Abstract
Introduction
Management of COVID‐19 in kidney transplant recipients should include treatment of the infection, regulation of immunosuppression, and supportive therapy. However, there is no consensus on this issue yet. This study aimed to our experiences with kidney transplant recipients diagnosed with COVID‐19.
Material and Methods
Kidney transplant recipients diagnosed with COVID‐19 from five major transplant centers in Istanbul, Turkey, were included in this retrospective cohort study. Patients were classified as having moderate or severe pneumonia for the analysis. The primary endpoint was all‐cause mortality. The secondary endpoints were acute kidney injury, the average length of hospital stay, admission to intensive care, and mechanical ventilation.
Results
Forty patients were reviewed retrospectively over a follow‐up period of 32 days after being diagnosed with COVID‐19. Cough, fever, and dyspnea were the most frequent symptoms in all patients. The frequency of previous induction and rejection therapy was significantly higher in the group with severe pneumonia compared to the moderate pneumonia group. None of the patients using cyclosporine A developed severe pneumonia. Five patients died during follow‐up in the intensive care unit. None of the patients developed graft loss during follow‐up.
Discussion
COVID‐19 has been seen to more commonly cause moderate or severe pneumonia in kidney transplant recipients. Immunosuppression should be carefully reduced in these patients. Induction therapy with lymphocyte‐depleting agents should be carefully avoided in kidney transplant recipients during the pandemic period.
Keywords: acute kidney injury, antiviral agents, COVID‐19, cytokine release syndrome, cytokine‐targeted therapy, kidney transplantation, respiratory distress syndrome, adult
1. INTRODUCTION
The strain of coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) that causes coronavirus disease 2019 (COVID‐19) was detected for the first time in Wuhan, China in December 2019. 1 The data on kidney transplant recipients diagnosed with COVID‐19 are limited, and recommendations are based on case series and expert opinion. 1 , 2 , 3 , 4 , 5 , 6 , 7 Although uremia and kidney transplantation are not considered a risk factor in COVID‐19, SARS‐CoV‐2 often causes a moderate or severe infection in kidney transplant recipients. 1 , 2 , 3 , 4 , 5 , 6 , 7 Lymphopenia, increased inflammatory markers, prothrombin time, and creatine phosphokinase are prognostic factors for COVID‐19. 8 , 9 Acute kidney injury is also associated with increased morbidity and mortality in COVID‐19 patients. 9 However, it is unclear which markers are useful for the monitoring of kidney transplant recipient patients.
Major complaints are fever, dry cough, fatigue, shortness of breath, and diarrhea. 1 , 2 , 3 , 4 , 5 , 6 Pneumonia is the most common manifestation of the infection, characterized by infiltrates in the lung. Cytokine storms also cause organ dysfunction, which may lead to death. 1 , 2 , 3 , 4 , 5 , 6 Diagnosis is usually based on fever, respiratory symptoms, contact history, typical chest radiology, and biochemical findings. 6 , 7 , 8 , 9 Positive reverse transcription‐polymerase chain reaction (RT‐PCR) results for COVID‐19 in oral and nasopharyngeal swabs confirm the diagnosis. 10 , 11 Management of COVID‐19 in kidney transplant recipients should include treatment of the infection, regulation of immunosuppression, and supportive therapy. 1 , 2 , 3 , 4 , 5 , 6 , 7 Local transplant centers have created their treatment regimens for COVID‐19 patients. However, there is no consensus on this issue yet. Kidney transplant recipients face several difficulties with this new infectious disease. This study aims to describe our experiences with kidney transplant recipients diagnosed with COVID‐19.
2. MATERIAL AND METHODS
2.1. Study population and design
Kidney transplant recipients diagnosed with COVID‐19 from five major transplant centers in Istanbul, Turkey, were included in this retrospective cohort study. Initially, 44 kidney transplant recipients diagnosed with COVID‐19 and identified between February 1, 2020 and May 4, 2020 were enrolled. The following exclusion criteria were applied for the study: (a) patients without typical findings (n = 1), (b) loss to follow‐up after diagnosis of COVID‐19 (n = 3). After the diagnosis of COVID‐19, the remaining 40 participants were followed up for a period of at least 15 days or until death (Figure 1). The Medical Ethics Committee of the Istanbul Faculty of Medicine approved this study.
FIGURE 1.
