Abstract
COVID‐19 is a novel coronavirus disease with a higher incidence of bilateral pneumonia and pleural effusion. The high pulmonary tropism and contagiousness of the virus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), have stimulated new approaches to combat its widespread diffusion. In developing new pharmacological strategies, the chemical characteristic of volatility can add therapeutic value to the hypothetical drug candidate. Volatile molecules are characterized by a high vapor pressure and are consequently easily exhaled by the lungs after ingestion. This feature could be exploited from a pharmacological point of view, reaching the site of action in an uncommon way but allowing for drug delivery. In this way, a hypothetical molecule for COVID‐19 should have a balance between its lung exhalation characteristics and both antiviral and anti‐inflammatory pharmacological action. Here, the feasibility, advantages, and disadvantages of a therapy based on oral administration of possible volatile drugs for COVID‐19 will be discussed. Both aerosolized antiviral therapy and oral intake of volatile molecules are briefly reviewed, and an evaluation of 1,8‐cineole is provided in view of a possible clinical use and also for asymptomatic COVID‐19.
Keywords: 1,8‐cineole; antiviral drug; asymptomatic COVID‐19; COVID‐19; lung elimination; monoterpenes; SARS‐CoV‐2; volatile drugs
1. INTRODUCTION
Since December 2019, a novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has been causing a pandemic pneumonia named COVID‐19. Infection started in Wuhan, the largest city of Hubei Chinese province, and rapidly spread throughout the world. SARS‐CoV‐2 is a coronavirus belonging to the β‐coronavirus cluster, which includes MERS‐CoV, the causative agent of Middle East respiratory syndrome (MERS) (Rabaan, Al‐Ahmed, Haque, et al., 2020). The mortality rate of COVID‐19 has been reported to range from 3.4% (WHO) up to 14% (Wu, Hao, Lau, Wong, et al., 2020) depending on different authors and modalities of data collection.
The unstoppable COVID‐19 has forced countries to develop new strategies to ensure both constant monitoring of the epidemic and an active response in terms of reorganization of intensive care (Remuzzi & Remuzzi, 2020) and to discover possible experimental drug therapies (Dong, Hu, & Gao, 2020). In this way, many authors are sharing clinical experience using different pharmacological approaches in COVID‐19 patients (Caputo, Lentini, & Habtemariam, 2020). A brief summary of some pharmacological therapies currently under investigation is reported in Table 1.
TABLE 1.
COVID‐19 pharmacological therapies registered on clinicaltrial.gov (accessed at April 8, 2020)
| Drug | Type of study | Estimated enrollment | Treatment | Primary outcome | ClinicalTrials.gov identifier | Location |
|---|---|---|---|---|---|---|
| Hydroxychloroquine Sulfate (os) | Two‐arm, open label, pragmatic randomized controlled trial. | 220 participants | 400 mg hydroxychloroquine sulfate (equaling 310 mg base) twice daily for seven days | Rate of decline in SARS‐CoV‐2 viral load | NCT04316377 | Norway |
|
Lopinavir/ritonavir (os) Arbidol (os) |
A randomized, open‐label, controlled study | 125 participants | Group A: Standard treatment + lopinavir/ritonavir (lopinavir 200 mg and ritonavir 50 mg oral, q12h, every time 2 tablets of each, taking for 7–14 days). In group B standard treatment + arbidol (arbidol 100 mg, oral, tid, 200 mg each time, taking for 7–14 days). Group C (control) only given ordinary treatment | The rate of virus inhibition | NCT04252885 | China |
|
Lopinavir/ritonavir (os) ribavirin (os) interferon Beta‐1B (s.c.) |
An open‐label randomized controlled trial | 70 participants |
Group A: Lopinavir/ritonavir 400 mg/100 mg twice daily for 14 days + ribavirin 400 mg twice daily for 14 days + interferon Beta‐1B 0.25 mg subcutaneous injection alternate day for 3 days. Group control: Lopinavir/ritonavir 400 mg/100 mg twice daily for 14 days |
Time to negative NPS 2019‐n‐CoV RT‐PCR | NCT04276688 | Hong Kong |
| Exosomes derived from allogenic adipose mesenchymal stem cells (MSCs‐Exo) (aerosol) | Pilot clinical study | 30 participants | Conventional treatment and aerosol inhalation of MSCs‐derived exosomes treatment participants will receive conventional treatment and 5 times aerosol inhalation of MSCs‐derived exosomes | Adverse reaction (AE) and severe adverse reaction and time to clinical improvement | NCT04276987 | China |
|
Xiyanping injection drugs Lopinavir tablet or ritonavir tablet (os) Alpha‐interferon nebulization (aerosol) |
A prospective, randomized, open, parallel controlled, Multicenter clinical study | 348 participants | Group A: Xiyanping injection, 10–20 ml daily, Qd, the maximum daily does not exceed 500 mg (20 ml) + Lopinavir tablet or ritonavir tablet + alpha‐interferon nebulization, for 7–14 days. Group control: Lopinavir/ritonavir tablets, 2 times a day, 2 tablets at a time; alpha‐interferon nebulization. | Clinical recovery time | NCT04275388 | China |
| Mesenchymal stem cells (MSCs) (i.v.) | Open label clinical trial | 20 participants | Mesenchymal stem cells (MSCs) therapy | Size of lesion area by chest radiograph or CT | NCT04252118 | China |
|
Lopinavir/ritonavir (os) ribavirin (os) |
A multicenter, double‐blinded, randomized, placebo‐controlled trial | 340 participants | Lopinavir/ritonavir plus ribavirin | Development of severe SARS | NCT00578825 | China |
| Nitric oxide gas (inhalated) | A multicenter, double‐blinded, randomized, placebo‐controlled trial | 104 participants | Group A: NO will be delivered at 80 ppm (ppm) in the first 48 hr of enrollment. Group control: Air without NO. | SARS‐free patients at 14 days | NCT04290871 | China/Italy |
| Hydroxychloroquine (os) | A multicenter, double‐blinded, randomized, placebo‐controlled trial | 1,500 participants | Hydroxychloroquine 200 mg tablet; 800 mg orally once, followed in 6 to 8 hr by 600 mg, then 600 mg once a day for 6 consecutive days. Group control: Placebo | Incidence of COVID19 disease | NCT04308668 | United States |
| Pirfenidone (os) | A randomized, open‐label study | 294 participants | Pirfenidone is administered orally 3 times a day, 2 tablets each time, for a period of 4 weeks or longer. Group control: Standard medication. | Lesion area of chest CT image at 4 weeks | NCT04282902 | China |
| Remdesivir (i.v.) | A Multicenter, adaptive, randomized blinded controlled trial | 394 participants | 200 mg of Remdesivir administered intravenously on Day 1, followed by a 100 mg once‐daily maintenance dose of Remdesivir for the duration of the hospitalization up to a 10 days total course. Group control: Placebo | Percentage of subjects reporting each severity rating on the 7‐point ordinal scale | NCT04280705 | United States |
|
Remdesivir (os) Lopinavir/ritonavir (os) Interferon Beta‐1A (s.c.) |
Multicenter, adaptive, randomized trial | 3,200 participants | Group 1: Remdesivir will be administered as a 200 mg intravenous loading dose on Day 1, followed by a 100 mg once‐daily intravenous maintenance dose for the duration of the hospitalization up to a 10 days total course. Group B: Lopinavir/ritonavir (400 lopinavir mg/100 mg ritonavir) will be administered every 12 hr for 14 days in tablet form. For patients who are unable to take medications by mouth, the lopinavir/ritonavir (400 lopinavir mg/100 mg ritonavir) will be administered as a 5‐ml suspension every 12 hr for 14 days via a preexisting or newly placed nasogastric tube. Group C:Lopinavir/ritonavir (400 lopinavir mg/100 mg ritonavir) will be administered every 12 hr for 14 days in tablet form. For patients who are unable to take medications by mouth, the lopinavir/ritonavir (400 lopinavir mg/100 mg ritonavir) will be administered as a 5‐ml suspension every 12 hr for 14 days via a preexisting or newly placed nasogastric tube. Interferon ß1a will be administered subcutaneously at the dose of 44 μg for a total of 3 doses in 6 days (Day 1, Day 3, and Day 6). Group D (control): Standard of care. |
Percentage of subjects reporting each severity rating on a 7‐point ordinal scale [time frame: Day 15] not hospitalized, no limitations on activities not hospitalized, limitation on activities; hospitalized, not requiring supplemental oxygen; hospitalized, requiring supplemental oxygen; hospitalized, on noninvasive ventilation or high flow oxygen devices; hospitalized, on invasive mechanical ventilation or ECMO; death. |
NCT04315948 | France |
| Chloroquine (os) | Double‐blind, randomized, placebo‐controlled trial | 10,000 participants | Chloroquine loading dose of 10 mg base/ kg followed by 150 mg daily (250 mg chloroquine phosphate salt) will be taken for 3 month. Group control: Placebo. | Number of symptomatic COVID‐19 infections will be compared in subjects randomized to chloroquine or placebo | NCT04303507 | UK |
|
Bromhexine (os) Arbidol (os) Recombinant human interferon α2b (spray) Favipiravir (os) |
Random, open, group sequential design | 60 participants | Group A: Bromhexine hydrochloride tablets + Favipiravir tablets + Arbidol hydrochloride Granulesa as standard treatment + recombinant human interferon α2b spray as standard treatment. Group control: Arbidol hydrochloride granules as standard treatment + recombinant human interferon α2b spray as standard treatment | Time to clinical recovery after treatment | NCT04273763 | China |
| Bevacizumab (i.v.) | Pilot study | 20 participants | Bevacizumab 500 mg i.v. | Partial arterial oxygen pressure (PaO2) to fraction of inspiration O2 (FiO2) ratio | NCT04275414 | China |
| Fingolimod (os) | Open label | 30 participants | Fingolimod 0,5 mg once daily, for three consecutive days | The change of pneumonia severity on X‐ray images | NCT04280588 | China |
| T89 (os) | An open‐label, randomized, blank‐controlled treatment clinical study | 120 participants | The subjects in the T89 treatment group will receive 30 pills of T89 orally, bid., for 10 days, except a standard background treatment (antiviral drug + antibacterial + oxygen therapy + traditional Chinese medicine decoction). The subjects in the blank control group will only receive a standard background treatment. | The time to oxygen saturation recovery to normal level | NCT04285190 | China |
|
ASC09/ritonavir (os) lopinavir/ritonavir (os) |
A randomized, open‐label, multicenter clinical trial | 160 participants | Group A: ASC09/ritonavir(300 mg/100 mg tablet), one tablet each time, twice daily, for 14 days, + conventional standardized treatment. Group control: Lopinavir/ritonavir tablets(200 mg/50 mg tablet), two tablets each time, twice daily, for 14 days, + conventional standardized treatment | The incidence of composite adverse outcome | NCT04261907 | China |
| Eculizumab (i.v.) | Pilot study | 900 mg IV every 7 days for 4 weeks THEN 1200 mg IV on the fifth week THEN 1200 mg IV every 14 days ongoing | Prevent compliment mediated mortality & injury | NCT04288713 | United States | |
|
Oseltamivir (os) Chloroquin (os) Lopinavir (os) ritonavir (os) Favipiravir (os) Oseltamivir (os) Darunavir (os) |
A 6‐week prospective, open label, randomized, in Multicenter study | 80 participants | Group A: Oseltamivir 300 mg per day plus Chloroquin 1,000 mg per day in mild COVID19. Group B: Lopinavir 10 mg/kg and ritonavir 2.5 mg/kg plus Favipiravir 2,400 mg, 2,400 mg, and 1,200 mg every 8 hr on Day 1, and a maintenance dose of 1,200 mg twice a day in mild COVID19. Group C: Lopipinavir 10 mg/kg and ritonavir 2.5 mg/kg plus Oseltamivir 4–6 mg /kg in mild COVID19. Group D: Lopipinavir 10 mg/kg and ritonavir 2.5 mg/kg plus Oseltamivir 4–6 mg /kg in moderate to critically ill COVID19. Group E: Favipiravir 2,400 mg, 2,400 mg, and 1,200 mg every 8 hr on Day 1, and a maintenance dose of 1,200 mg twice a day plus Lopipinavir 10 mg/kg and ritonavir 2.5 mg/kg in moderate to critically ill COVID19. Group F: Combination of Darunavir 400 mg every 8 hr ritonavir ritonavir 2.5 mg/kg plus Oseltamivir 4–6 mg /kg plus Chloroquine 500 mg per day in moderate to critically ill COVID19. Group G: Favipiravir 2,400 mg, 2,400 mg, and 1,200 mg every 8 hr on Day 1, and a maintenance dose of 1,200 mg twice a day plus Darunavir 400 mg every 8 hr ritonavir ritonavir 2.5 mg/kg plus Chloroquine 500 mg per day in moderate to critically ill COVID19. Group H: Patient who unwilling to treatment and willing to quarantine in mild COVID19 | SARS‐CoV‐2 eradication time | NCT04303299 | Thailand |
