Abstract
Disseminated intravascular coagulation (DIC) is linked with severe COVID‐19, prompting considerable concern. DIC can be a devastating systemic disorder. It is often markedly manifest on the skin as acrocyanosis or as petechiae and purpura with progression to hemorrhagic bullae. Subcutaneous hematomas may occur, as may thrombotic findings including necrosis and gangrene. The most common cause is infection, with special emphasis now on COVID‐19. We have reviewed the medical literature under the search terms “Disseminated intravascular coagulation” and “consumption coagulopathy” for the past two decades in the English language using Medline and Google Scholar to update special concerns and considerations, focusing on those with COVID‐19. Skin findings with DIC may be prominent. The severity of cutaneous lesions often correlates with the gravity of systemic disease. DIC is most effectively treated by addressing the underlying cause and resuscitating the patient using supportive measures. It is pivotal to recognize and treat DIC early, before deadly complications, such as multiple organ failure, arise.
Keywords: bullae, COVID‐19, disseminated intravascular coagulation, petechiae, purpura fulminans, sepsis, skin
1. INTRODUCTION
Disseminated intravascular coagulopathy (DIC) is linked with severe COVID‐19, as are other COVID‐19‐associated coagulopathies. 1 Disseminated intravascular coagulation (DIC) is a clinically striking condition of inflammatory, systemic hemostasis first reported by Dupuy in 1834. 1 , 2 , 3 This activation overcomes anti‐coagulation mechanisms, leading to fibrin clot formation within small and medium sized vessels. 1 , 2 , 4 The pathogenesis involves endothelial injury, exposing sub‐endothelial collagen, so that microthrombi are formed as the underlying pathology. 5 , 6 Hemostasis, along with inflammation, may lead to organ failure, particularly in those that are critically ill. 1 , 7 Depletion of coagulation factors leads to risk of serious hemorrhagic complications. 1 , 2 DIC may be caused by malignancy, infection, hypoxia, hypoxemia, trauma, burns, vascular disorders, immunologic disorders, toxins, hepatic disease or peripartum complications. 1 , 2 , 5 , 8 , 9 , 10 , 11 , 12 , 13 DIC may occur with metastatic cutaneous melanoma. 2 Acute myeloid leukemia and prostate cancer are the most common causative malignancies. 8 The severity of skin findings in DIC has not been classified. The development of DIC in patients with COVID‐19 unfavorably alters its clinical course. 3 Routine monitoring of hemostasis tests is important in selected COVID‐19 patients.
2. CUTANEOUS MANIFESTATIONS
Clinical patterns with DIC are varied, exemplified by those identified with COVID‐19, from petechial to acro‐ischemia presentations including finger/toe cyanosis, skin bulla and dry gangrene, some resembling chilblains disease (Figures 1 and 2). 7 Hypercoagulable states in DIC are associated with a set of common cutaneous manifestations. 14 In a study of 36 patients with DIC, cutaneous findings were the presenting sign in 47% of them. 8 They were caused by either initial thrombosis or hemorrhage secondary to consumption of coagulation factors. 8 , 9 Skin manifestations caused by hemorrhage occurred with the following frequencies: petechiae (61%), purpura (83%), palpable purpura (32%), hemorrhagic bullae (19%), subcutaneous dissecting hematomas (31%), or bleeding from wound/IV sites (39%). 8 , 15 Skin manifestations caused by thrombosis included acral cyanosis (25%) and gangrene (17%). 9 Purpura fulminans is a combination of hemorrhagic and thrombotic findings.
FIGURE 1.
