularly rilpivirine and efavirenz), while 33.8% were taking protease inhibitors eithers separately or as boosters. Also, 203 out of 378 had viral load suppression (<50 copies per mL) while the data on at least 90% of the remaining were not available. Given these, it is apparent that majority of the patients in these cases were virologically suppressed on ART. This nonetheless did not prevent progression to severe COVID‐19 or even death in some patients. A χ 2 analysis of 105 patients whose individual information were available showed that though a great proportion of patient with viral load <50 copies successfully recovered (91.5% vs 81.8% of non‐virologically suppressed patients) there is no evidence of association between viral suppression status and severity of COVID‐19 (χ 2 = 1.068; P = .302) or clinical outcome of patients (χ 2 = 1.069; P = .301). Except for only 2 out of 105, all patients where on ART (92 of whom where virologically suppressed), yet 22.8% of them had severe/critical COVID‐19 while nine died. This therefore raises significant concerns over earlier suggestion that PLWH are somewhat immune to severe cases of COVID‐19. Suwanwongse and Shabarek 41 previous expressed similar concern when they reported as high as 78% mortality among HIV/SARS‐CoV‐2 coinfected patients in their facility. The main reason earlier studies hypothesized that ART could confer protection against COVID‐19 in PLWH was that antiviral agents, such as remdesivir, tenofovir, and lopinavir showed antiviral activity against SARS‐CoV‐2 in vitro studies. Despite several observations that some antiviral agents somewhat improve symptoms of COVID‐19, 42 at present standard ART does not seem to shield PLWH from COVID‐19. Also, given the profound contribution of the immune system, particularly the massive release of cytokines and chemokines, to the severity of COVID‐19, earlier reports proposed that HIV‐related immunosuppression could paradoxically protect against severe manifestation of COVID‐19. 37 , 43 , 44 , 45 Out of 182 with individual data on CD4 count, 106 had CD4 count ≥200 cells per µL—59.4% of whom had CD4 > 500 cells per µL. Our analysis show an evidence of strong association between immune suppression status (CD4 count <200 or ≥200 cells per µL) and increased severity of disease (n = 119; χ 2 = 7.772; P = .005) but not clinical outcome (n = 109; χ 2 = 1.191; P= .275). A binary regression analysis further showed that CD4 count <200 cells per µL increases the risk of progression to severe COVID‐19 by almost 5 (odds ratio = 4.92; 95% confidence interval, 1.48‐16.37). About 61% of cases with CD4 <200 cells per μL had complicated COVID‐19 manifestation of which two died. Thus, contrary to earlier suggestions that coinfection patients may have mortality benefits from HIV‐related immunosuppression, low CD4 count below 200 per µL may rather predispose them to severe forms of the disease. Two hundred and sixty‐nine of the patients were reported to be hospitalized while 109 were reported to be outpatients. The large number of hospitalized patients is mainly due to the fact that most of the case and series reports considered in this paper were of inpatients. As such we are unable to estimate if coinfection influence the hospitalization rate. Currently several medical comorbidities including hypertension, diabetes, respiratory disease, CVD, kidney disease, liver disease, hepatitis infection, etc. have been identified as risks factors associated with severe disease manifestation and poor prognosis. 26 , 27 , 38 Out of 237 cases with information on comorbidities, 151 (63.7%) had at least one comorbidity. Hypertension was the most prevalent comorbidity observed in coinfected patients followed by diabetes (Figure 4). In the original case reports included in this study, a great part of the of the dead were reported to have multiple comorbidities. Thus, it is possible the higher overall case‐fatality rate reported earlier (14%) was compounded by these other comorbidities and not sole contribution of HIV infection. Nevertheless, HIV infection is generally characterized by various comorbidities 46 and associated morbidity and mortality are inevitable.
Table 1.