| Recombinant human interferon α1β (aerosol) | Randomized, open, blank control study | 328 participants | Group A: Standard treatment + recombinant human interferon α1β 10ug bid was administered by nebulization for 10 days. Group control: Standard treatment. | The incidence of side effects | NCT04293887 | China |
|
Carrimycin (os) lopinavir/ritonavir (os) Arbidol (os) chloroquine phosphate (os) |
Randomized open label study | 520 participants | Group A: Carrimycin + basic treatment. Group control: Lopinavir/ritonavir tablets or Arbidol or chloroquine phosphate. | Fever to normal time (day) | NCT04286503 | China |
|
Lopinavir/ritonavir (os) Xiyanping (i.v.) |
Multicenter Randomzied Opena label clinical study | 80 participants | Group A: Lopinavir/ritonavir tablets combined with Xiyanping injection. Group control: Lopinavir/ritonavir tablets | Clinical recovery time | NCT04295551 | China |
|
Danoprevir (os) ritonavir (os) interferon (aerosol) Pegasys (s.c.) Novaferon (aerosol) Lopinavir + ritonavir (os) Chinese medicines (os) interferon (aerosol) |
An open and controlled clinical trial | 50 participants | Group A: Danoprevir one tablet (100 mg/tablet) at a time, twice a day, up to 14 days. Ritonavir one tablet (100 mg/tablet) at a time, twice a day, up to 14 days. With or without spray inhalation of interferon, 50 μg/time for adults, twice a day up to 14 days. Group B: Pegasys 180 μg, subcutaneous injection once a week. Group C: Spray inhalation of Novaferon, 20 μg, twice a day, up to 14 days. Group D: Lopinavir/ritonavir, 200 mg/50 mg, per tablet, oral, 2 tablets at a time, twice a day, up to 14 days. Group E: Chinese medicines, up to 14 days. Spray inhalation of interferon, 50 μg/time for adults, twice a day up to 14 days. | Rate of composite adverse outcomes | NCT04291729 | China |
|
Lopinavir /ritonavir (os) interferon beta‐1b (s.c.) |
Randomized placebo‐controlled clinical trial | 194 participants | Group A: Lopinavir /ritonavir 400 mg +100 mg/ml twice daily for 14 days and interferon beta‐1b 0.25 mg subcutaneous every alternate day for 14 days. Group control: Lopinavir /ritonavir 400 mg +100 mg/ml twice daily for 14 days and interferon beta‐1b 0.25 mg subcutaneous every alternate day for 14 days | 90‐day mortality | NCT02845843 | Saudi Arabia |
| CD24Fc (i.v.) | A randomized, double‐blind, placebo‐controlled, multisite trial | 230 participants | Group A: CD24Fc is given on Day 1. Group control: Placebo is given on Day 1. | Improvement of COVID‐19 disease status | NCT04317040 | |
| Hydroxychloroquine (os) | Double blinded, randomized controlled trial | 500 participants | Group A: Hydroxychloroquine 400 mg day for 10 days. Group control: Placebo | All‐cause hospital mortality | NCT04315896 | Mexico |
|
Alfa interferon (aerosol) lopinavir/ritonavir (os) |
Randomized open label study | 150 participants | Group A: Oxygen therapy, antiviral therapy (alfa interferon via aerosol inhalation, and lopinavir/ritonavir, 400 mg/100 mg, p.o. bid) + traditional Chinese medicines (TCMs) granules: 20 g, p.o, bid, for 14 days. Group control: Oxygen therapy, antiviral therapy (alfa interferon via aerosol inhalation, and lopinavir/ritonavir, 400 mg/100 mg, p.o, bid) for 14 days. | Time to complete remission of 2019‐nCoV infection‐associated symptoms | NCT04251871 | China |
| Methylprednisolone (i.v.) | Randomized open label study | 80 participants | Group A: Methylprednisolone 40 mg q12h for 5 days + standard care. Group control: Standard care. | Lower Murray lung injury score | NCT04244591 | China |
| Nitric oxide gas (inhalated) | Open label study | 460 participants | Group A: NO will be delivered at 160 ppm (ppm) for 15 min in each cycle. Group control: No intervention | COVID‐19 diagnosis | NCT04312243 | United States |
| Losartan (os) | Randomized controlled trial | 200 participants | Group A: Losartan; 25 mg daily; oral administration. Group control: Placebo | Sequential organ failure assessment (SOFA) respiratory score | NCT04312009 | United States |
|
Darunavir (os) Cobicistat (os) |
Randomized open label study | 30 participants | Group A: Darunavir and cobicistat one tablet per day for 5 days + conventional treatments. Group control: Conventional treatment. | The virological clearance rate of throat swabs, sputum, or lower respiratory tract secretions at Day 7 | NCT04252274 | China |
|
Favipiravir (os) Tocilizumab (i.v.) |
Multicenter, randomized and controlled clinical trial | 150 participants |
Group A: Favipiravir: On the first day, 1,600 mg each time, twice a day; from the second to the seventh day, 600 mg each time, twice a day. Oral administration, the maximum number of days taken is not more than 7 days + Tocilizumab: The first dose is 4 ~ 8 mg/kg and the recommended dose is 400 mg. Group B: Favipiravir on the first day, 1,600 mg each time, twice a day; from the second to the seventh day, 600 mg each time, twice a day. Oral administration, the maximum number of days taken is not more than 7 days. Group C: Tocilizumab The first dose is 4 ~ 8 mg/kg and the recommended dose is 400 mg. |
Clinical cure rate | NCT04310228 | China |
| Tetrandine (os) | Randomized open label study | 60 participants | Group A: Tetrandrine 60 mg QD for 1 week. Group control: No intervention | Survival rate | NCT04308317 | China |
| Aviptadil (i.v. and endotracheal nebulization) | Ransomized crossover trial | 20 participants | Group A: Aviptadil by intravenous infusion followed by Aviptadil by endotracheal nebulization. Group B: Aviptadil endotracheal nebulization followed by Aviptadil intravenous infusion | Mortality | NCT04311697 | United States/Israel |
| Arbidol (os) | Randomized, open label, Multicenter study | 380 participants | Group A: Arbidol tablets: Take 2 tablets/time, 3 times/day for 14–20 days + conventional treatment. Group control: Conventional treatment | Virus negative conversion rate in the first week | NCT04260594 | China |
| Thalidomide (os) | Prospective, Multicenter, randomized, double‐blind, placebo, parallel controlled clinical study | 100 participants | Group A: thalidomide100mg for 14 days. Group control: Placebo 100 mg for 14 days. | Time to clinical recovery (TTCR) | NCT04273529 | China |
| Remdesivir (i.v.) | Randomized open label study | 400 participants | Group A: Remdesivir 200 mg i.v. on Day 1 followed by RDV 100 mg on Days 2, 3, 4, and 5 + standard of care. Group B: Remdesivir 200 mg i.v. on Day 1 followed by RDV 100 mg on Days 2, 3, 4, 5, 6, 7, 8, 9, and 10. + standard of care. Group control: Standard of care. | Proportion of participants with normalization of fever and oxygen saturation through Day 14 | NCT04292899 | United States |
| Vitamin C (i.v.) | Prospective randomized clinical trial | 140 participants | Group A: 12 g vitamin C will be infused in the experimental group twice a day for 7 days by the infusion pump with a speed of 12 ml/hr. group control: Placebo infusion | Ventilation‐free days | NCT04264533 | China |
| Siltuximab (i.v.), | Randomized, open‐label study | 100 participants | Group A: A single‐dose of 11 mg/kg of siltuximab will be administered by intravenous infusion. Group control: A dose of 250 mg/24 hr of methylprednisolone during 3 days followed by 30 mg/24 hr during 3 days will be administered by intravenous infusion. | Proportion of patients requiring ICU admission at any time within the study period | NCT04329650 | Spain |
| Hyperimmune plasma | Single group assignment | 49 participants | Administration of hyperimmune plasma on critical patients at Day 1 and based on clinical response on Days 3 and 5 | Death from any cause | NCT04321421 | Italy |
| Vitamin C (i.v.) | Single group assignment | 500 participants | 10 g of vitamin C intravenously in addition to conventional therapy. | In‐hospital mortality | NCT04323514 | Italy |
| DAS181 (nebulized) | Single group assignment | 4 participants | Patient receives nebulized DAS181 (4.5 mg BID/day, a total 9 mg/day) for 10 days. | Improved clinical status | NCT04324489 | China |
| Recombinant human angiotensin‐converting enzyme 2 (rhACE2) (i.v.) | Open label, randomized, controlled, pilot clinical study | 24 participants | Group A: 0.4 mg/kg rhACE2 IV BID for 7 days. Group control: Standard care. | Time course of body temperature (fever) [ | NCT04287686 | China |
| Traditional Chinese medicine prescription | Retrospective cohort study | 50 participants | Traditional Chinese medicine prescriptions have been recommended according to the guidelines for the treatment of COVID‐19 issued by National Health Commission of the PRC. | Length of hospital stay (days) | NCT04323332 | China |
| Ciclesonide (inhaled) | Open‐labeled, multicenter, randomized clinical trial | 141 participants | Group A: Ciclesonide 320ug oral inhalation q12h for 14 days. Group B (active comparator): Ciclesonide 320ug oral inhalation q12h for 14 days + Hydroxychloroquine 400 mg QD for 10 days. Group control: No ciclesonide and hydroxychloroquine | Rate of SARS‐CoV‐2 eradication at Day 14 from study enrollment | NCT04330586 | Korea |
| Sarilumab (i.v.) | Randomized, double‐blind, placebo‐controlled trial | 400 participants | Group A: High dose of single intravenous (IV) dose of sarilumab. Group B: Low dose of single intravenous (IV) dose of sarilumab. Group control: Single intravenous (IV) dose of placebo. | Percent change in C‐reactive protein (CRP) levels | NCT04315298 | United States |
|
Anakinra (s.i.), Siltuximab (i.v.) Tocilizumab (i.v.) |
Prospective, randomized, factorial design, interventional trial | 342 participants | Group A: Anakinra as a daily subcutaneous injection of 100 mg for 28 days or until hospital discharge. Group B: Siltuximab as single IV infusion at a dose of 11 mg/kg. Group C: Anakinra as a daily subcutaneous injection of 100 mg for 28 days or until hospital discharge + Siltuximab as single IV infusion at a dose of 11 mg/kg. Group D: Tocilizumab as single IV infusion at a dose of 8 mg/kg with a maximum infusion of 800 mg/injection. Group E: Anakinra as a daily subcutaneous injection of 100 mg for 28 days or until hospital discharge + Tocilizumab as single IV infusion at a dose of 8 mg/kg with a maximum infusion of 800 mg/injection. Group control: Usual care. | Time to clinical improvement | NCT04330638 | Belgium |
| Peginterferon lambda‐1a (s.i.) | Open‐label randomized controlled trial | 120 participants | Group A: Peginterferon lambda‐1a (180 μg subcutaneous injection) single dose + standard of care treatment for COVID‐19 infection. Group control: Standard of care treatment for COVID‐19 infection | Duration of viral shedding of SARS‐CoV‐2 by qRT‐PCR | NCT04331899 | United States |
| Sildenafil (os) | Open label pilot study | 10 participants |
Sildenafil citrate tablets 0.1 g/day for 14 days |
Rate of disease remission | NCT04304313 | China |
| Mavrilimumab (i.v.) | Open label clinical trial | 10 participants | Single IV dose of mavrilimumab | Time to resolution of fever | NCT04337216 | United States |
|
Levamisole (os) budesonide/Formoterol inhaler Lopinavir/ritonavir hydoxychloroquine |
Double blind, parallel, randomized clinical trial | 30 participants | Group A: Levamisole 50 mg tablet has to be taken 1–2 tablets every 8 hr budesonide + Formoterol has to be inhaled 1–2 puff every 12 hr + Hydroxy Chloroquine 200 mg single dose Lopinavir/ritonavir 2 tablets every 12 hr. Group control: Hydroxy Chloroquine 200 mg single dose Lopinavir/ritonavir 2 tablets every 12 hr | Clear chest CT‐scan | NCT04331470 | Iran |
| Defibrotide (i.v.) | Prospective, interventional, single‐arm, multicentric, open label trial | 50 participants | Defibrotide 25 mg/kg body weight total dose in 2 hr duration infusion each, every 6 hr (Defibrotide 6.25 mg/kg body weight each dose) treatment duration = 7 days | Reduction of the progression of acute respiratory failure | NCT04335201 | Italy |
| Ruxolitinib | Single group assignment | 20 participants | Ruxolitinib 10 mg twice a day | Recovery of pneumonia | NCT04334044 | Mexico |
| Huaier granule (os) | Open label clinical trial | 550 participants | Standard treatment + Huaier granule 20 g po tid for 2 weeks | Mortality rate | NCT04291053 | China |
| IFX‐1 (i.v.) | Pragmatic adaptive open label, randomized phase II/III Multicenter study | 130 participants | Group A: Best supportive care + IFX‐1. Group control: Best supportive care | Change in PaO2/FiO2 | NCT04333420 | The Netherlands |
| Piclidenoson (os) | Randomized open label pilot trial | 40 participants | Piclidenoson 2 mg orally every 12 hr for up to 21 days | Duration of viral shedding in days | NCT04333472 | Israel |