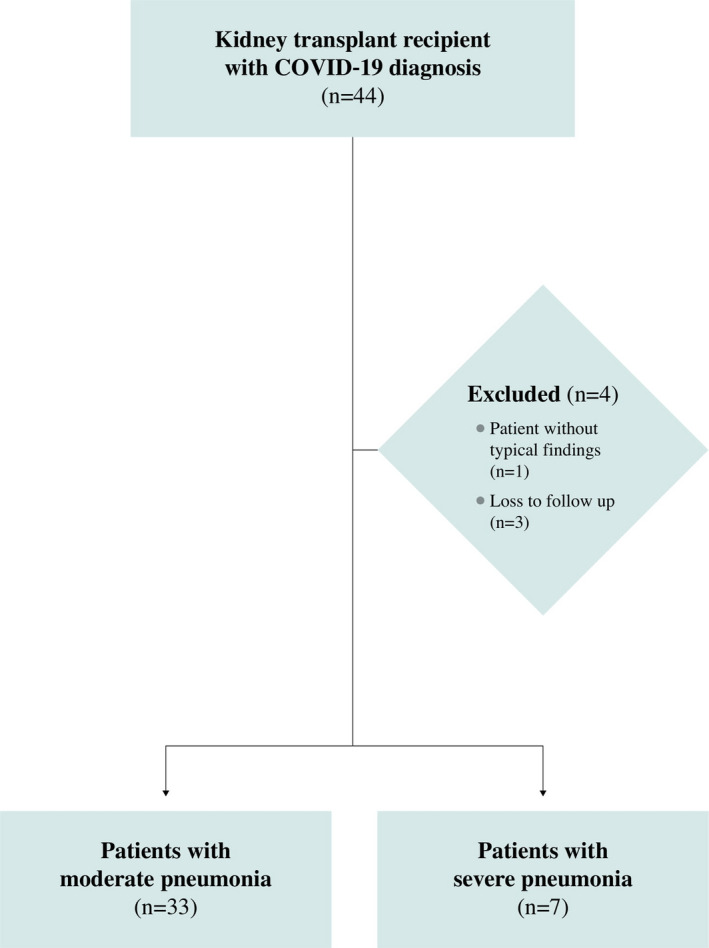
Flowchart of the patients in the study. COVID‐19, coronavirus disease 2019
The patients were categorized into two groups for analysis. Patients with respiratory failure requiring mechanical ventilation, septic shock, or multiple organ dysfunction were defined as having severe pneumonia. All other cases were classified as having moderate pneumonia.
2.2. Data collection
The demographics, clinical data, comorbidities, laboratory and radiological results, data on anti‐viral and anti‐cytokine treatments, and management of immunosuppression were extracted from electronic medical records. Nasal and oropharyngeal swabs were collected and tested for SARS‐CoV‐2 RNA with RT‐PCR assay. All patients underwent non‐contrast chest computerized tomography (CT) scanning in the supine position during end‐inspiration. All cases demonstrated early or progressive radiographic deterioration on CT at the initial admission.
2.3. Patient management
The COVID‐19 diagnosis was based on contact history, symptoms, laboratory, and radiological findings. A positive RT‐PCR test was used to confirm the diagnosis. Indications for hospitalization were moderate, severe pneumonia and cytokine release syndrome (persistent fever, blood lymphocyte count <800/mm3, serum C‐reactive protein > 40 mg/L, aspartate aminotransferase >45 IU/L, ferritin > 500 ng/mL, D‐dimer > 1000 ng/mL, and triglyceride > 150 mg/dL). Criteria for admission to the intensive care unit (ICU) were a partial pressure of arterial oxygen and inspiratory oxygen fraction (PaO2/FiO2) ratio less than 300, oxygen saturation under 90% and PaO2 below 70 mm Hg despite 5 L/min oxygen therapy, and/or persistent hypotension (systolic blood pressure < 90 mm Hg or mean arterial pressure < 65 mm Hg).
2.4. Anti‐viral and cytokine‐targeted therapy
All of the patients were initially treated with hydroxychloroquine (400 mg BID for the first day, and then 200 mg BID for four days; oral) and azithromycin (500 mg QD for the first day, and then 250 mg QD for four days; oral). Tocilizumab (400 mg QD for two days; intravenous) or anakinra (100 mg QD for 7‐14 days or until hospital discharge; subcutaneous) were used in the treatment of cytokine release syndrome. Resistant cases were treated with favipiravir (1600 mg BID for the first day, and then 600 mg BID for four days; oral). Antibiotic therapy was administered based on the infection specialist's decision in the presence of confirmed or suspected invasive bacterial infection. The patients were also monitored for adverse drug reactions during the hospital stay. The QT interval of all patients was regularly monitored.
2.5. Adjustment of immunosuppressive regimen
A standard protocol was used to manage immunosuppression, anti‐viral, and cytokine‐targeted therapy (Figure 2). Antimetabolites (mycophenolate derivatives and azathioprine) were discontinued in all cases. Steroid doses were increased to a stress dose in the early period and then continued at a maintenance dose. Calcineurin doses were halved in patients with a stable clinic course and were discontinued in hypoxemic patients. The calcineurin and mammalian target of rapamycin (mTOR) levels were also monitored twice a week.