Clinical characteristics of HIV/SARS‐CoV‐2 coinfected patients
| Country | HIV/COVID‐19 coinfection | At least one comorbidity (n = 237) | ART before COVID‐19 (n = 244) | Viral load < 50 (n = 216) | CD4 count > 200 (n = 188) | Reference |
|---|---|---|---|---|---|---|
| Austria | 1 | 1 | 1 | 1 | 1 | 47 |
| China | 9 | 1 | 4 | 0 | 2 | 33 , 34 , 45 , 48 , 49 , 50 , 51 , 52 |
| Cyprus | 1 | NA | 1 | 1 | 1 | 53 |
| Germany | 33 | 20 | 33 | 30 | 32 | 54 |
| Italy | 47 | 30 | 36 | 44 | 0 | 28 |
| Japan | 1 | NA | NA | 0 | 0 | 55 |
| Singapore | 1 | 0 | 1 | 1 | 1 | 56 |
| S. Africa | 1 | 1 | 1 | 1 | 0 | 57 |
| Spain | 56 | 34 | 55 | 54 | 49 | 29 , 36 |
| Turkey | 4 | 1 | 3 | 0 | 3 | 30 |
| Uganda | 1 | 0 | 1 | 0 | 1 | 8 |
| UK | 101 | 6 | 18 | 17 | 14 | 26 , 31 |
| USA | 122 | 57 | 74 | 54 | 60 | 27 , 37 , 40 , 41 , 58 , 59 , 60 , 61 , 62 |
| Overall | 378 | 151 | 228 | 203 | 164 |
Abbreviations: COVID‐19, coronavirus disease 2019; HIV, human immunodefeciency virus; NA, not available; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Figure 1.
Demographic characteristics of HIV/SARS‐CoV‐2 coinfected patients
Figure 2.
Disease Severity associated with HIV/SARS‐CoV‐2 coinfected patients
Figure 3.
Outcome reported endpoint for HIV/SARS‐CoV‐2 coinfected patients
Figure 4.
Common comorbidities associated with HIV/SARS‐CoV‐2 coinfection. CVD, cardiovascular diseases; HLD, hyperlipidemia
4. CONCLUSION
It is generally accepted that, at present, our knowledge about the coronavirus is not definite and keeps evolving with emerging evidence, nonetheless, the preliminary analysis of available evidence shows that PLWH are not protected from COVID‐19 or severity of the disease. Also, HIV‐related immunosuppression may increase risk of severity of COVID‐19 instead confer protection. While the study does not show excess morbidity and mortality among PLWH, especially those with viral load suppression on ART, people living with HIV ought to be vigilant and adhere strictly to guidelines and recommendations of how to keep themselves safe from SARS‐CoV‐2 infection.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
ONK and PA contributed to the ideas and organization of the content and wrote the manuscript. All authors have read and approved this final version of the manuscript.
Supporting information
Supporting information
ACKNOWLEDGMENTS
We acknowledge the selfless efforts of healthcare professionals and all other essential service providers in the fight against COVID‐19.
Kanwugu ON, Adadi P. HIV/SARS‐CoV‐2 coinfection: A global perspective. J Med Virol. 2021;93:726–732. 10.1002/jmv.26321
Osman N. Kanwugu and Parise Adadi contributed equally to this work.
REFERENCES
- 1. World Health Organization . Coronavirus disease 2019 (COVID‐19): situation reports. 2020.
- 2. University JH . Johns Hopkins Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu/map.html2020)
- 3. Sun Y, Koh V, Marimuthu K, et al. Epidemiological and clinical predictors of COVID‐19. Clin Infect Dis. 2020. 10.1093/cid/ciaa322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected: interim guidance, 13 March 2020: World Health Organization, 2020.
- 5. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases with COVID‐19: a retrospective review of medical records in a single medical center, Wuhan, China. Intl J Infect Dis. 2020;94:128‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu L, Chen S, Fu Y, et al. Risk factors associated with clinical outcomes in 323 COVID‐19 hospitalized patients in Wuhan, China. Clin Infect Dis. 2020. 10.1093/cid/ciaa539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baluku JB, Mwebaza S, Ingabire G, Nsereko C, Muwanga M. HIV and SARS‐CoV‐2 co‐infection: a case report from Uganda. J Med Virol. 2020. 10.1002/jmv.26044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. The Joint United Nations Programme on HIV/AIDS (UNAIDS) . Global HIV & AIDS statistics — 2020 fact sheet, 2020. https://www.unaids.org/en/resources/fact-sheet
- 10. Amimo F, Lambert B, Magit A. What does the COVID‐19 pandemic mean for HIV, tuberculosis, and malaria control? Trop Med Health. 2020;48:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sun S, Hou J, Chen Y, Lu Y, Brown L, Operario D. Challenges to HIV care and psychological health during the COVID‐19 pandemic among people living with HIV in China. AIDS Behav. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pinto RM, Park S. COVID‐19 pandemic disrupts HIV continuum of care and prevention: implications for research and practice concerning community‐based organizations and frontline providers. AIDS Behav. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cairns G. Disruption to HIV treatment in Africa during COVID‐19 pandemic could double HIV deaths, modelling studies warn. Accessed June 2, 2020. https://www.aidsmap.com/news/may-2020/disruption-hiv-treatment-africa-during-covid-19-pandemic-could-double-hiv-deaths