| YinHu QingWen decoction (os) | Adaptive, randomized, single‐blind, three‐arm parallel controlled clinical trial | 300 participants | Group A: YinHu QingWen decoction (Granula) dissolved into 600 ml decoction and divided to 3 times (once with 200 ml). It will be given a 200 ml per time, three times a day, for 10 days + standard western medicine treatment. Group B: 10% dose of YinHu QingWen decoction (Granula) dissolved into 600 ml decoction and divided to 3 times (once with 200 ml). It will be given a 200 ml per time, three times a day, for 10 days + standard western medicine treatment. Group control: Chinese medicine treatment + standard western medicine treatment. | Mean clinical recovery time (hr) | NCT04278963 | China |
| Tranexamic acid (os or i.v.) | Randomized, placebo‐controlled, double blind comparison | 60 participants | Group A: Oral dosing of tranexamic acid at dose of 1,300 mg p.o. three times per day × 5 days; alternative dosing intravenously with loading dose of 10 mg/kg followed by 1 mg/kg/hr infusion × 5 days. Group control: 2 tablets of placebo three times per day × 5 days; alternative dosing intravenous normal saline at volumes similar to those use for experimental arm | Admission to intensive care unit 7 days after randomization | NCT04338126 | United States |
| Sargramostim (inhalation or i.v.) | Prospective, randomized, open‐label, interventional trial | 80 participants | Group A: Sargramostim inhalation via mesh nebulizer and/or IV administration upon clinical deterioration. Group control: Standard of care | Improvement in oxygenation at a dose of 250 mcg daily during 5 days improves oxygenation in COVID‐19 patients with acute hypoxic respiratory failure | NCT04326920 | Belgium |
| Valsartan (os) | Double‐blind, placebo‐controlled randomized clinical trial | 651 participants | Group A: Valsartan at a dosage and frequency titrated to blood pressure with 80 mg or 160 mg tablets up to a maximum dose of 160 mg b.i.d. group control: Matching 80 mg or 160 mg placebo tablets at a dosage and frequency titrated to systolic blood pressure | First occurrence of intensive care unit admission, mechanical ventilation, or death within 14 days. | NCT04335786 | The Netherlands |
| BLD‐2660 (os) | Randomized, double‐blind, placebo‐controlled trial | 120 participants | Group A: BLD‐2660. Control group: Placebo. | Antiviral activity | NCT04334460 | |
| Nintedanib (os) | Single‐center, randomized, placebo‐controlled trial | 96 participants | Group A: Nintedanib cloth sulfonate 150 mg, twice a day, about 12 hr apart. Continuous medication for 8 weeks. Group control: One capsule at a time, twice a day, with an interval of about 12 hr each time. Continuous medication for 8 weeks. | Changes in forced vital capacity (FVC) | NCT04338802 | China |
| Emapalumab (i.v.) | Randomized, open‐label, parallel group, 3‐arm, Multicenter trial | 54 participants | Group A: Emapalumab i.v infusion every 3rd day for a total 5 infusions. Day 1:6 mg/kg. Days 4, 7, 10, and 13:3 mg/kg. Group active comparator: Anakinra i.v infusion four times daily for 15 days. 400 mg/day in total, divided into 4 doses given every 6 hr. Group control: Standard of care. | Treatment success | NCT04324021 | Italy |
|
PD‐1 blocking antibody (i.v.) Thymosin (s.c.) |
Open label clinical trial | 120 participants | Group A: PD‐1 blocking antibody 200 mg iv, one time + standard treatment. Group B: Thymosin 1.6 mg sc qd, last for 5 days + standard treatment. Group C: Standard treatment. | Lung injury score | NCT04268537 | China |
| Tofacitinib (os) | Prospective cohort study | 50 participants | Tofacitinib 10 mg twice a day will be administered within 24 hr from hospital admission for 14 days | Need of mechanical ventilation | NCT04332042 | Italy |
| Fuzheng Huayu (os) | Randomized, double blind, placebo‐controlled, multicenter trial | 136 participants | Group A: 1 N‐acetylcysteine capsule and 4 Fuzheng Huayu tablets three times a day for 24 weeks. Group control: 1 N‐acetylcysteine capsule and 4 placebo tablets three times a day for 24 weeks. | High‐resolution computed tomography (HRCT) score; lung function including FVC, FVC as a percentage of projected value and Dlco | NCT04279197 | China |
|
Lopinavir/ritonavir (os) Hydroxychloroquine sulfate (os) Baricitinib (os) Sarilumab (s.c.) |
Open label clinical trial | 1,000 participants | Group A: Lopinavir/ritonavir tablet 200 mg/50 mg 2 tables by mouth, every 12 hr for 10 days. Group B: Hydroxychloroquine sulfate tablet 200 mg 2 tablets by mouth, every 12 hr for 10 days. Group C: Baricitinib 2 mg po daily for 10 days. Group D: Sarilumab 200 mg subcutaneous injection once. | Clinical status of subject at Day 15 | NCT04321993 | Canada |
| Angiotensin‐(1,7) (i.v.) | Randomized, controlled, investigator initiated, adaptive, single blinded, interventional trial | 60 participants | Group A: Angiotensin 1–7 endogenous peptide dilution: Angiotensin‐(1–7) 0.5 mg/L NaCl 0.9%. Group control: Infusion of NaCl 0.9% without diluted peptide in it. | Ventilator free days | NCT04332666 | Belgium |
| Anluohuaxian (os) | Multicenter, open, randomized controlled trial | 750 participants | Group A: Anluohuaxian 6 g each time, twice a day. Group control: Regular treatment | Changes in high‐resolution computer tomography of the lung | NCT04334265 | China |
| Camostat Mesilate (os) | Quadruple blinded, randomized, placebo‐controlled, phase IIa trial | 180 participants | Group A: Camostat Mesilate 2 × 100 mg pills 3 times daily for 5 days. Group control: Placebo 2 pills 3 times daily for 5 days. | Days to clinical improvement from study enrolment | NCT04321096 | Denmark |
|
Emtricitabine/tenofovir disoproxil (os) Hydroxychloroquine (os) |
Double blind randomized clinical trial controlled with placebo | 4,000 participants | Group A: Emtricitabine/tenofovir disoproxil, 200 mg/245 mg tablets. A dose of one tablet once a day will be administered + placebo of Hydroxychloroquine. Group B: Hydroxychloroquine, 200 mg tablets. A dose of one tablet once a day will be administered + placebo of Emtricitabine/tenofovir disoproxil. Group C: Tenofovir Disoproxil Fumarate 245 mg/Emtricitabine 200 mg + Hydroxychloroquine 200 mg. Group D: Placebo of Tenofovir Disoproxil Fumarate 245 mg/Emtricitabine 200 mg + placebo of Hydroxychloroquine 200 mg | Number of confirmed symptomatic infections of SARS‐CoV‐2 (COVID‐19) | NCT04334928 | Spain |
| Escin (os, i.v.) | Double‐blind, placebo‐controlled clinical trial | 120 participants | Group A: Standard therapy + Escin tablet 40 mg*3, os for 12 days. Group B: Standard treatment + sodium Escinate 20 mg iv/day for 12 days. Group control: Standard therapy | Mortality rate | NCT04322344 | Italy |
| Nitric oxide releasing solution (NORS) | Multicenter, randomized, single blind, controlled, clinical trial | 200 participants | Group A: Prevention: Standard precautions + NORS treatment will consist of daily self‐administration of three routes; nitric oxide gargle (NOG) every morning, nitric oxide Nasophyaryngeal irrigation (NONI) every evening, and nitric oxide nasal spray (NONS) up to 5 times per day. Group B: Treatment NORS treatment will consist of daily self‐administration of three routes; nitric oxide gargle (NOG) every morning, nitric oxide Nasophyaryngeal irrigation (NONI) every evening, and nitric oxide nasal spray (NONS) up to 5 times per day. + standard treatment. Group control: Standard treatment | Prevention primary endpoint and treatment sub study primary endpoint | NCT04337918 | United States |
| Sirolimus (os) | Randomized, double blind, placebo controlled trial | 30 participants | Group A: Sirolimus 6 mg daily on Day 1 followed by 2 mg daily for the next 13 days for a total treatment duration of 14 days or hospital discharge, whatever happens sooner. Group control: Placebo. | Progression to advanced respiratory support | NCT04341675 | United States |
| Atovaquone/azithromycin (os) | Open‐label, nonrandomized, single group trial | 25 participants | Atovaquone 750 mg PO Q12H for up to 10 days azithromycin 500 mg PO daily 1 followed by 250 mg PO daily for up to 10 days (Days 2–10) | Virology cure rate | NCT04339426 | United States |
| TJ003234 (i.v.) | Randomized, double‐blind, placebo‐controlled, multicenter study | 144 participants | GROUP A: TJ003234 3 mg/kg i.v. single infusion. Group B: TJ003234 6 mg/kg i.v. single infusion. Group control: Placebo i.v. single infusion. | Proportion (%) of subjects experiencing deterioration in clinical status | NCT04341116 | United States |
|
Nitazoxanide (os) Hydroxychloroquine (os) |
Open‐label randomized controlled trial | 86 participants | Group A: Hydroxychloroquine 200 mg twice daily every 12 hr for 10 days plus Nitazoxanide 500 mg twice daily every 12 hr for 10 days. Group B: Hydroxychloroquine 200 mg twice daily every 12 hr for 10 days | Mechanical ventilation requirement | NCT04341493 | Mexico |
| Meplazumab (i.v.) | Open label clinical trial | 20 participants | 10 mg Meplazumab by intravenous infusion, every day for 2 days | 2019 nCoV nucleic acid detection | NCT04275245 | China |
Scientists and pharmaceutical companies are starting to develop several types of vaccines (Gao, Bao, Mao, et al., 2020). However, it seems that more time is required for the results to establish a reasonable level of safety. Effectiveness of vaccines is another important aspect because of the high amount of variability, linked to the mutation rate of the novel virus, which could make a previously helpful vaccine or new antiviral molecule ineffective. Government regulatory agencies are called upon to guarantee an accurate and careful evaluation process to satisfy all the necessary safety, quality, and efficacy requirements, avoiding any shortcuts in assessment (Jiang, 2020).
In the absence of a known efficient pharmacological therapy and because of the lack of time due to this public health emergency, it is reasonable to explore any possible strategy of pharmacological intervention (Ebrahimi, 2020; Phadke & Saunik, 2020; Rothlin, Vetulli, Duarte, & Pelorosso, 2020). Screening existing drugs or molecules with known pharmacological and safety profiles is a consolidated approach. This has already happened with the choice of tocilizumab, approved for rheumatoid arthritis, to block the massive release of cytokine IL6, induced by the coronavirus at the cellular level, and thus prevent its lethal effects (Wu, Wang, Kuo, et al., 2020). In China, more than 80 clinical trials are testing both the newest molecules and the oldest remedies, even those from Traditional Chinese Herbal Medicine (Maxmen, 2020), as reported in Table 1. The situation that is developing is one in which there are many studies with therapeutic possibilities but limited time. Here, an approach is discussed considering the possibility of slowing the engraftment of the virus at the level of the pulmonary alveoli through a pharmacological strategy with nonspecific mechanisms of action or toward specific molecular targets.
Pathophysiology of COVID‐19 pneumonia involves an immune response that could result in pulmonary damage reducing lung capacity with functional impairment (Li, Fan, Lai, et al., 2020). The spread of the virus occurs due to close contact with an infected person, exposed to respiratory droplets resulting from coughing, sneezing, or aerosols. The virus penetrates the human body through the lungs by inhalation (Shereen, Khan, Kazmi, Bashir, & Siddique, 2020). Pathogenetic mechanism of SARS‐Cov‐2 involves binding with the ACE‐2 receptor for cells attachment and penetration (Renu, Prasanna, & Abilash, 2020).
Lungs are an internal organ with a high total capacity of 6 L, an average surface area of approximately 50 m2, and peculiar pharmacokinetics involving the absorption, metabolism, and elimination of substances in its lumen. The importance of this organ as a site of action for targeted drug therapies is clear, especially in asymptomatic people to reduce the viral load even before the onset of pathogenesis and symptoms, the Achille's heel of COVID‐19 pandemic (Arons, Hatfield, Reddy, et al., 2020; Gandhi, Yokoe, & Havlir, 2020; McHugh, 2020).
Considering what above, here data from inhaled antiviral aerosol studies are reviewed and the potential use of volatile molecules orally ingested, easily eliminated and exhaled by the lungs, are argued. This last pharmacological strategy has never considered before in clinical treatment and could be analyzed with some limitations in this time of emergency.