FIGURE 2.
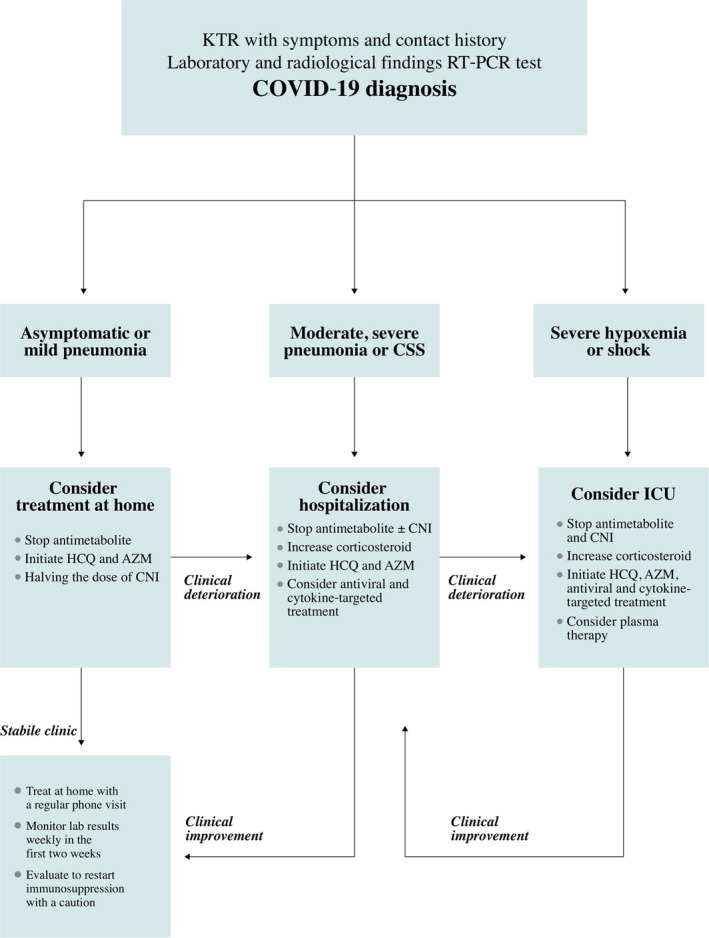
Treatment scheme for COVID‐19 in kidney transplant recipients. AZM, azithromycin; CNI, calcineurin inhibitors; COVID‐19, coronavirus disease 2019; CSS, cytokine storm syndrome; HCQ, hydroxychloroquine; ICU, intensive care unit; KTR, kidney transplant recipient; RT‐PCR, reverse transcription‐polymerase chain reaction
2.6. Anticoagulation and oxygen treatment
Low‐molecular‐weight heparin was used in all patients unless there were contraindications. Doses were adjusted according to the patients' risk of bleeding and coagulation. Oxygen treatment was provided to patients whose oxygen saturation was below 92% via a nasal cannula, and with a mask with a reservoir, if this was insufficient. If respiratory failure continued despite these treatments, mechanical ventilation was provided after non‐invasive ventilation.
2.7. Outcomes
The primary endpoint was all‐cause mortality. The secondary endpoints were acute kidney injury, the average length of hospital stay, admission to intensive care, and mechanical ventilation.
2.8. Statistical analysis
Categorical variables were summarized with counts and percentages. Quantitative variables were summarized with means and standard deviations or medians and interquartile range statistics, where appropriate. The chi‐square and Fisher's exact tests were performed for qualitative variables, whereas the Mann‐Whitney U test was used for quantitative variables with the non‐parametric distribution. Logistic regression analysis was used to identify patient loss and the associated risk in terms of odds ratio and 95% confidence intervals. Variables were selected by backward elimination using likelihood ratio tests. A P‐value of <.05 was considered significant.
3. RESULTS
3.1. Demographics and clinical characteristics
Forty patients (20 female) were reviewed retrospectively over a median follow‐up period of 32 days (interquartile range, 14‐51 days) after being diagnosed with COVID‐19. The demographic characteristics and immunosuppression regimen of patients according to their groups, are shown in Table 1. Only one patient (3%) in the moderate pneumonia group was followed up in the outpatient clinic, and all other patients were hospitalized. No differences were observed between the groups in terms of age, gender, post‐transplant follow‐up time, etiology of chronic kidney disease (CKD), donor type, and comorbidities (Table 1). Moderate pneumonia was detected in 33 (82.5%) kidney transplant recipients and severe pneumonia in 7 (17.5%). The frequency of previous induction (n = 18 [54.6%] and n = 7 [100%]; P = .016, respectively) and rejection therapy (n = 4 [12.1%] and n = 3 [42.9%]; P = .023, respectively) was significantly higher in the group with severe pneumonia compared to the moderate pneumonia group. Although no significant difference was found between the groups in terms of tacrolimus usage (n = 25 [75.8%] and n = 6 [85.7%]; P = .525, respectively), none of the patients using cyclosporine A (n = 5 [15.2%] and n = 0 [0%]; P < .001, respectively), developed severe pneumonia.