- 14. Holt E. HIV services take a backseat to COVID‐19 in Russia. 2020. Accessed June 2, 2020. http://www.ipsnews.net/2020/05/hiv-services-take-a-backseat-to-covid-19-in-russia/
- 15. Adadi P, Kanwugu ON. Living with HIV in the time of COVID‐19: a glimpse of hope. J Med Virol. 2020:jmv.26118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. MacIntyre CR, Heslop DJ. Public health, health systems and palliation planning for COVID‐19 on an exponential timeline. Med J Aust. 2020:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kowalska JD, Skrzat‐Klapaczyńska A, Bursa D, et al. HIV care in times of the COVID‐19 crisis—where are we now in Central and Eastern Europe? Intl J Infect Dis. 2020;S1201‐9712(20):30319‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. The Lancet HIV . Lockdown fears for key populations. Lancet HIV. 2020;7(6):e373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guo Wei WH, Hua B, Jie L, Xiangnan W, Kai Z, Amakobe S. A rapid assessment of the impact of the new coronavirus pneumonia epidemic on the health needs of HIV‐infected patients. Chinese J Epidemiol. 2020;41:5. [Google Scholar]
- 20. Gokengin D, Oprea C, Begovac J, et al. HIV care in Central and Eastern Europe: How close are we to the target? Int J Infect Dis. 2018;70:121‐30. [DOI] [PubMed] [Google Scholar]
- 21. Luis H, Fridayantara WD, Mahariski P, et al. Crisis for people living with HIV in Indonesia. Lancet HIV. 2020;7(6):e384‐e385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shiau S, Krause KD, Valera P, Swaminathan S, Halkitis PN. The burden of COVID‐19 in people living with HIV: a syndemic perspective. AIDS Behav. 2020:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feldman C. Potential impact of SARS‐CoV‐2 infection in HIV‐positive patients in South Africa. Wits J Clin Med. 2020;2(SI):19‐24. [Google Scholar]
- 24. Morel T, Maher D, Nyirenda T, Olesen OF. Strengthening health research capacity in sub‐Saharan Africa: mapping the 2012‐2017 landscape of externally funded international postgraduate training at institutions in the region. Global Health. 2018;14(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chu KM, Jayaraman S, Kyamanywa P, Ntakiyiruta G. Building research capacity in Africa: equity and global health collaborations. PLoS Med. 2014;11(3):e1001612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA. 2020;323:e206775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gervasoni C, Meraviglia P, Riva A, et al. Clinical features and outcomes of HIV patients with coronavirus disease 2019. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vizcarra P, Pérez‐Elías MJ, Quereda C, et al. Description of COVID‐19 in HIV‐infected individuals: a single‐centre, prospective cohort. Lancet HIV. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Altuntas Aydin O, Kumbasar Karaosmanoglu H, Kart Yasar K. HIV/SARS‐CoV‐2 co‐infected patients in Istanbul, Turkey. J Med virol. 2020. [DOI] [PubMed] [Google Scholar]
- 31. Childs K, Post FA, Norcross C, et al. Hospitalized patients with COVID‐19 and HIV: a case series. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen J, Qi T, Liu L, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID‐19) in Taizhou, Zhejiang, China. Infection. 2020;80:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang L, Duan Y, Zhang W, et al. Epidemiologic and clinical characteristics of 26 cases of COVID‐19 arising from patient‐to‐patient transmission in Liaocheng, China. Clin Epidemiol. 2020;12:387‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kato H, Shimizu H, Shibue Y, et al. Clinical course of 2019 novel coronavirus disease (COVID‐19) in individuals present during the outbreak on the Diamond Princess cruise ship. J Infect Chemother 2020. 2020 Aug;26:865‐869. 10.1016/j.jiac.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Blanco JL, Ambrosioni J, Garcia F, et al. COVID‐19 in patients with HIV: clinical case series. Lancet HIV. 2020;7(5):e314‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shalev N, Scherer M, LaSota ED, et al. Clinical characteristics and outcomes in people living with HIV hospitalized for COVID‐19. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guan W, Liang W, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Center JHCR . Mortality analyses. 2020. Accessed August 6, 2020. https://coronavirus.jhu.edu/data/mortality