2. AEROSOLIZED ANTIVIRAL DRUGS
Inhalation is the preferred route of administration for many drugs that have a direct effect on the airways, particularly in conditions such as asthma and chronic obstructive pulmonary disease (COPD; Berger, 2009). The inhalation route is also used to facilitate systemic administration in other pathologies (e.g., to avoid daily insulin injections). The main advantage is the great bioavailability in the lungs at doses with less risk of systemic side effects. The size of the particles administered by inhalation is fundamental in determining the deposit site within the respiratory tract. Table 2 reports other factors such as mechanical, chemical, immune, and behavioral factors have been identified as critical to determine the correct drug delivery (Newman, 2017). The optimal size for airway deposition is 2–5 μm as mass median aerodynamic diameter. Larger particles tend to settle in the upper airways, and smaller particles remain suspended and then exhaled (Sturton, Persson, & Barnes, 2008). For example, promising therapy has been tested for tuberculosis showing the efficacy of pyrazinoic acid through both aerodynamic and stoichiometric properties of the nebulized formulation. In this case, the diameter used for the particles was between 3.3 and 5.4 μm (Durham, Zhang, German, et al., 2015).
TABLE 2.
critical factors for pulmonary drug delivery (modified from Newman, 2017)
| Barriers to successful pulmonary drug delivery | |
|---|---|
| Mechanical barriers | Impaction of inhaled drug particles and droplets in mouth and nose |
| Impaction losses in large airways restrict delivery to peripheral lung regions | |
| Effects of disease: airway narrowing, mucus hypersecretion and mucus plugging | |
| Removal of drug by lung mucociliary clearance | |
| Chemical barriers | Drug degradation by proteolytic enzymes |
| Effects of other chemicals, for example, surfactant | |
| Immunological barriers | Particle engulfment by alveolar macrophages |
| Behavioral barriers | Nonadherence to treatment regimen |
| Poor inhaler technique | |
There are numerous ways of administering inhaled drugs (Virchow, Crompton, Dal Negro, Pedersen, et al., 2008):
pressurized metered inhalers
expansion chambers
powder inhalers
nebulizers
gas
Some data have suggested the use of aerosolization in antiviral therapy or symptomatic treatment, and this is confirmed by some trials recently registered for COVID‐19 treatment (Table 1).
Debs, Montgomery, Brunette, De Bruin, and Shanley (1988) conducted in vivo studies examining the effect of oral of aerosol administration of the antiviral agent ganciclovir in an experimental model of murine cytomegalovirus (MCMV) pneumonia. The authors reported the same outcome for the two groups but suggested a more specific inhibition of replication of MCMV in the lungs with the aerosolized drugs. A more recent in vivo study has tested through aerosol an experimental synthetic ligand (PUL‐042) for Toll‐like receptor (TLR) 2/6 and TLR 9 in a mouse pneumonia experimental model. The concomitant aerosol of both immune stimulant and antiviral oseltamivir has resulted in a greater rate of survival in patients with influenza pneumonia compared to controls (Leiva‐Juarez, Kirkpatrick, Gilbert, Scott, et al., 2018). The interest in this new type of inhaled immune stimulant that targets the TLR pathway has resulted in recent trials with PUL‐042 to reduce the severity COVID‐19 pneumonia in SARS‐CoV‐2 positive patients (ClinicalTrial.gov id: NCT04312997 and NCT04313023).
It has been argued that aerosol delivery of antiviral drugs or vaccines may lead to some advantages in safety and efficacy in treating influenza (Morgan, Hemmink, Porter, et al., 2016; Strong, Ito, Murray, & Rapeport, 2018; Wong, Christopher, Viswnaan, Schnell, et al., 2010). For antiviral drugs, the main advantage of the inhalation route is the lack of first pass metabolism, which leads to increased bioavailability. For example, the old drug ribavirin (RBV) has been proposed for aerosol therapy in critical care situations, but it lacks of strong recommendation and is restricted to high‐risk patients (Diot & Plantier, 2016; Velkov, Rahim, Zhou, Chan, et al., 2015). A recent comparative retrospective cohort analysis has found no significant differences in clinical outcome between oral and inhaled RBV therapy but higher costs for aerosol therapy emerged as a problem (Trang, Whalen, Hilts‐Horeczko, Doernberg, et al., 2018). The inhaled RBV therapy combined with intravenous immunoglobulin is applied in bone marrow transplant patients in cases of viral pneumonia because of its poor systemic absorption, protecting against haemolytic anemia frequently noted after oral administration (Velkov et al., 2015).
One of the most well‐known antiviral drugs, zanamivir (Relenza®, GlaxoSmithKline), has showed low oral bioavailability: to solve it, a new inhaled formulation has been approved with a 15% of the inhaled dose reaching the lower respiratory tract (Peng, Milleri, & Stein, 2000). A comparative clinical study has underlined the greater effect of aerosol compared to oral oseltamivir in reducing symptoms of influenza A or B (Kawai, Ikematsu, Iwaki, Maeda, et al., 2008).
IFN‐γ is not an antiviral drug but useful in the treatment of some respiratory diseases due to its immunomodulatory pharmacological activity. Recently, a novel nebulized formulation of interferon gamma (IFN‐γ) has been tested using special vibrating mesh‐type nebulizers. This experiment was conducted following the regulatory standard requirements of methodologies for the assessment of pulmonary drug delivery. The increase bioavailability through special nebulization system has improved the delivery of this large molecule achieving optimal bioavailability in the lower respiratory tract, while maintaining its pharmacological activity (Hilberg et al., 2012; Moon, Smyth, Watts, & Williams 3rd., 2019). Aerosolized IFN‐γ has been tested in clinical trials showing great tolerability with some improvement in the reduction of cavity lesion size and bacterial loads (Condos, Hull, Schluger, Rom, & Smaldone, 2004; Condos, Rom, & Schluger, 1997; Moss, Mayer‐Hamblett, Wagener, Daines, et al., 2005).
An important limit to improve aerosol therapy could be represented by the risk of dispersion of the virus into the environment already reported for hypoxemic COVID‐19 patients requiring oxygen therapy (Ari, 2020; Li, Fink, & Ehrmann, 2020). The authors reviewed the available data on the use of the high‐flow nasal cannula for oxygen administration. Analysis of the generation and dispersion of bio‐aerosols showed a risk similar to standard oxygen masks and therefore of aerosol treatment. Aerosol generators posed a potential risk of intra‐hospital contamination and may carry bacteria and increase the risk of bacterial lung infection in patients with viral pneumonia (Ari, 2020; Pitchford, Corey, Highsmith, et al., 1987; Wexler et al., 1991).
3. DRUG EXHALATION BY THE LUNGS
Lungs are the organ most affected by COVID‐19 pandemic. Assuming a strategy based on seeking localized pharmacological action, could the lung elimination process be considered from a “drug delivery” point of view rather than a “drug elimination” one?
The ability of a drug to cross cell membranes depends on its partition coefficient depending on its chemical–physical characteristics; hydrophilic groups refer to those that are capable of forming hydrogen bonds with water, such as the carboxylic, alcoholic, amino, aldehyde and ketone groups and electrically charged groups. The partition coefficient of a drug (i.e., its ability to cross cell membranes) can vary due to metabolization processes. Generally, metabolic transformations lead to multiple hydrophilic compounds with partition coefficients lower than those of the original drugs. Furthermore, it is important to remember that many drugs are organic molecules that contain acidic or basic residues, that is, groups which, depending on the pH of the solution in which they are found, can be electrically neutral or charged. For these drugs, the partition coefficient is also dependent on the pH of the environment and the pKA of the reactive groups.
The diffusion of drugs follows Fick's law, so a drug with an adequate partition coefficient can diffuse through cell membranes (Krämer, Lombardi, Primorac, Thomae, & Wunderli‐Allenspach, 2009). The laws governing the diffusion between two compartments separated by a membrane are described by Fick's law: molar flow = (c 1 − c 2) ⊇ D ⊇ A/d wherein by molar flow is meant the speed (moles per second) of the passage of a solute from compartment 1 to compartment 2; c 1 and c 2 are the concentrations of the compound in the two compartments; D is the diffusion coefficient, which depends on the chemical–physical characteristics of solvent and solute (in the case of passage through biological membranes, D is mainly determined by the partition coefficient); A is the area of the membrane that separates the two compartments, and d is its thickness. In the case of the plasma membrane, d can be considered a constant; in the case of a tissue, d depends on the number of cell layers to be overcome.
Knowledge of this physiological mechanism could be useful in the review of possible molecules with low pharmacodynamic specificity toward the virus but with a high tropism for pulmonary elimination. The site of action in this case becomes the first element of pharmacological advantage over the aetiopathological agent.
4. VOLATILE MOLECULES
Pulmonary elimination is inversely proportional to the blood solubility of a molecule. Other chemical characteristics, such as vapor pressure and molecular dimension, increase lung clearance via exhalation: higher lipophilic properties, higher vapor pressure, and lower molecular weight contribute to easier elimination through exhalation. The main classes of volatile organic compounds exhalated by the lungs can be summarized as saturated (ethane, pentane and aldehydes) and unsaturated hydrocarbons (isoprene), and oxygen‐ (acetone), sulfur‐ (ethyl mercaptan and dimethyl sulphide) and nitrogen‐ (dimethylamine, ammonia) containing compounds (Dent, Sutedja, & Zimmerman, 2013).
The pharmacokinetic pathway of lung elimination is known, for example, for chloral hydrate a highly lipophilic and small molecule used as sedative. This drug is now used only in a few cases due to its narrow therapeutic index, but it had been preferred in the pediatric population for its easy oral administration and short half‐life (Necula, Stamate, Blebea, & Cozma, 2019). Oral administration represents a clear advantage, increasing patient compliance. Disulfiram is used in the treatment of alcohol dependence: its metabolites, carbon disulphide (CS2), and acetone, are transported from the blood into alveolar air and exhaled with the breath. Disulfiram increases the acetaldehyde blood concentration by aldehyde dehydrogenase inhibition of ethanol metabolism, with an increase also in lung exhalation and acetaldehyde toxicity (Torsten, Gómez‐Moreno, & Aguilar‐Salvatierra, 2017). The particular mechanisms of this drug cause many of the unwanted effects of a hangover immediately after alcohol consumption to break the ethanol addiction. Curiously, disulfiram has been tested in vivo against the viruses that cause severe acute respiratory syndrome (SARS) and MERS, but the studies did not provide adequate clinical evidence (Lin, Moses, Hsieh, Cheng, et al., 2018).
Many volatile molecules come from natural sources. The most common example of this, experienced by each of us, is the pulmonary excretion of garlic diallyl‐derivatives, such as diallyl disulphide, and other organic sulfur compounds, such as allyl methyl sulphide and methyl mercaptan, in expired air and affecting social relations.
A number of molecules with typical volatile characteristics belong to the small terpenoid class of monoterpenes consisting of two isoprene units that can be linear (acyclic) or contain one or two rings. Terpenoids represent the major component of essential oils, and the large number of pharmacological properties of each compound has already been discussed (Kozioł, Stryjewska, Librowski, Sałat, et al., 2014; Wojtunik‐Kulesza, Kasprzak, Oniszczuk, & Oniszczuk, 2019). The most well‐known are α‐pinene, limonene, γ‐terpinene, terpinolene, α‐terpineol, linalool, thymol, menthol, and carveol. Common pharmacological effects of different types of cyclic monoterpenes are antibacterial, antiviral, and antifungal effects (Dehshikh, Sourestani, Dehsheikh, Mottaghipisheh, et al., 2020).
The chemical–physical properties of eucalyptol (1,8‐cineole) allow significant concentrations to be achieved in the lungs through pulmonary exhalation. In addition, taking note of its characterized kinetics of pulmonary elimination and according to Fick's law, the time of persistence should also be sufficient for eucalyptol to perform a considerable pharmacological action. Furthermore, due to the negligible side effects, the risk/benefit ratio suggests that its pharmacological effects are worth testing.
4.1. Eucalyptol (1,8‐cineole)
Eucalyptol (Figure 1) is a natural saturated bicyclic monoterpenoid that is extracted from various species of Eucalyptus (e.g., Eucalyptus globulus Labill., Eucalyptus polybractea R.T. Baker and Eucalyptus smithii R.T. Baker, Fam. Myrtaceae). The essential oil of eucalyptus contains not less than 70% of eucalyptol as reported in Ph. Eur. 10th ed. Pure eucalyptol is a clear liquid at room temperature, and the melting point is 1.5°C with a flash point of 49°C (Prasanthi & Lakshmi, 2012).
FIGURE 1.