TABLE 1.
Patients' demographic characteristics and immunosuppression regimen
| All patients (n = 40) | Moderate pneumonia (n = 33) | Severe pneumonia (n = 7) | P‐value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (mean ± SD, y) | 44.9 ± 14.8 | 43.3 ± 14.9 | 49.3 ± 14.8 | .388 |
| Sex (n, %) | ||||
| Male | 20 (50%) | 17 (51.5%) | 3 (42.9%) | .05 |
| Female | 20 (50%) | 16 (48.5%) | 4 (57.1%) | |
| Post‐transplant follow‐up (Median‐IQR 25‐75, mo) | 74.5 (31.5‐128.3) | 77 (29.5‐133) | 74 (49‐108) | .746 |
| Etiology of CKD (n, %) | ||||
| Hypertensive nephropathy | 4 (10%) | 4 (12.1%) | 0 | .416 |
| Diabetic nephropathy | 2 (5%) | 1 (3%) | 1 (14.3%) | |
| Chronic glomerulonephritis | 13 (32.5%) | 10 (30.3%) | 3 (42.9%) | |
| Other | 6 (15%) | 6 (18.2%) | 0 | |
| Unknown | 15 (37.5%) | 12 (36.4%) | 3 (42.9%) | |
| Comorbidities (n, %) | ||||
| Pre‐existing lung disease | 3 (7.5%) | 3 (9.1%) | 0 | .552 |
| Previous heart disease | 3 (7.5%) | 3 (9.1%) | 0 | .552 |
| Chronic hypertension | 26 (65%) | 21 (63.6%) | 5 (71.4%) | .529 |
| Usage of RAS blockage | 18 (45%) | 15 (45.5%) | 3 (42.9%) | .617 |
| Type of donor (n, %) | ||||
| Living | 35 (87.5%) | 30 (90.9%) | 5 (71.4%) | .204 |
| Deceased | 5 (12.5%) | 3 (9.1%) | 2 (28.6%) | |
| Immunosuppression | ||||
| Induction therapy (n, %) | ||||
| ATLG | 22 (55%) | 15 (45.5%) | 7 (100%) | .016 |
| Basiliximab | 3 (7.5%) | 3 (9.1%) | 0 | |
| Anti‐rejection therapy (n, %) | 7 (17.5%) | 4 (12.1%) | 3 (42.9%) | .023 |
| Maintenance immunosuppression at admission (n, %) | ||||
| Tacrolimus | 31 (77.5%) | 25 (75.8%) | 6 (85.7%) | .525 |
| Cyclosporine A | 5 (12.5%) | 5 (15.2%) | 0 | <.001 |
| mTOR inhibitors | 4 (10%) | 3 (9.1%) | 1 (14.3%) | .502 |
| Mycophenolate derivatives | 36 (90%) | 29 (87.9%) | 7 (100%) | .624 |
| Steroids | 40 (100%) | 33 (100%) | 7 (100%) | 1 |
P‐values compared moderate pneumonia and severe pneumonia, obtained from the chi‐square test, Fisher's exact test, or Mann‐Whitney U test.
P‐values in bold showed statistically significant differences.
Abbreviations: ATLG, anti‐T‐lymphocyte globulin; CKD, chronic kidney disease; mTOR, mammalian target of rapamycin.
3.2. Clinical presentations and laboratory results
Cough (n = 30 [75%]), fever (n = 25 [62.5%]), and dyspnea (n = 21 [52.5%]) were the most frequent symptoms in all patients. Although all serum acute phase reactants were more increased in patients with severe pneumonia compared to the other group (Table 2), a significant difference was also found in the serum lactate dehydrogenase (249 [interquartile range, 174‐340] and 559 [interquartile range, 275‐666]; P = .048, respectively) and alanine aminotransferase values only (15 [interquartile range, 8‐23] and 27 [interquartile range, 23‐39]; P = .015, respectively). The frequency of graft dysfunction was similar between the groups (n = 12 [36.4%] and n = 2 [28.6%]; P = .615, respectively). None of the patients developed graft loss during follow‐up (Table 2).
TABLE 2.