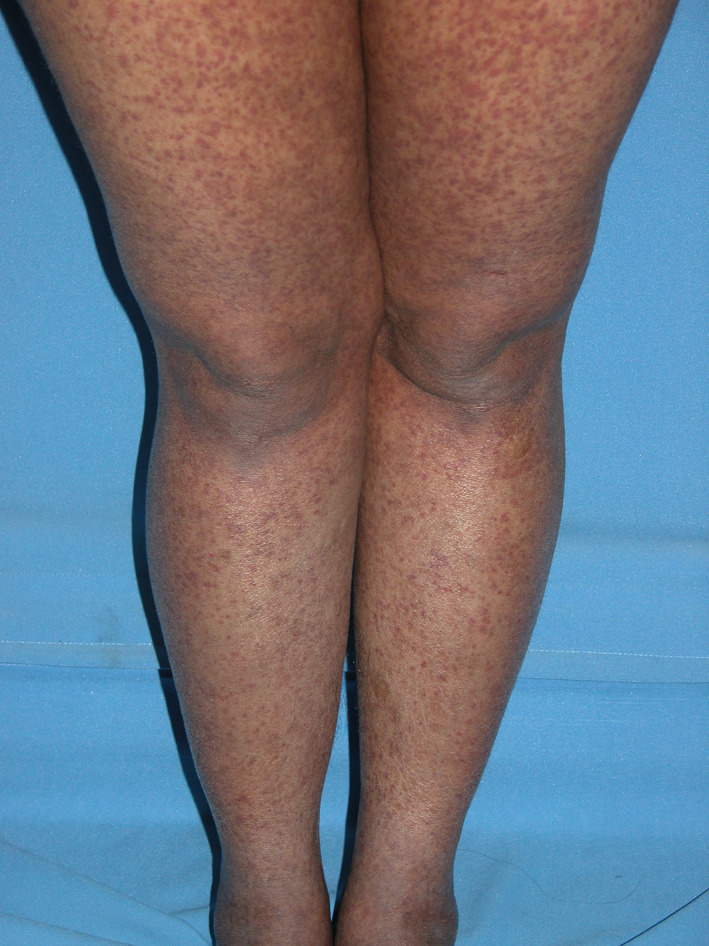
COVID‐19 patient with purpuric eruption, legs (Reprinted from Reference 7)
FIGURE 2.
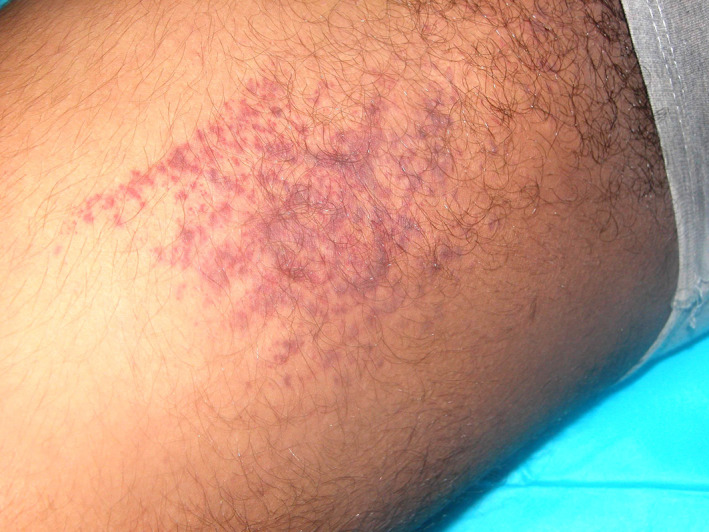
COVID‐19 patient with purpuric eruption, thigh (Reprinted from Reference 7)
In DIC, petechiae are miniscule, pin‐point sized, macules caused by hemorrhage from skin capillaries. 8 , 9 They are sometimes papular, palpable petechiae (Figures 1 and 2). Purpura is evident as nonblanching, erythematous macules, patches, or plaques varying in size from 5 to 20 mm. They may be flat or raised, palpable purpura with clear or necrotic centers. 8 Purpura and petechiae become palpable due to increased hemorrhage and edema. 9 Purpura is similar to petechiae, but larger and in this setting often has sharply demarcated borders. Petechiae and purpura are usually limited to the extremities. 8 They have a propensity to occur in wounds and at pressure points. 9 The purpura may become large and widespread enough to converge and develop into hemorrhagic bullae (Figure 3). 8 When of less than 24 hours duration, petechiae and purpura are characterized by thrombi in the superficial vascular plexus at the junction of the papillary and reticular dermis. Older lesions contain epidermal necrosis, subepidermal bullae, and glandular necrosis. Other hypercoagulable states that cause purpura include thrombotic thrombocytopenic purpura, warfarin skin necrosis, heparin‐induced thrombocytopenia, calciphylaxis, and catastrophic antiphospholipid syndrome. 14 , 16 Palpable purpura may be caused by septic emboli, allergic cutaneous vasculitis, drug reaction (penicillin, sulfonamides), or systemic necrotizing angitis. 8 , 17 , 18 , 19 Palpable purpura is the hallmark of leukocytoclastic vasculitis. Hemorrhagic bullae are filled with blood. Large hemorrhagic bullae may undergo necrosis and gangrenous change and require distinction from erythema multiforme or bullous pemphigoid. 8 Bullae in DIC must also be differentiated from Steven‐Johnson syndrome, the flaccid bullae in toxic epidermal necrolysis (TEN) and dissecting hematomas, which are extensive subcutaneous accumulations of blood that are rapidly progressive and painful. 8 , 19 , 20
FIGURE 3.
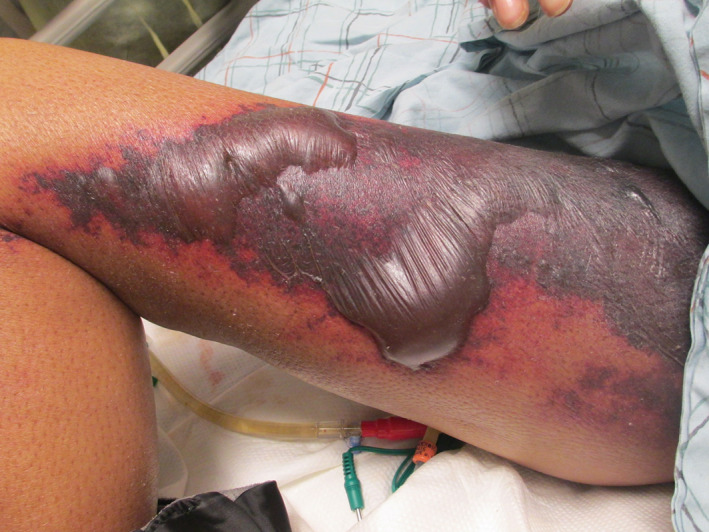
Hemorrhagic bullae in a patient with disseminated intravascular coagulation
DIC may also be evident with cyanosis, which appears as a non‐blanching, central gray patch surrounded by irregular, erythematous or purplish borders. It is most commonly located on the digits, but may also be found on the ears and nose. Acral cyanosis may evolve into gangrene. Indwelling arterial and venous catheters are associated with an enhanced risk of acral cyanosis and gangrene. Acral cyanosis must be distinguished from Raynaud's phenomenon, chilblains, and acrocyanosis. 8