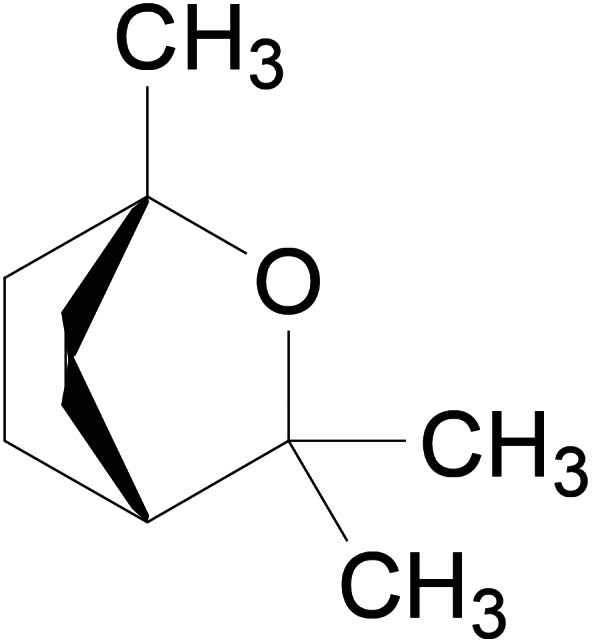
1,8‐Cineole
Eucalyptus essential oil appears in almost all European national pharmacopeias and is traditionally used as a mucolytic, as an antiseptic and to treat asthma, fever, flu, bronchitis and whooping cough. EMA indicates its traditional use of this essential oil as a treatment for the cough associated with a cold. In Germany, 1,8‐cineole is a licensed medicinal product formulated in gut soluble capsules containing 100 mg/capsule and is indicated for acute and chronic bronchitis, sinusitis, and respiratory infections.
In vitro studies have underlined the pharmacology of eucalyptol as a bronchodilator, in enhancing the activity of mucociliary cells with a corresponding effect on clearance and in decreasing mucus production (Galan, Ezeudu, Garcia, Geronimo, et al., 2020). In vitro investigation has shown up to a 92% inhibition of the release of pro‐inflammatory cytokines tumor necrosis factor‐alpha (TNF‐α) and interleukin‐1‐beta (IL‐1b) by lipopolysaccharide (LPS)‐stimulated monocytes treated with 1.5 μg/ml of eucalyptol (Juergens, Dethlefsen, Steinkamp, Gillissen, et al., 2003). Another study reported eucalyptol extract significantly to inhibit the nuclear factor (NF)‐κB p65 gene promoter in LPS‐stimulated human cell lines resulting in a decrease in inflammation compared to that of the control (Greiner, Müller, Zeuner, Hauser, et al., 2013). Another in vitro study has showed an antiviral response stimulation of 1,8‐cineole in human stem cells both by increasing activity of antiviral transcription factor interferon regulatory factor 3 and by reducing proinflammatory NF‐κB activity (Müller et al., 2016).
An interesting experiment was conducted analyzing human blood of asthmatic patients and healthy subjects, both pretreated with 200 mg eucalyptol thrice daily for 3 days, ex vivo. Up to 40.1% inhibition of LTB4 and PGE2 from monocytes from asthmatic patients (n = 10) and up to 57.9% inhibition of those from healthy subjects (n = 12) have been reported ex vivo (Juergens, Stöber, Schmidt‐Schilling, Kleuver, et al., 1999). A placebo‐controlled clinical trial analyzing 242 patients with acute bronchitis has measured the effect of 200 mg eucalyptol thrice daily for 10 day on the “Bronchitis‐Sum‐Score” bronchitis endpoint on day fourth and 10th. The group treated with eucalyptol showed a significant reduction in the score compared to that of the placebo group (3.55 vs. 2.91) on day 4, but no significant differences were reported on day 10 (Fischer & Dethlefsen, 2013a, 2013b).
A double‐blind placebo‐controlled trial has considered glucocorticosteroid reduction endpoints for 32 asthmatic patients given 200 mg of eucalyptol, or placebo, thrice daily for 12 weeks. The results demonstrated a significant reduction of glucocorticosteroid medication in the treatment group, even when the use of salbutamol was doubled in this group, compared to the baseline condition, but the score of dyspnea was significantly greater in the placebo arm (Juergens et al., 2003).
Assumption of same dosage of eucalyptol for 6 months has been tested as an adjunctive therapy in a more recent multicenter placebo‐controlled double‐blind trial considering 247 asthmatic patients already under medication. The authors reported a significant improvement in lung function in the treatment group compared to the placebo, by reducing dyspnea demonstrated by primary end‐points, such as forced expiratory volume 1 s, asthma symptoms and quality of life (Worth & Dethlefsen, 2012). The same authors conducted a similar trial on 242 smoking or former smoking patients with moderate/severe COPD with concomitant use of standard therapy. The group treated with eucalyptol showed a significant reduction in the frequency, duration, and severity of COPD exacerbations compared with those of the control group. Secondary end‐points, lung function, dyspnea, and quality of life, improved without reaching significance (Worth, Schacher, & Dethlefsen, 2009). Data from a COPD experimental animal model exposed to cigarette smoke have highlighted normal lung parenchyma and significantly less leukocyte infiltration by 40–50% in mice treated with 3 and 10 mg/kg eucalyptol compared to placebo. In the same study, the eucalyptol group showed a significant 60% reduction in myeloperoxidase activity and 50 and 40% decreases in IL‐1β and interleukin‐6 (IL‐6) expression, respectively; TNF‐α levels were reduced by 80% in the higher dosage group. In addition to the anti‐inflammatory effect, a predictable disinfectant effect in reducing bacterial colonies was also measured in this experimental COPD model of mice treated with 260 mg of eucalyptol per day (Yu, Sun, Su, He, et al., 2019).
In vitro antiviral activity of nebulized eucalyptol essential oil has been explored by Usachev, Pyankov, Usacheva, and Agranovski (2013). The authors analyzed the effect of eucalyptus aerosol on two viral model systems of nebulized influenza virus A strain NWS/G70C (H11N9), simulating hazardous bioaerosols in indoor and outdoor environments, by a plaque assay technique. The results underlined that 99% viral inactivation is achieved after 15 s of aerosolization and exposure for 5 min. The antiviral activity was exerted by both the aerosol form and vaporized phase. The antiviral effect was measured in an in vitro experimental model of Herpes simplex virus, and 1,8‐cineole had an antiviral IC50 equal to about a quarter of the maximum nonlethal dose measured in a cytotoxicity assay (Astani, Reichling, & Schnitzler, 2010). A similar in vitro investigation confirmed the antiviral activity against the infectious bronchitis virus (IBV) with an antiviral IC50 equal to about a sixth of the maximum nonlethal dose (Yang et al., 2010).
Antiviral activity has been investigated in an influenza‐virus‐induced pneumonia experimental in vivo model showing a decreasing level of IL‐4, IL‐5, IL‐10, and MCP‐1 in nasal lavage fluids and of IL‐1β, IL‐6, TNF‐α, and IFN‐γ in lung tissues of mice. A reduction of expression of NF‐kB p65, intercellular adhesion molecule (ICAM)‐1, and vascular cell adhesion molecule (VCAM)‐1 in lung tissues has been reported underlining a reduction of pulmonary inflammatory process (Li et al., 2016).
An in vivo experiment with similar experimental model with mouse type A influenza virus (Victoria/3/75,H3N2) has revealed that the synergistic effect of 1,8‐cineole with oseltamivir. Combinations of 1,8‐cineole (30, 60, and 120 mg/kg/day) and oseltamivir (0.1, 0.2, and 0.4 mg/kg/day) have increased in a dose‐dependent manner the number of survivors and mean survival time compared to the monotherapy alone. Improvements of lung parameters (lung index, viral titers, and pathology) and reduction of cytokines (IL‐10, TNF‐α, IL‐1β, and IFN‐γ) expression in lung have been reported (Lay et al., 2017).
A small number of both in vivo and in vitro pharmacokinetic studies have considered oral administration. In a rabbit model given 200 mg/kg of eucalyptol, a peak plasma concentration was reached after 1 hr (Bhowal & Gopal, 2015). The oxidative metabolic pathway of eucalyptol produces 2‐hydroxy‐1,8‐cineole and 3‐hydroxy‐1,8‐cineole, conjugated to glucuronide products. It has been reported that chronic administration of 800 mg/day does not imply accumulation (Juergens et al., 2003). By extrapolation from data on other monoterpenes with similar chemical structures, it could be supposed that lung elimination of the unchanged form of eucalyptol could range from 1 to 10% in the expired air (Kohlert, van Rensen, März, Schindler, et al., 2000). However, in an interesting and unconventional way, an association with a macrolide such as clarithromycin or with pharmacoenhancer cobicistat (integral part of antiretroviral therapy for human immunodeficiency virus HIV) can inhibit the metabolism of P450 to increase the blood concentration and exhalation of 1,8‐cineole by decreasing the hepatic clearance (von Hentig, 2015; Westphal, 2000, Miyazawa, M., Shindo, M., & Shimada, T. 2001).
The mechanism of action for antiviral 1,8‐cineole has not been fully elucidated. Considering other similar monoterpenes, an effect directed toward the virus can be asserted for 1,8‐cineole, contrarily to most known antivirals such as aciclovir have an intracellular target of a viral enzyme such as DNA polymerase in the case of aciclovir or RNA polymerase as in the case of remdesevir (Astani & Schnitzler, 2014). In vitro experimental model proposed by Astani and colleagues on herpes simplex virus type 1 revealed the complete antiviral action by β‐pinene and limonene was exerted at noncytotoxic doses, but the antiviral action was not effective on previously infected cells. Similar evidence regarding the direct antiviral effect of monoterpenoid substances was achieved by Lai (Lai et al., 2012) and Zamora and colleagues (Pliego Zamora, Edmonds, Reynolds, Khromykh, & Ralph, 2016). The authors tested the efficacy of a mixture of monoterpenes, including 1,8‐cineole, on an immunodeficient mouse model of West Nile Virus infection, detecting a decreased viral load but not an increase in apoptosis or inhibitions of cell enzymes from infected cells. It could therefore be argued that monoterpenes can interfere with the virion envelope and inhibit entry into host cells as well as having a direct virucidal effect given by an unspecific effect of the particular chemical nature of these substances (Swamy, Akhtar, & Sinniah, 2016).
The EMA monograph on eucalyptus essential oil (eucalyptol content of at least 70%) indicates oral use of up to 200 mg for a maximum of 5 times a day for adults and adolescents. The use in children under 30 months is contraindicated and it is not recommended under 12 years of age (EMA, 2014a). Side effects of 1,8‐cineole containing preparations are listed in EMA regulatory assessment of Eucalyptus spp. Cases of acute toxicity were reported following accidental ingestion or overdosing leading inflammatory diseases of gastrointestinal tract and rare cases of nausea, vomiting and diarrhea. Numerous other side effects have been reported such as laryngospasm and consciousness, but most are given by the pediatric population to which this class of substances is contraindicated. Another important factor highlighted in the adverse events reports submitted is a certain idiosyncrasy (EMA, 2014b).
5. DISCUSSION
The novel SARS‐CoV‐2 infection is straining global health systems. The massive spread of the virus is requiring new response paradigms from the scientific community. The first step of the pharmacological strategy was to consider molecules already on the market for a reasoned use in the treatment of viral pneumonia COVID‐19 (Li & De Clercq, 2020). As a consequence, most of the antiviral drugs currently registered in clinical trials are medicines with other indications, but which can potentially benefit patients affected by COVID‐19 (Caputo et al., 2020).
Based on clinical experience from SARS and MERS, and considering the characteristic of this single‐stranded RNA beta‐coronavirus, a number of antiviral nucleoside analogue drugs, such as favipiravir, which selectively inhibits viral RNA‐dependent RNA polymerase, or ritonavir, a protease inhibitor used against the hepatitis C virus, are currently in trials (Gul, Htun, Shaukat, Imran, & Khan, 2020). Remdesevir has just received a recommendation from the EMA's human medicines committee (CHMP) for the treatment of COVID‐19 in adults and adolescents from 12 years of age with pneumonia who require supplemental oxygen. Other specific antiviral drugs are being analyzed in order to find a specific pharmacological response to this pandemic (Li & De Clercq, 2020).
Alongside this approach, some nonspecific pharmacological strategies with secondary mechanisms of action against the virus have been considered, such as the immune modulator chloroquine (Touret & de Lamballerie, 2020). This strategy has shown promising in vivo and in vitro data and some clinical evidence, the latest reported by Gao, Tian, and Yang (2020) describing the superiority of chloroquine over the control group in a Chinese clinical trial. However, now a series of tests is not confirming these preliminary data (Molina, Delaugerre, Le Goff, et al., 2020) and clinical trials with this drug have been temporarily stopped and resumed (WHO, 2020a, 2020b). Others nonspecific pharmacological approaches are currently under investigation including the use of inhaled medicines (Table 1). For example, nitric oxide gas is a selective pulmonary vasodilator administered to COVID‐19 patients as a rescue therapy for refractory hypoxemia due to acute respiratory distress syndrome. Some evidence from in vitro and clinical data have pointed out that inhaled nitric oxide gas (iNO) has exerted nonspecific antiviral activity (ClinicalTrial.gov id: NCT04290871).