Patients' clinical characteristics and laboratory results
| All patients (n = 40) | Moderate pneumonia (n = 33) | Severe pneumonia (n = 7) | P‐value | |
|---|---|---|---|---|
| Time | ||||
| Post‐infection follow‐up (Median‐IQR 25‐75, d) | 32 (23‐44) | 31 (22‐41) | 44 (41‐51) | .087 |
| Duration of hospitalization (Median‐IQR 25‐75, d) | 9 (5‐12) | 7 (4‐12) | 13 (11‐28) | .002 |
| Clinical characteristics | ||||
| Contact history (n, %) | 20 (50%) | 15 (45.5%) | 5 (71.4%) | .204 |
| Presentation symptoms (n, %) | ||||
| Fever | 25 (62.5%) | 19 (57.6%) | 6 (85.7%) | .168 |
| Cough | 30 (75%) | 25 (75.8%) | 5 (71.4%) | .575 |
| Dyspnea | 21 (52.5%) | 14 (42.4%) | 7 (100%) | .006 |
| Diarrhea | 10 (25%) | 9 (27.3%) | 1 (14.2%) | .51 |
| Initial examination findings (Median‐IQR 25‐75) | ||||
| Pulse rate (/min) | 88 (79‐98) | 84 (78‐98) | 94 (86‐101) | .344 |
| SpO2 value (%) | 96 (93‐98) | 96 (94‐98) | 90 (88‐96) | .007 |
| Respiratory rate (/min) | 18 (16‐24) | 18 (16‐23) | 22 (18‐26) | .646 |
| Blood pressure (Median‐IQR 25‐75, mm Hg) | ||||
| Systolic | 120 (114‐135) | 120 (110‐140) | 120 (118‐133) | .5 |
| Diastolic | 70 (68‐80) | 70 (65‐80) | 80 (69‐83) | .475 |
| Laboratory results | ||||
|
Laboratory results at admission (Median‐IQR 25‐75) | ||||
| Serum creatinine (mg/dL) | 1.6 (1.2‐2.2) | 1.7 (1.4‐2.4) | 1.2 (1.1‐2.1) | .288 |
| Leucocyte count (/mm3) | 5150 (4013‐7035) | 4635 (3975‐7110) | 6250 (4190‐6825) | .75 |
| Lymphocyte count (/mm3) | 725 (503‐1080) | 755 (498‐1100) | 660 (420‐1035) | .566 |
| Hemoglobin count (g/dL) | 11.2 (9.7‐12.7) | 11.1 (9.6‐12.8) | 11.3 (9.2‐12.5) | .865 |
| Platelet count (/mm3) | 192 (154‐238) | 192 (160‐237) | 192 (138‐266) | .932 |
| Serum CRP levels (mg/L) | 45 (24‐88) | 44 (15‐78) | 66 (35‐167) | .235 |
| Serum ALT levels (IU/L) | 16 (10‐27) | 15 (8‐23) | 27 (23‐39) | .015 |
| Serum AST levels (IU/L) | 23 (15‐34) | 22 (13‐30) | 34 (20‐43) | .064 |
| Serum LDH levels (IU/L) | 257 (198‐370) | 249 (174‐340) | 559 (275‐666) | .048 |
| Serum D‐dimer levels (ng/mL) | 720 (510‐1374) | 665 (505‐1469) | 1075 (598‐1343) | .593 |
| Serum ferritin levels (ng/mL) | 358 (173‐992) | 319 (161‐871) | 634 (264‐1906) | .329 |
| Serum procalcitonin (ng/mL) | 0.2 (0.1‐0.3) | 0.2 (0.1‐0.3) | 0.4 (0.2‐1.1) | .13 |
| Graft dysfunction at admission (n, %) | 14 (35%) | 12 (36.4%) | 2 (28.6%) | .615 |
P‐values compared moderate pneumonia and severe pneumonia, obtained from the chi‐square test, Fisher's exact test, or Mann‐Whitney U test.
P‐values in bold showed statistically significant differences.
Abbreviations: SpO2 = blood oxygen saturation levels; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C‐reactive protein; LDH, lactate dehydrogenase.
3.3. Outcomes
Five patients (12.5%) died during follow‐up in the ICU. Two patients died due to respiratory failure, and three patients due to multiorgan failure associated with cytokine release syndrome. The dosage of calcineurin inhibitors was halved in 23 (69.7%) patients with moderate pneumonia and two (28.6%) with severe pneumonia. Eighteen patients (45%) were treated with favipiravir and eight patients (20%) with cytokine‐targeted therapy. Anti‐viral (n = 12 [36.4%] and n = 6 [85.7%]; P = .024, respectively) and cytokine‐targeted therapy (n = 5 [15.2%] and n = 3 [42.9%]; P = .024, respectively) were more widely used in the severe pneumonia group. Twenty‐four patients (60%) received antibiotics. Six of the seven patients (85.7%) with severe pneumonia who were followed up in the ICU received mechanical ventilation (Table 3). No arrhythmia or other adverse events were detected during follow‐up. No significant pharmacological interaction was observed between the drugs and calcineurin inhibitors during the follow‐up. No significant differences were observed in the calcineurin levels.