It is important to differentiate the thrombotic/coagulopathic vasculopathic acral ischemia as seen in DIC from chilblain‐like lesions (blue “COVID toes”), also associated with the COVID‐19 pandemic 4. This acral ischemia due to thrombosis in COVID‐19 patients with severe disease reflects a hypercoagulable state and highly elevated D‐dimer levels. Unlike the other skin findings in COVID‐19, chilblain‐like lesions are unassociated with severe disease but may represent a clue to COVID‐19 diagnosis. 20 They clinically and histologically strongly resemble idiopathic and autoimmune related chilblains. 21 There are no data to our knowledge to support that acral ischemia as seen in DIC and chilblain‐like findings are different degrees of involvement within the same spectrum. 7 , 8 , 20
Gangrene of hands, feet, tip of the nose, ear, and posterior scalp may occur in DIC. 15 Symmetrical peripheral gangrene (SPG) is gangrene of the acral parts of the body. The hypercoagulable state of DIC results in microvascular occlusion beginning distally and advancing proximally. 22 SPG manifests as fever, followed by cyanosis, pallor, and pain, and is often caused by Gram‑positive and Gram‑negative organisms, rarely tuberculosis. 22 , 23 There is an association between cold ambient temperature and the development of gangrene, postulated to be related to increased vasospasm. 21 SPG is almost universally associated with DIC. Gangrene has been found to have a mortality of 35%. 23 , 24 , 25 Gangrene may lead to osteomyelitis. 15 In one study of 12 patients, 3 died within 1 week and 8 of the remaining 9 required amputation. 24 There is also some risk of auto‐amputation of digits. 8
Purpura fulminans, the most fatal presentation of DIC, is a dermatologic emergency. 14 It manifests as multiple purple/erythematous, nonblanching, confluent macules, hemorrhagic bullae, and skin necrosis. 5 , 14 Purpura fulminans is rapid in onset and extensive. 5 , 8 , 12 Late findings in purpura fulminans include geographic plaques, hemorrhagic bullae, gangrene and firm eschars. 12 , 26 , 27 The distal extremities are most commonly involved, but any part of the body is susceptible. 5 , 23 It is associated with high mortality due involvement of visceral organs. 5 , 9 , 28 Multiple organ failure makes DIC difficult to distinguish from TEN. 19 , 20 Other causes of purpura fulminans include congenital protein C or S deficiency, sepsis, trauma, malignancy, obstetric complications, hepatic failure, toxins, or immunologic reactions. 14