Lungs are the target organ of COVID‐19 disease and the virus load in the pulmonary airways promotes person‐to‐person contagion. Here, the possibility of a nonspecific pharmacological effect focused on the pulmonary site of action has been discussed, with the aim of finding new therapeutic approaches (Geller, Varbanov, & Duval, 2012). In addition to pharmaceutical aerosols targeting drugs to the lower respiratory tract, here the possibility of exploiting the pulmonary elimination mechanism to concentrate orally administrated molecules with nonspecific action in the lungs is considered. A number of limitations of this strategy are defined by the particular characteristics that the candidate molecules must possess (Table 3); on the other hand, the pulmonary elimination process favors a local concentration of the xenobiotic, which consequently can better reach a dose‐dependent pharmacological effect on the lungs. This peculiar pharmacokinetic aspect linked to the characteristics of easy oral administration and antiviral properties, even nonspecific, of a molecule may provide a therapeutic advantage to reach and treat this aggressive viral pneumonia.
TABLE 3.
Possible characteristics of volatile and aerosolized molecules for COVID‐19
| Volatile molecules for COVID‐19 | Aerosolized molecules for COVID‐19 | |
|---|---|---|
| Requirements | Small molecules | |
| High lipophily | Any molecules | |
| High vapor pressure | Ad hoc formulation | |
| Virucidal properties at lower toxic concentration | No toxic at therapeutic concentration | |
| No toxic at therapeutic concentration | ||
| Advantages | Oral administration | |
| Lung exhalation and tropism for lower respiratory tract | Faster onset of action | |
| Easy formulation and production | Delivery on the lung | |
| Cheap preparation | Diminished first pass metabolism | |
| Deeper delivery in lungs | ||
| Possible indications | Initial stages of viral pneumonia | Initial stages of viral pneumonia |
| Prophylaxis | Prophylaxis | |
| In association with other therapies | In association with other therapies | |
| Contraindications | First pass metabolism | Costly preparations |
| Contraindicated for children | Difficulties on formulations | |
| Risk of cross‐contamination | ||
| Toxicity of some propellants |
Starting from the hypothesis of looking for a molecule with these pharmacokinetic and pharmacodynamic characteristics, we may search among monoterpenes, whose chemical and pharmacological characterizations are well known. The 1,8‐cineole is the main monoterpene of the essential oil of eucalyptus. EMA community herbal monograph on eucalyptus essential oil has established a dose regimen of 200 mg orally up to 5 times/day, and its safety has been adequately assessed. It has been reported to have a large spectrum of antimicrobial effect against several type of bacteria, viruses and fungi, which is assumed to be a nonspecific pharmacological effect given its chemical characteristics. Some in vitro investigation has highlighted the antiviral properties of an aerosolized solution of 1,8‐cineole on nebulized viruses with interesting IC50 (Usachev et al., 2013), confirming the antiviral properties already described (Astani et al., 2010; Yang et al., 2010). Furthermore, its anti‐inflammatory effect in the lung has been well characterized, and specific clinical trials of its traditional use by oral intake have highlighted its efficacy and safety in asthmatic patients. The pharmacological characteristics of this monoterpene could make it a candidate to test for the treatment of COVID‐19, and its use as a prophylactic or when the viral load is particularly low is also hypothesized. The easy oral administration and the pulmonary elimination of this substance which can exert its loco potential virucidal effect already highlighted in vitro and in vivo, makes this molecule an interesting candidate for a clinical trial (Sharifi‐Rad, Salehi, Schnitzler, et al., 2017). The pharmacological action seems not to be specific toward a viral molecular target, but through a direct virucidal nonspecific mechanism of action. This also makes it a candidate as a direct antiviral for SARS‐Cov‐2 in which research is utterly delineating specific molecular targets for drug development. It could be asserted that possible pharmacological strategy could be given by a nonspecific but localized therapy in the lungs, contrary to the current research approach that is looking for specificity and a systemic response (McHugh, 2020).
Another point of view is to consider the volatile molecule particularly useful as antiviral prophylaxis, where the viral load is particularly low. This last is the case of asymptomatic virus transmitters which represents a major obstacle to the containment strategy (Gao, Xu, Sun, et al., 2020; Park, Cornforth, Dushoff, & Weitz, 2020; Rothe, Schunk, Sothmann, et al., 2020; Zhou, Li, Chen, et al., 2020), as demonstrated by the case‐study of COVID‐19 epidemic in the municipality of Vo', Italy (Lavezzo et al., 2020). It has been reported that asymptomatic persons play a crucial role in the transmission and diffusion of SARS‐Cov‐2 (Gandhi et al., 2020). Some authors have explored the possibility of therapy for asymptomatic COVID‐19 infection patients (Wang et al., 2020) and hydroxychloroquine is proposed as prophylaxis (D'Cruz, 2020; Lother, Abassi, Agostinis, et al., 2020).
The treatment in asymptomatic cases leaves numerous new openings on possible preventive drug therapy effective in deactivating the virus and stopping the contagion by transmitters that show no symptoms. Measuring prophylactic antiviral capacity in this case would open new ways of taking the drug by limiting it to an occasional and noncontinuous frequency depending on the results of the possible virucidal ability of the drug, given the long latency of SARS‐Cov‐2 (Lauer, Grantz, Bi, et al., 2020; Liu, Magal, Seydi, & Webb, 2020). The latency of SARS‐Cov‐2 virus allows an initial therapeutic window in which the low viral load may not require drugs with full systemic but partial antiviral action specific for the respiratory tract. The importance of identifying molecules with oral absorption but pulmonary elimination would allow easier administration and greater compliance than inhaled drug (Fabbri, Piattella, Caramori, & Ciaccia, 1996; Kallstrom, 2004; Shahiwala, 2011). Therefore, specific studies are required to measure the pharmacokinetic of this approach.
A further advantage is given by the great flexibility of 1,8‐cineole, which can be proposed as virucidal both by inhalation or spray for the upper respiratory tract in order to avoid the spread of the virus, and orally for the lower respiratory tract in order to avoid the beginning of the pathogenesis of COVID. In fact, an aspect of transmission by asymptomatic person is the high viral load shedding in the upper respiratory tract and nasal cavity that spreads through the respiratory droplets (Wölfel, Corman, Guggemos, et al., 2020).
Another point to consider is the great availability of different monoterpenes with similar characteristics that can be taken into consideration in a more detailed scientific analysis and for subsequent use in therapy. The use of these molecules as a starting point for the synthesis of new compounds could be advantageous; however, 1,8‐cineole is supported by EMA monograph which makes it a candidate ready for use if it proves to be effective.
The use of naturally derived substances in this particular emergency situation is also reflected in recent clinical trials that focus on a possible role of traditional Chinese medicine (TCM) in support of standard therapy. Some Chinese guidelines have described the use of traditional herbal medicine for prophylactic purposes (Jin, Cai, Cheng, Cheng, et al., 2020). Furthermore, the recent precious gift made by the People's Republic of China to Italy as emergency support contained TCM products (Ansa, 2020).
6. CONCLUSION
A possible antiviral strategy can focus specifically on the respiratory tract, sacrificing the molecule for a chemical characteristic of volatility instead of a specific antiviral molecular activity, however, with virucidal activity even if nonspecific. Volatile molecule can be exhaled by the lungs promoting a local pharmacological action. Searching for possible antiviral molecules has highlighted possible candidates among monoterpene molecules with virucidal action. Aiming to search for molecules with nonspecific action allows to bypass possible changes of viral molecular targets that may develop in the future (Dawood, 2020).
In light of the above, it is clear that trials on small volatile molecules with antiviral characteristics could lead to positive results. These can be expected both from a prophylaxis and in slowing down and/or inhibiting the progression of the disease (against the virus) from the initial stages to the more severe phases in association with other therapies (Table 3). This perspective is reinforced by the negligible side effects and, therefore, the positive risk/benefit ratio. A suggested approach could be to start with the already characterized volatile compounds from the points of view of safety and known effectiveness against viruses, for example, 1,8‐cineole. In conclusion, a deeper clinical investigation could be reasonable but also greater pharmacological research aimed at identifying nontoxic formulation with volatile and nonspecific antiviral characteristics.
CONFLICT OF INTEREST
The author declares no conflicts of interest.
ACKNOWLEDGMENTS
The author wishes to thank Dr. E. Aiello for valuable suggestions and language input in preparation of the manuscript.
This research did not receive any direct grant from funding agencies in the public, commercial, or nonprofit sectors.
Colalto C. Volatile molecules for COVID‐19: A possible pharmacological strategy? Drug Dev Res. 2020;81:950–968. 10.1002/ddr.21716
REFERENCES
- Ansa . (2020). Retrieved from https://www.ansa.it/sito/notizie/speciali/aziende_emergenza_covid19/2020/03/20/coronavirus-siena-riceve-donazione-da-gemellata-nantong_20413074-57a5-4798-a314-1235937f52c4.html
- Ari, A. (2020). Practical strategies for a safe and effective delivery of aerosolized medications to patients with COVID‐19. Respiratory Medicine, 167, 105987. 10.1016/j.rmed.2020.105987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arons, M. M. , Hatfield, K. M. , Reddy, S. C. , Kimball, A. , James, A. , Jacobs, J. R. , & Jernigan, J. A. (2020). Presymptomatic SARS‐CoV‐2 infections and transmission in a skilled nursing facility. New England Journal of Medicine, 382, (22), 2081–2090. 10.1056/nejmoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astani, A. , Reichling, J. , & Schnitzler, P. (2010). Comparative study on the antiviral activity of selected monoterpenes derived from essential oils. Phytotherapy Research, 24(5), 673–679. 10.1002/ptr.2955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astani, A. , & Schnitzler, P. (2014). Antiviral activity of monoterpenes beta‐pinene and limonene against herpes simplex virus in vitro. Iranian Journal of Microbiology, 6(3), 149–155. [PMC free article] [PubMed] [Google Scholar]
- Berger, W. (2009). Aerosol devices and asthma therapy. Current Drug Delivery, 6(1), 38–49. 10.2174/156720109787048203 [DOI] [PubMed] [Google Scholar]
- Bhowal, M. , & Gopal, M. (2015). Eucalyptol: Safety and pharmacological profile. Journal of Pharmaceutical Sciences, 5, 125–131. 10.5530/rjps.2015.4.2 [DOI] [Google Scholar]
- Caputo, L. , Lentini, G. , & Habtemariam, S. (2020). Repurposing therapeutic agents and herbal medicines to defeat viral nemesis. Drug Development Research, 30. Published online ahead of print, 2020 Mar 30. 10.1002/ddr.21668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condos, R. , Hull, F. P. , Schluger, N. W. , Rom, W. N. , & Smaldone, G. C. (2004). Regional deposition of aerosolized interferon‐gamma in pulmonary tuberculosis. Chest, 125, 2146–2155. 10.1378/chest.125.6.2146 [DOI] [PubMed] [Google Scholar]
- Condos, R. , Rom, W. N. , & Schluger, N. W. (1997). Treatment of multidrug‐resistant pulmonary tuberculosis with interferon‐gamma via aerosol. Lancet, 349(9064), 1513–1515. 10.1016/S0140-6736(96)12273-X [DOI] [PubMed] [Google Scholar]
- Dawood, A. A. (2020). Mutated COVID‐19 may foretell mankind in a great risk in the future. New Microbes and New Infections, 35, 100673. 10.1016/j.nmni.2020.100673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz, M. (2020). The ICMR bulletin on targeted hydroxychloroquine prophylaxis for Covid‐19: Need to interpret with caution. Indian Journal of Medical Ethics, V(2), 100–102. 10.20529/IJME.2020.040 [DOI] [PubMed] [Google Scholar]
- Debs, R. J. , Montgomery, A. B. , Brunette, E. N. , De Bruin, M. , & Shanley, J. D. (1988). Aerosol administration of antiviral agents to treat lung infection due to murine cytomegalovirus. The Journal of Infectious Diseases, 157(2), 327–323. 10.1093/infdis/157.2.327 [DOI] [PubMed] [Google Scholar]
- Iriti, M. (2020). Monoterpenes: essential oil components with valuable features. Mini‐Reviews in Medicinal Chemistry, 20. 10.2174/1389557520666200122144703. [DOI] [PubMed] [Google Scholar]
- Dent, A. G. , Sutedja, T. G. , & Zimmerman, P. V. (2013). Exhaled breath analysis for lung cancer. Journal of Thoracic Disease, 5, (suppl 5), S540–S550. 10.3978/j.issn.2072-1439.2013.08.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diot, P. , & Plantier, L. (2016). A new era for ribavirin aerosols to treat respiratory syncitial virus infections in immunocompromised patients? Respiratory Care, 61(5), 714–715. 10.4187/respcare.04797 [DOI] [PubMed] [Google Scholar]
- Dong, L. , Hu, S. , & Gao, J. (2020). Discovering drugs to treat coronavirus disease 2019 (COVID‐19). Drug Discoveries & Therapeutics, 14(1), 58–60. 10.5582/ddt.2020.01012 [DOI] [PubMed] [Google Scholar]
- Durham, P. G. , Zhang, Y. , German, N. , Mortensen, N. , Dhillon, J. , Mitchison, D. A. , … Hickey, A. J. (2015). Spray dried aerosol particles of pyrazinoic acid salts for tuberculosis therapy. Molecular Pharmaceutics, 12(8), 2574–2581. 10.1021/acs.molpharmaceut.5b00118. [DOI] [PubMed] [Google Scholar]
- Ebrahimi, S. A. (2020). Noscapine, a possible drug candidate for attenuation of cytokine release associated with SARS‐CoV‐2. Drug Development Research. Online ahead of print. 10.1002/ddr.21676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMA , Committee on Herbal Medicinal Products (HMPC). (2014a). Assessment report on eucalytus globulus Labill., Eucalyptus polybractea R.T. Baker and/or Eucalyptus smithii R.T. Baker, aetheroleum. EMA/HMPC/307782/2012.