TABLE 3.
Patients' treatment regimens and outcomes
| All patient s (n = 40) | Moderate pneumonia (n = 33) | Severe pneumonia (n = 7) | P‐value | |
|---|---|---|---|---|
| Treatment modalities and outcomes | ||||
| Withdrawal of IS agent (n, %) | ||||
| Calcineurin inhibitors | 11 (27.5%) | 7 (21.2%) | 4 (57.1%) | .075 |
| Antimetabolites | 40 (100%) | 33 (100%) | 7 (100%) | 1 |
| mTOR inhibitors | 4 (10%) | 3 (9.1%) | 1 (14.3%) | 1 |
| Treatment of infection (n, %) | ||||
| Favipiravir | 18 (45%) | 12 (36.4%) | 6 (85.7%) | .024 |
| Anti‐cytokine agents (n, %) | ||||
| Tocilizumab | 5 (12.5%) | 2 (6.1%) | 3 (42.9%) | .024 |
| Anakinra | 3 (7.5%) | 3 (9.1%) | 0 | |
| Antibiotics (n, %) | 24 (60%) | 21 (63.6%) | 3 (42.9%) | .273 |
| Ventilation devices (n, %) | ||||
| Nasal cannula | 37 (92.5%) | 30 (90.9%) | 7 (100%) | 0.407 |
| Non‐invasive ventilation | 4 (10%) | 1 (3%) | 3 (42.9%) | .013 |
| Mechanical ventilation | 6 (15%) | 0 | 6 (85.7%) | <.001 |
| Follow‐up in intensive care unit | 7 (17.5%) | 0 | 7 (100%) | <.001 |
| Number of patient fatalities (n, %) | 5 (12.5%) | 0 | 5 (71.4%) | <.001 |
P‐values compared moderate pneumonia and severe pneumonia, obtained from the chi‐square test, Fisher's exact test, or Mann‐Whitney U test.
P‐values in bold showed statistically significant differences.
Abbreviations: IS, immunosuppression; mTOR, mammalian target of rapamycin.
Multivariate logistic regression analysis also revealed that previous anti‐rejection therapy (9.75 [95% CI, 1.223 to 77.724; P = .032]) was the independent predictor for mortality (Table 4). The use of cyclosporine (0.077 [95% CI, 0.018‐0.324; P ≤ .001], respectively) was associated with a lower incidence of death.
TABLE 4.
Logistic regression analysis of mortality risk factors for kidney transplant recipients
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Odds ratio | Confidence interval | P | Odds ratio | Confidence interval | P | |
| Age | 0.992 | 0.931‐1.058 | .812 | |||
| Male sex | 1.588 | 0.236‐10.704 | .635 | |||
| Post‐transplant time | 0.996 | 0.98‐1.012 | .638 | |||
| Deceased donor | 0.516 | 0.046‐5.84 | .593 | |||
| ATLG usage as an induction therapy | 1.83 | 0.522‐6.413 | .345 | |||
| Usage of cyclosporine | 0.167 | 0.065‐0.43 | <.001 | 0.077 | 0.018‐0.324 | <.001 |
| Anti‐rejection therapy | 11.625 | 1.467‐92.139 | .02 | 9.75 | 1.223‐77.724 | .032 |
| SpO2 value | 0.915 | 0.723‐1.158 | .46 | |||
| Serum LDH levels | 1.006 | 0.098‐1.014 | .052 | |||
| Serum ALT levels | 1.009 | 0.974‐1.045 | .622 | |||
| Graft dysfunction at admission | 0.538 | 0.051‐5.74 | .608 | |||
| CNI withdrawal after admission | 0.198 | 0.028‐1.396 | .104 | |||
| Anti‐viral treatment | 6 | 0.606‐59.444 | .126 | |||
| Antibiotics | 0.13 | 0.013‐1.301 | .087 | |||
| Anti‐cytokine agents | 0.31 | 0.042‐2.278 | .25 | |||
P‐values in bold showed statistically significant differences.
Abbreviations: ALT, alanine aminotransferase; ATLG, anti‐T‐lymphocyte globulin; CNI, calcineurin inhibitors; LDH, lactate dehydrogenase; RT‐PCR, reverse transcription‐polymerase chain reaction; SpO2, blood oxygen saturation levels.