- 40. Benkovic S, Kim M, Sin E. 4 Cases: HIV and SARS‐CoV‐2 co‐infection in patients from Long Island, New York. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suwanwongse K, Shabarek N. Clinical features and outcome of HIV/SARS‐CoV‐2 co‐infected patients in the Bronx, New York City. J Med Virol. 2020. 10.1002/jmv.26077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Costanzo M, De Giglio MAR, Roviello GN. SARS‐CoV‐2: recent reports on antiviral therapies based on lopinavir/ritonavir, darunavir/umifenovir, hydroxychloroquine, remdesivir, favipiravir and other drugs for the treatment of the new coronavirus. Curr Med Chem. 2020. [DOI] [PubMed] [Google Scholar]
- 43. Mascolo S, Romanelli A, Carleo MA, Esposito V. Could HIV infection alter the clinical course of SARS‐CoV‐2 infection? When less is better. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laurence J Why aren't people living with HIV at higher risk for developing severe coronavirus disease 2019 (COVID‐19)? AIDS Patient Care STDs 2020. [DOI] [PubMed]
- 45. Su J, Shen X, Ni Q, et al. Infection of severe acute respiratory syndrome coronavirus 2 in a patient with acquired immunodeficiency syndrome. AIDS. 2020. [DOI] [PubMed]
- 46. Jones R, Nelson M, Bracchi M, Asboe D, Boffito M. COVID‐19 in patients with HIV. Lancet HIV. 2020;7:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Müller H, Kniepeiss D, Stauber R, et al. Recovery from COVID‐19 following hepatitis C, human immunodeficiency virus infection, and liver transplantation. Am J Transpl. 2020. 10.1111/ajt.16107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu Q, Chen T, Zhang H. Recovery from COVID‐19 in two patients with coexisted HIV infection. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chen J, Cheng X, Wang R, Zeng X. Computed tomography imaging of an HIV‐infected patient with coronavirus disease 2019 (COVID‐19). J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang M, Luo L, Bu H, Xia H. One case of coronavirus disease 2019 (COVID‐19) in a patient co‐infected by HIV with a low CD4(+) T‐cell count. Int J Infect Dis. 2020;96:148‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhao J, Liao X, Wang H, et al. Early virus clearance and delayed antibody response in a case of COVID‐19 with a history of co‐infection with HIV‐1 and HCV. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhu F, Cao Y, Xu S, Zhou M. Co‐infection of SARS‐CoV‐2 and HIV in a patient in Wuhan city, China. J Med Virol. 2020;92:529‐530. 10.1002/jmv.25732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Iordanou S, Koukios D, Matsentidou‐Timiliotou C, Markoulaki D, Raftopoulos V. Severe SARS‐CoV‐2 pneumonia in a 58‐year‐old patient with HIV: a clinical case report from the Republic of Cyprus. J Med Virol. 2020. 10.1002/jmv.26053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Härter G, Spinner CD, Roider J, et al. COVID‐19 in people living with human immunodeficiency virus: a case series of 33 patients. Infection. 2020:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nakamoto T, Kutsuna S, Yanagawa Y, et al. A case of SARS‐CoV‐2 infection in an untreated HIV patient in Tokyo, Japan. J Med Virol. 2020. 10.1002/jmv.26102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Louisa SJ, Lin Serene WX, Gollamudi S. A case of HIV and SARS‐CoV‐2 co‐infection in Singapore. J Acquir Immune Defic Syndr. 2020;84(4):e23‐e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Parker A, Shaw J, Karamchand S, et al. HIV and SARS‐CoV‐2 co‐infection: the diagnostic challenges of dual pandemics. SAMJ. 2020;110:6. [DOI] [PubMed] [Google Scholar]
- 58. Kumar RN, Tanna SD, Shetty AA, Stosor V. COVID‐19 in an HIV‐positive kidney transplant recipient. Transpl Infect Dis. 2020:13338. 10.1111/tid.13338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ridgway JP, Farley B, Benoit JL, et al. A case series of five people living with HIV hospitalized with COVID‐19 in Chicago, Illinois. AIDS Patient Care STDS. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patel RH, Pella PM. COVID‐19 in a patient with HIV infection. J Med Virol. 2020:jmv.26049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mahmood K, Rashed ER, Oliveros E, et al. Predisposition or protection? COVID‐19 in a patient on LVAD support with HIV/AIDS. JACC Case Rep. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Okoh AK, Bishburg E, Grinberg S, Nagarakanti S. COVID‐19 pneumonia in patients with HIV—A case series. J Acquir Immune Defic Syndr. 1999:2020. 10.1097/QAI.0000000000002411 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