- EMA , Committee on Herbal Medicinal Products (HMPC). (2014b). Community herbal monograph on Eucalyptus globulus Labill., Eucalyptus polybractea R.T. Baker and/or Eucalyptus smithii R.T. Baker, aetheroleum. EMA/HMPC/307781/2012.
- Fabbri, L. M. , Piattella, M. , Caramori, G. , & Ciaccia, A. (1996). Oral vs inhaled asthma therapy. Pros, cons and combinations. Drugs, 52(suppl 6), 20–28. 10.2165/00003495-199600526-00005 [DOI] [PubMed] [Google Scholar]
- Fischer, J. , & Dethlefsen, U. (2013a). Efficacy of cineole in patients suffering from acute bronchitis: A placebo‐controlled double‐blindtrial. Cough, 9(1), 25. 10.1186/1745-9974-9-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, J. , & Dethlefsen, U. (2013b). Efficacy of cineole in patients suffering from acute bronchitis: A placebo‐controlled double‐blind trial. Cough, 9(1), 25. 10.1186/1745-9974-9-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry, N. M. , & Malcolm, B.J. (2020). Eucalyptol (1,8‐cineole): an underutilized ally in respiratory disorders?. Journal of Essential Oil Research, 32(2), 103–110. 10.1080/10412905.2020.1716867. [DOI] [Google Scholar]
- Gandhi, M. , Yokoe, D. S. , & Havlir, D. V. (2020). Asymptomatic transmission, the Achilles' heel of current strategies to control Covid‐19. The New England Journal of Medicine, 382, 2158–2160. 10.1056/NEJMe2009758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, J. , Tian, Z. , & Yang, X. (2020). Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Bioscience Trends, 14(1), 72–73. 10.5582/bst.2020.01047 [DOI] [PubMed] [Google Scholar]
- Gao, Z. , Xu, Y. , Sun, C. , Wang, S. , Guo, Y. , Qiu, S. , & Ma, K. (2020). A systematic review of asymptomatic infections with COVID‐19. Journal of Microbiology, Immunology, and Infection. Online ahead of print. 10.1016/j.jmii.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller, C. , Varbanov, M. , & Duval, R. E. (2012). Human coronaviruses: Insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses, 4(11), 3044–3068. 10.3390/v4113044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner, J. F. , Müller, J. , Zeuner, M. T. , Hauser, S. , Seidel, T. , Klenke, C. , & Kaltschmidt, B. (2013). 1,8‐Cineol inhibits nuclear translocation of NF‐κB p65 and NF‐κB‐dependent transcriptional activity. Biochimica et Biophysica Acta, 1833(12), 2866–2878. 10.1016/j.bbamcr.2013.07.001 [DOI] [PubMed] [Google Scholar]
- Gul, M. H. , Htun, Z. M. , Shaukat, N. , Imran, M. , & Khan, A. (2020). Potential specific therapies in COVID‐19. Therapeutic Advances in Respiratory Disease, 14, 1753466620926853. 10.1177/1753466620926853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilberg, O. , Andersen, C. U. , Henning, O. , Lundby, T. , Mortensen, J. , & Bendstrup, E. (2012). Remarkably efficient inhaled antifungal monotherapy for invasive pulmonary aspergillosis. The European Respiratory Journal, 40(1), 271–273. 10.1183/09031936.00163511 [DOI] [PubMed] [Google Scholar]
- Jiang, S. (2020). Don't rush to deploy COVID‐19 vaccines and drugs without sufficient safety guarantees. Nature, 579(7799), 321. 10.1038/d41586-020-00751-9 [DOI] [PubMed] [Google Scholar]
- Jin, Y‐H , Cheng, Z‐S , Cheng, H. , Deng, T. , Fan, Y‐P , Wang, Xing‐Huan (2020). A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019‐nCoV) infected pneumonia (standard version). Military Medical Research, 7(1). 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juergens, U. R. , Dethlefsen, U. , Steinkamp, G. , Gillissen, A. , Repges, R. , & Vetter, H. (2003). Anti‐inflammatory activity of 1.8‐cineol (eucalyptol) in bronchial asthma: A double‐blind placebo‐controlled trial. Respiratory Medicine, 97(3), 250–256. 10.1053/rmed.2003.1432. [DOI] [PubMed] [Google Scholar]
- Juergens, U. R. , Stöber, M. , Schmidt‐Schilling, L. , Kleuver, T. , & Vetter, H. (1999). Antiinflammatory effects of eucalyptol (1.8‐cineole) in bronchial asthma: Inhibition of arachidonic acid metabolism in human blood monocytes ex vivo. European Journal of Medical Research, 3(9), 407–412. [PubMed] [Google Scholar]
- Kallstrom, T. J. (2004). Evidence‐based asthma management. Respiratory Care, 49(7), 783–792. [PubMed] [Google Scholar]
- Kawai, N. , Ikematsu, H. , Iwaki, N. , Maeda, T. , Kanazawa, H. , Kawashima, K. , & Kashiwagi, S. (2008). A comparison of the effectiveness of zanamivir and oseltamivir for the treatment of influenza a and B. The Journal of Infection, 56(1), 51–57. 10.1016/j.jinf.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Kohlert, C. , van Rensen, I. , März, R. , Schindler, G. (2000). Bioavailability and pharmacokinetics of natural volatile terpenes in animals and humans. Planta Medica, 66(6), 495–505. [DOI] [PubMed] [Google Scholar]
- Kozioł, A. , Stryjewska, A. , Librowski, T. , Sałat, K. , Jarosz, M. , Moniczewski, A. , & Lochyński, S. (2014). An overview of the pharmacological properties and potential applications of natural monoterpenes. Mini Reviews in Medicinal Chemistry, 14(14), 1156–1168. 10.2174/1389557514666141127145820. [DOI] [PubMed] [Google Scholar]
- Krämer, S. D. , Lombardi, D. , Primorac, A. , Thomae, A. V. , & Wunderli‐Allenspach, H. (2009). Lipid‐bilayer permeation of drug‐like compounds. Chemistry & Biodiversity, 6(11), 1900–1916. 10.1002/cbdv.200900122 [DOI] [PubMed] [Google Scholar]
- Lai, W. L. , Chuang, H. S. , Lee, M. H. , Wei, C. L. , Lin, C. F. , & Tsai, Y. C. (2012). Inhibition of herpes simplex virus type 1 by thymol‐related monoterpenoids. Planta Medica, 78(15), 1636–1638. 10.1055/s-0032-1315208 [DOI] [PubMed] [Google Scholar]
- Lauer, S. A. , Grantz, K. H. , Bi, Q. , Jones, F. K. , Zheng, Q. , Meredith, H. R. , & Lessler, J. (2020). The incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: Estimation and application. Annals of Internal Medicine, 172(9), 577–582. 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavezzo, E. , Franchin, E. , Ciavarella, C. , Cuomo‐Dannenburg, G. , Barzon, L. , Del Vecchio, C. , … Crisanti, A. (2020). Suppression of a SARS‐CoV‐2 outbreak in the Italian municipality of Vo’. Nature. 10.1038/s41586-020-2488-1. [DOI] [PubMed] [Google Scholar]
- Lay, Y. N. , Li, Y. , Fu, L. C. , Zhao, F. , Liu, N. , Zhnag, F. X. , & Xu, P. P. (2017). Combinations of 1,8‐cineol and oseltamivir for the treatment of influenza virus A (H3N2) infection in mice. Journal of Medical Virology, 89(7), 1158–1167. 10.1002/jmv.24755 [DOI] [PubMed] [Google Scholar]
- Leiva‐Juarez, M. M. , Kirkpatrick, C. T. , Gilbert, B. E. , Scott, B. , Tuvim, M. J. , Dickey, B. F. , & Markesich, D. (2018). Combined aerosolized Toll‐like receptor ligands are an effective therapeutic agent against influenza pneumonia when co‐administered with oseltamivir. European Journal of Pharmacology, 5(818), 191–197. 10.1016/j.ejphar.2017.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, G. , & De Clercq, E. (2020). Therapeutic options for the 2019 novel coronavirus (2019‐nCoV). Nature Reviews. Drug Discovery, 19(3), 149–150. 10.1038/d41573-020-00016-0 [DOI] [PubMed] [Google Scholar]
- Li, G. , Fan, Y. , Lai, Y. , Han, T. , Li, Z. , Zhou, P. , & Wu, J. (2020). Coronavirus infections and immune responses. Journal of Medical Virology, 92(4), 424–432. 10.1002/jmv.25685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Fink, J. B. , & Ehrmann, S. (2020). High‐flow nasal cannula for COVID‐19 patients: Low risk of bio‐aerosol dispersion. The European Respiratory Journal, 55(5), 2000892. 10.1183/13993003.00892-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Lai, Y. , Wang, Y. , Liu, N. , Zhang, F. X. , & Xu, P. P. (2016). 1, 8‐Cineol protect against influenza‐virus‐induced pneumonia in mice. Inflammation, 39(4), 1582–1593. 10.1007/s10753-016-0394-3 [DOI] [PubMed] [Google Scholar]
- Lin, M‐H. , Moses, D. C. , Hsieh, C‐H. , Cheng, S‐C. , Chen, Y‐H. , Sun, C‐Y. , & Chou, C‐Y. (2018). Disulfiram can inhibit MERS and SARS coronavirus papain‐like proteases via different modes. Antiviral Research, 150, 155–163. 10.1016/j.antiviral.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z. , Magal, P. , Seydi, O. , & Webb, G. (2020). A COVID‐19 epidemic model with latency. Infectious Disease Modelling, 5, 323–337. 10.1016/j.idm.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lother, S. A. , Abassi, M. , Agostinis, A. , Bangdiwala, A. S. , Cheng, M. P. , Drobot, G. , & Boulware, D. R. (2020). Post‐exposure prophylaxis or pre‐emptive therapy for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): Study protocol for a pragmatic randomized‐controlled trial. Prophylaxie post‐exposition ou traitement préventif pour le syndrome respiratoire aigu sévère du coronavirus 2 (SARS‐CoV‐2): protocole d'étude pour une étude randomisée contrôlée pragmatique. Canadian Journal of Anaesthesia, 1–11. 10.1007/s12630-020-01684-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxmen, A. (2020). More than 80 clinical trials launch to test coronavirus treatments. Nature, 578(7795), 347–348. 10.1038/d41586-020-00444-3 [DOI] [PubMed] [Google Scholar]
- McHugh, K. J. (2020). Employing drug delivery strategies to create safe and effective pharmaceuticals for COVID‐19. Bioengineering & Translational Medicine, 5(2), e10163. 10.1002/btm2.10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa, M. , Shindo, M. , & Shimada, T. (2001). Oxidation of 1, 8‐cineole, the monoterpene cyclic ether originated from Eucalyptus polybractea, by cytochrome P450 3A enzymes in rat and human liver microsomes. Drug Metabolism and Disposition, 29, 200–205. [PubMed] [Google Scholar]
- Molina, J. M. , Delaugerre, C. , Le Goff, J. , Mela‐Lima, B. , Ponscarme, D. , Goldwirt, L. , & de Castro, N. (2020). No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID‐19 infection. Médecine et Maladies Infectieuses, 50(4), 384. 10.1016/j.medmal.2020.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon, C. , Smyth, H. D. C. , Watts, A. B. , & Williams, R. O., 3rd. (2019). Delivery technologies for orally inhaled products: An update. AAPS PharmSciTech, 20(3), 117. 10.1208/s12249-019-1314-2 [DOI] [PubMed] [Google Scholar]
- Morgan, S. B. , Hemmink, J. D. , Porter, E. , Harley, R. , Shelton, H. , Aramouni, M. , & Tchilian, E. (2016). Aerosol delivery of a candidate universal influenza vaccine reduces viral load in pigs challenged with pandemic H1N1 virus. Journal of Immunology, 196(12), 5014–5023. 10.4049/jimmunol.1502632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss, R. B. , Mayer‐Hamblett, N. , Wagener, J. , Daines, C. , Hale, K. , Ahrens, R. , & Starko, K . (2005). Randomized, double‐blind, placebo‐controlled, dose‐escalating study of aerosolized interferon gamma‐1b in patients with mild to moderate cystic fibrosis lung disease. Pediatric Pulmonology, 39(3), 209–218. 10.1002/ppul.20152 [DOI] [PubMed] [Google Scholar]
- Müller, J. , Greiner, J. F. , Zeuner, M. , Brotzmann, V. , Schäfermann, J. , Wieters, F. , & Kaltschmidt, C. (2016). 1,8‐Cineole potentiates IRF3‐mediated antiviral response in human stem cells and in an ex vivo model of rhinosinusitis. Clinical Science, 130(15), 1339–1352. 10.1042/CS20160218 [DOI] [PubMed] [Google Scholar]