4. DISCUSSION
COVID‐19 is a life‐threatening respiratory disease that has affected countries all over the world. SARS‐CoV‐2 has been seen to often lead to lower respiratory tract infections requiring hospitalization in kidney transplant recipients. In this study, we analyzed 40 kidney transplant recipients diagnosed with COVID‐19 over a median follow‐up period of 32 days. All of our patients had moderate to severe pneumonia. All patients except one were followed up in the hospital or ICU. 87.5% of our patients had received kidney transplants from living donors. Severe pneumonia was common in patients receiving anti‐rejection and induction therapy. Hypoxemia was more common in patients with severe pneumonia, and the serum lactate dehydrogenase and alanine aminotransferase values were higher in this group. Five patients died in intensive care during follow‐up. Both cytokine‐targeted therapy and favipiravir were used more commonly in the group with severe pneumonia. Multivariate logistic regression analysis demonstrated that the use of cyclosporine was associated with a lower incidence of death. Conversely, rejection therapy was identified as a risk factor for death.
Early mortality in kidney transplant recipients varies at a rate of 6%‐25%. 1 , 2 , 3 , 4 , 5 , 6 In our study, almost all of our patients were hospitalized with moderate or severe pneumonia. Five patients died during the follow‐up. The lower mortality rate compared with the data from Italy and the USA may be due to several reasons. 2 , 4 The low average age and lower comorbidity rate of the study population could be important reasons. While the average age of our cohort was 44.9, it was reported as being 60 in Akalin et al's series. 2 In the same study, the frequency of hypertension and diabetes mellitus were 94% and 69%, respectively. We found these frequencies to be 65% and 5%, respectively. Another critical point is that our study group consisted of mostly living‐related transplantation patients. This condition may have led to lower induction use and low cumulative immunosuppression, which may have led to a low mortality rate.
Similarly to our study, previous studies have demonstrated that induction and anti‐rejection therapy may be a risk factor for mortality in COVID‐19. 1 , 2 , 3 , 4 , 5 , 6 For this reason, it may be a rational approach to avoid induction therapy in patients with low immunological risk during the COVID‐19 pandemic. Clinicians should be aware that potent immunosuppressants, especially anti‐T‐lymphocyte globulin, are very risky.
The number of coronavirus infections is increasing in kidney transplant recipients. However, the optimal immunosuppression strategy during the COVID‐19 pandemic remains unknown. The ideal approach for continued immunosuppression treatment is to protect against graft rejection and to maintain enough immunity to prevent overwhelming infection. By our protocol, antimetabolites were discontinued initially, calcineurin doses were halved, and steroids were increased in the stress dose. No permanent kidney damage or graft loss occurred during follow‐up. However, the majority of patients in our study population had low immunological risk. Recent studies have demonstrated that acute kidney injury occurs in 21%‐30% of kidney transplant recipients. 2 , 3 Therefore, discontinuation of all immunosuppression in patients with high immunological risk should be treated with caution. It could cause rejection and graft loss.
Concurrently, cyclosporine A inhibits the replication of coronaviruses and may be an effective treatment in patients with macrophage activation syndrome. 12 , 13 , 14 In our study, the use of cyclosporine was associated with a lower incidence of death. Hence, it would be beneficial to investigate the role of cyclosporine A in the treatment of this disease.
The use of tocilizumab has been reported in the treatment of cytokine release syndrome triggered by COVID‐19. 2 , 4 , 5 Previous studies have also demonstrated the use of lopinavir‐ritonavir and darunavir‐ritonavir in the treatment of COVID‐19. Drug‐drug interaction between anti‐viral drugs and calcineurin inhibitors has been reported in these studies. 2 , 4 , 5 To the best of our knowledge, the use of favipiravir and anakinra in the treatment of COVID‐19 in kidney transplant recipients has not been previously reported. Our study was not conducted to investigate the effectiveness of these drugs. However, unlike other anti‐virals, favipiravir does not exhibit drug–drug interaction between calcineurin inhibitors. Therefore, regular monitoring of the calcineurin inhibitor levels may not be necessary when using favipiravir. We did not encounter any problems with the CNI levels in patients taking favipiravir.
With regard to the hydroxychloroquine treatment given to all patients, we did not observe any side effects such as arrhythmia, QT prolongation, nausea, diarrhea, retinal toxicity, cutaneous rash, or hypoglycemia.
Our retrospective study has many limitations; the sample size is small, and the follow‐up period is short. Other limitations were that there is no control group and PCR samples were only collected from patients with moderate or severe symptoms rather than all patients. The many restrictions and prejudices involved have meant that we are unable to draw definitive conclusions from these experiences. Hence, our findings are preliminary and will need to be confirmed in large‐scale prospective cohort studies with longer follow‐up. The use of a standard treatment model may be considered as the strength of the study.