- Necula, V. , Stamate, M. C. , Blebea, C. , & Cozma, S. (2019). Safety and effectiveness of chloral hydrate in outpatient paediatric sedation for objective hearing tests. International Journal of Pediatric Otorhinolaryngology, 126, 109605. 10.1016/j.ijporl.2019.109605 [DOI] [PubMed] [Google Scholar]
- Newman, S. P. (2017). Drug delivery to the lungs: Challenges and opportunities. Therapeutic Delivery, 8(8), 647–661. 10.4155/tde-2017-0037 [DOI] [PubMed] [Google Scholar]
- Park, S. W. , Cornforth, D. M. , Dushoff, J. , & Weitz, J. S. (2020). The time scale of asymptomatic transmission affects estimates of epidemic potential in the COVID‐19 outbreak. Epidemics, 31, 100392. 10.1016/j.epidem.2020.100392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, A. W. , Milleri, S. , & Stein, D. S. (2000). Direct measurement of the anti‐influenza agent zanamivir in the respiratory tract following inhalation. Antimicrobial Agents and Chemotherapy, 44(7), 1974–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phadke, M. , & Saunik, S. (2020). COVID‐19 treatment by repurposing drugs until the vaccine is in sight. Drug Development Research. Online ahead of print. 10.1002/ddr.21666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchford, K. C. , Corey, M. , Highsmith, A. K. , Perlman, R. , Bannatyne, R. , Gold, R. , … Ford‐Jones, E. L. (1987). Pseudomonas species contamination of cystic fibrosis patients' home inhalation equipment. The Journal of Pediatrics, 111(2), 212–216. 10.1016/s0022-3476(87)80069-0 [DOI] [PubMed] [Google Scholar]
- Pliego Zamora, A. , Edmonds, J. H. , Reynolds, M. J. , Khromykh, A. A. , & Ralph, S. J. (2016). The in vitro and in vivo antiviral properties of combined monoterpene alcohols against West Nile virus infection. Virology, 495, 18–32. 10.1016/j.virol.2016.04.021 [DOI] [PubMed] [Google Scholar]
- Prasanthi, D. , & Lakshmi, P. K. (2012). Terpenes: Effect of lipophilicity in enhancing transdermal delivery of alfuzosin hydrochloride. Journal of Advanced Pharmaceutical Technology & Research, 3(4), 216–223. 10.4103/2231-4040.104712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabaan, A. A. , Al‐Ahmed, S. H. , Haque, S. , Sah, R. , Tiwari, R. , Malik, Y. S. , & Rodriguez‐Morales, A. J. (2020). SARS‐CoV‐2, SARS‐CoV, and MERS‐COV: A comparative overview. Le Infezioni in Medicina, 28(2), 174–184. [PubMed] [Google Scholar]
- Remuzzi, A. , & Remuzzi, G. (2020). COVID‐19 and Italy: What next? Lancet, 395(10231), 1225–1228. 10.1016/S0140-6736(20)30627-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renu, K. , Prasanna, P. L. , & Abilash, V. G. (2020). Coronaviruses pathogenesis, comorbidities and multi‐organ damage—A review. Life Sciences, 255, 117839. 10.1016/j.lfs.2020.117839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe, C. , Schunk, M. , Sothmann, P. , Bretzel, G. , Froeschl, G. , Wallrauch, C. , & Hoelscher, M. (2020). Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany. The New England Journal of Medicine, 382(10), 970–971. 10.1056/NEJMc2001468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothlin, R. P. , Vetulli, H. M. , Duarte, M. , & Pelorosso, F. G. (2020). Telmisartan as tentative angiotensin receptor blocker therapeutic for COVID‐19. Drug Development Research. Online ahead of print. 10.1002/ddr.21679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahiwala, A. (2011). Formulation approaches in enhancement of patient compliance to oral drug therapy. Expert Opinion on Drug Delivery, 8(11), 1521–1529. 10.1517/17425247.2011.628311 [DOI] [PubMed] [Google Scholar]
- Sharifi‐Rad, J. , Salehi, B. , Schnitzler, P. , Ayatollahi, S. A. , Kobarfard, F. , Fathi, M. , … Sharifi‐Rad, M. (2017). Susceptibility of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p‐cymene and essential oils of Sinapis arvensis L., Lallemantia royleana Benth. and Pulicaria vulgaris Gaertn. Cellular and Molecular Biology, 63(8), 42–47. 10.14715/cmb/2017.63.8.10 [DOI] [PubMed] [Google Scholar]
- Shereen, M. A. , Khan, S. , Kazmi, A. , Bashir, N. , & Siddique, R. (2020). COVID‐19 infection: Origin, transmission, and characteristics of human coronaviruses. Journal of Advanced Research, 24, 91–98. 10.1016/j.jare.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong, P. , Ito, K. , Murray, J. , & Rapeport, G. (2018). Current approaches to the discovery of novel inhaled medicines. Drug Discovery Today, 23(10), 1705–1717. 10.1016/j.drudis.2018.05.017 [DOI] [PubMed] [Google Scholar]
- Sturton, G. , Persson, C. , & Barnes, P. J. (2008). Small airways: An important but neglected target in the treatment of obstructive airway diseases. Trends in Pharmacological Sciences, 29(7), 340–345. 10.1016/j.tips.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Swamy, M. K. , Akhtar, M. S. , & Sinniah, U. R. (2016). Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evidence‐Based Complementary and Alternative Medicine, 2016, 3012462. 10.1155/2016/3012462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsten, M. , Gómez‐Moreno, G. , & Aguilar‐Salvatierra, A. (2017). Drug‐related oral malodour (halitosis): A literature review. European Review for Medical and Pharmacological Sciences, 21(21), 4930–4934. [PubMed] [Google Scholar]
- Touret, F. , & de Lamballerie, X. (2020). Of chloroquine and COVID‐19. Antiviral Research, 177, 104762. 10.1016/j.antiviral.2020.104762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trang, T. P. , Whalen, M. , Hilts‐Horeczko, A. , Doernberg, S. B. , & Liu, C. (2018). Comparative effectiveness of aerosolized versus oral ribavirin for the treatment of respiratory syncytial virus infections: A single‐center retrospective cohort study and review of the literature. Transplant Infectious Disease, 20(2), e12844. 10.1111/tid.12844 [DOI] [PubMed] [Google Scholar]
- Usachev, E. V. , Pyankov, O. V. , Usacheva, O. V. , & Agranovski, I. E. (2013). Antiviral activity of tea tree and eucalyptus oil aerosol and vapour. Journal of Aerosol Science, 59, 22–30. 10.1016/j.jaerosci.2013.01.004 [DOI] [Google Scholar]
- Velkov, T. , Rahim, N. , Zhou, Q. , Chan, H.‐K. , & Li, J. (2015). Inhaled anti‐infective chemotherapy for respiratory tract infections: Successes, challengesand the road ahead. Advanced Drug Delivery Reviews, 85, 65–82. 10.1016/j.addr.2014.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virchow, J. C. , Crompton, G. K. , Dal Negro, R. , Pedersen, R. , Magnan, A. , Seidenberg, J. , & Barnes, P. J. (2008). Importance of inhaler devices in the management of airway disease. Respiratory Medicine, 102(1), 10–19. 10.1016/j.rmed.2007.07.031 [DOI] [PubMed] [Google Scholar]
- von Hentig, N. (2015). Clinical use of cobicistat as a pharmacoenhancer of human immunodeficiency virus therapy. HIV AIDS, 8, 1–16. 10.2147/HIV.S70836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Xu, K. , Yang, M. , & Quin, C. (2020). Development of an inactivated vaccine candidate for SARS‐CoV‐2. Science, 369, (6499), 77–81. 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Xu, X. , Ruan, J. , Lin, S. , Jiang, J. , & Ye, H. (2020). Quadruple therapy for asymptomatic COVID‐19 infection patients. Expert Review of Anti‐Infective Therapy, 1, 1–8. 10.1080/14787210.2020.1758066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal, J. F. (2000). Macrolide‐induced clinically relevant drug interactions with cytochrome P‐450A (CYP) 3A4: An update focused on clarithromycin, azithromycin and dirithromycin. British Journal of Clinical Pharmacology, 50(4), 285–295. 10.1046/j.1365-2125.2000.00261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wexler, M. R. , Rhame, F. S. , Blumenthal, M. N. , Cameron, S. B. , Juni, B. A. , & Fish, L. A. (1991). Transmission of gram‐negative bacilli to asthmatic children via home nebulizers. Annals of Allergy, 66(3), 267–271. [PubMed] [Google Scholar]
- WHO . (2020a). Director‐General's opening remarks at the media briefing on COVID‐19. World Health Organization, March 3, 2020. Available from https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19‐‐‐3‐march‐2020
- WHO . (2020b). Director‐General's opening remarks at the media briefing on COVID‐19. Available at https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---25-may-2020
- Wojtunik‐Kulesza, K. A. , Kasprzak, K. , Oniszczuk, T. , & Oniszczuk, A. (2019). Natural monoterpenes: Much more than only a scent. Chemistry & Biodiversity, 16(12), e1900434. 10.1002/cbdv.201900434 [DOI] [PubMed] [Google Scholar]
- Wölfel, R. , Corman, V. M. , Guggemos, W. , Seilmaier, M. , Zange, S. , Müller, M.A. , & Wendtner, C. (2020). Virological assessment of hospitalized patients with COVID‐2019. Nature, 581(7809), 465–469. 10.1038/s41586-020-2196-x [DOI] [PubMed] [Google Scholar]
- Wong, J. P. , Christopher, M. E. , Viswnaan, S. , Schnell, G. , Dai, X. , Van Loon, D. , & Stephen, E. R. (2010). Aerosol and nasal delivery of vaccines and antiviral drugs against seasonal and pandemic influenza. Expert Review of Respiratory Medicine, 4(2), 171–177. 10.1586/ers.10.15 [DOI] [PubMed] [Google Scholar]
- Worth, H. , & Dethlefsen, U. (2012). Patients with asthma benefit from concomitant therapy with cineole: A placebo‐controlled, double‐blind trial. The Journal of Asthma, 49(8), 849–853. 10.3109/02770903.2012.717657 [DOI] [PubMed] [Google Scholar]
- Worth, H. , Schacher, C. , & Dethlefsen, U. (2009). Concomitant therapy with Cineole (Eucalyptole) reduces exacerbations in COPD: A placebo‐controlled double‐blind trial. Respiratory Research, 10, 69. 10.1186/1465-9921-10-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, P. , Hao, X. , Lau, E. H. Y. , Wong, J. Y. , Leung, K. S. M. , Wu, J. T. , … Leung, G. M. (2020). Real‐time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveillance, 25(3), 1–6. 10.2807/1560-7917.ES.2020.25.3.2000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, R. , Wang, L. , Kuo, H. D. , Shannar, A. , Peter, R. , Chou, P. J. , & Kong, A. N. (2020). An update on current therapeutic drugs treating COVID‐19. Current Pharmacology Reports, 6(3), 1–15. 10.1007/s40495-020-00216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Z. , Wu, N. , Fu, Y. , Yang, G. , Wang, W. , Zu, Y. , & Efferth, T. (2010). Anti‐infectious bronchitis virus (IBV) activity of 1,8‐cineole: Effect on nucleocapsid (N) protein. Journal of Biomolecular Structure & Dynamics, 28(3), 323–330. 10.1080/07391102.2010.10507362. [DOI] [PubMed] [Google Scholar]
- Yu, N. , Sun, Y. T. , Su, X. M. , He, M. , Dai, B. , & Kang, J. (2019). Eucalyptol protects lungs against bacterial invasion through attenuating ciliated cell damage and suppressing MUC5AC expression. Journal of Cellular Physiology, 234(5), 5842–5850. 10.1002/jcp.26359 [DOI] [PubMed] [Google Scholar]
- Zhou, R. , Li, F. , Chen, F. , Liu, H. , Zheng, J. , Lei, C. , & Wu, X. (2020). Viral dynamics in asymptomatic patients with COVID‐19. International Journal of Infectious Diseases, 96, 288–290. 10.1016/j.ijid.2020.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]