In conclusion, COVID‐19 has been seen to more commonly cause moderate or severe pneumonia in kidney transplant recipients, possibly due to immunosuppressive therapy. The mortality rate of these patients is higher than that of the general population; therefore, immunosuppression should be carefully reduced in these patients. No anti‐viral and cytokine‐targeted therapy have been approved yet for the treatment of COVID‐19. Hence, all drugs should be used with caution in these patients. Induction therapy with lymphocyte‐depleting agents should be carefully avoided in kidney transplant recipients during the pandemic period.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
ED and AT involved in study design. MU, SY and AS involved in carried out experiments. ED and AT involved in data analysis. ED involved in creation figures. ED, MU, EP, AS, BY, ABD, OM, SY, ZAU, ARU, MED, MM, BA, NG, SS, OAO, HY and AT involved in the paper drafting and revision. All authors approved the final version of the manuscript.
ACKNOWLEDGEMENTS
I would like to mention those healthcare professionals who have been victims of COVID‐19 in Turkey. In Turkey, 7428 health workers have been infected and some of these individuals have died from the disease. In particular, I wish to commemorate our teacher, Professor Murat Dilmener, and Cemil Tasçıoğlu, both of whom died of COVID‐19. Both spent many years working as clinicians and teachers at Istanbul University. We remember them with respect and appreciation.
Demir E, Uyar M, Parmaksiz E, et al. COVID‐19 in kidney transplant recipients: A multicenter experience in Istanbul. Transpl Infect Dis. 2020;22:e13371. 10.1111/tid.13371
REFERENCES
- 1. Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID‐19 infection in kidney transplant recipients. Kidney International. 2020;97 (6):1076–1082. 10.1016/j.kint.2020.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and Kidney Transplantation. New England Journal of Medicine. 2020;382 (25):2475–2477. 10.1056/nejmc2011117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Columbia University Kidney Transplant Program . Early Description of Coronavirus 2019 Disease in Kidney Transplant Recipients in New York. Journal of the American Society of Nephrology. 2020;31 (6):1150–1156. 10.1681/asn.2020030375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short‐term outcome of 20 kidney transplant patients admitted for SARS‐CoV2 pneumonia. Kidney International. 2020;97 (6):1083–1088. 10.1016/j.kint.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montagud‐Marrahi E, Cofan F, Torregrosa JV, et al. Preliminary data on outcomes of SARS‐CoV‐2 infection in a Spanish single center cohort of kidney recipients. American Journal of Transplantation. 2020; 10.1111/ajt.15970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johnson KM, Belfer JJ, Peterson GR, Boelkins MR, Dumkow LE. Managing COVID‐19 in Renal Transplant Recipients: A Review of Recent Literature and Case Supporting Corticosteroid‐sparing Immunosuppression. Pharmacotherapy: The Journal of Human Pharmacology and Drug Therapy. 2020;40 (6):517–524. 10.1002/phar.2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. López V, Vázquez T, Alonso‐Titos J, et al. Recomendaciones en el manejo de la pandemia por coronavirus SARS‐CoV‐2 (Covid‐19) en pacientes con trasplante renal. Nefrología. 2020;40 (3):265–271. 10.1016/j.nefro.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang D, Hu B, Hu C, et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA. 2020;323 (11):1061. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. The Lancet. 2020;395 (10229):1054–1062. 10.1016/s0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ai T, Yang Z, Hou H, et al. Correlation of Chest CT and RT‐PCR Testing in Coronavirus Disease 2019 (COVID‐19) in China: A Report of 1014 Cases. Radiology. 2020;200642 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cheng MP, Papenburg J, Desjardins M, et al. Diagnostic Testing for Severe Acute Respiratory Syndrome–Related Coronavirus 2. Annals of Internal Medicine. 2020;172 (11):726–734. 10.7326/m20-1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. de Wilde AH, Zevenhoven‐Dobbe JC, van der Meer Y, et al. Cyclosporin A inhibits the replication of diverse coronaviruses. Journal of General Virology. 2011;92 (11):2542–2548. 10.1099/vir.0.034983-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ravelli A. Macrophage activation syndrome. Curr Opin Rheumatol. 2002;14:548‐552. [DOI] [PubMed] [Google Scholar]
- 14. Willicombe M, Thomas D, McAdoo S. COVID‐19 and Calcineurin Inhibitors: Should They Get Left Out in the Storm?. Journal of the American Society of Nephrology. 2020;31 (6):1145–1146. 10.1681/asn.2020030348 [DOI] [PMC free article] [PubMed] [Google Scholar]