DIC may manifest at varying degrees of severity. One study found that 58% of patients presented with petechiae, 31% with purpura, 19% with palpable petechiae or purpura, 11% with acral cyanosis. 8 19% of purpura progressed to hemorrhagic bullae. Two patients progressed to purpura fulminans. Palpable purpura was usually found in association with N. meningitidis infection. The combination of septic shock, palpable purpura, and acral cyanosis is very suggestive of DIC.
3. ETIOLOGY
Sepsis, particularly bacterial sepsis, is the condition most commonly associated with DIC (Table 1). 4 More than half of COVID‐19 pneumonias admitted to intensive care at a large Texas hospital developed clinically significant thromboses that were associated with hypercoagulable thromboelastographic parameters alone. 1 In fact, 30‐50% of patients with gram‐negative sepsis have DIC, but it is just as common in those with gram‐positive sepsis. 4 , 29 Systemic coagulation is activated by lipopolysaccharide, endotoxin, or exotoxin. 4 Trauma, particularly brain trauma, results in DIC by the release of fats and phospholipids, hemolysis, and endothelial damage. 4 Both solid and hematologic malignancies cause DIC due to expression of tissue factor on tumor cells. 4 , 30 Obstetrical complications, such as placental abruption and amniotic fluid embolism, cause a self‐limited DIC due to leakage of a thromboplastin‐like substance. 4 , 31 Micro‐angiopathic hemolytic anemias area distinct entity, comprised of thrombotic thrombocytopenic purpura, hemolytic uremic syndrome, chemotherapy induced micro‐angiopathic hemolytic anemia, malignant hypertension, and the hemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome, that result in systemic coagulation due to endothelial damage. 4 Endothelial damage results in platelet adhesion and fibrin clot formation. DIC can be caused a variety of other systemic disorders. Purpura fulminans is associated with a deficiency of protein C or protein S. It is commoner in children than adults, usually linked in the former with scarlet fever, varicella, or pneumococcal infection.
TABLE 1.
Common etiologies of disseminated intravascular coagulopathy
| Type | Cause |
|---|---|
| Infection |
Bacterial (gram‐negative sepsis, gram‐positive sepsis) Rickettsiosis (rocky mountain spotted fever, scrub typhus) Viral (HIV, CMV, HSV, rotavirus, influenza, varicella‐zoster virus, Ebola, dengue, COVID‐19) Fungal (histoplasmsis, candida sepsis, aspergillosis, Candida auris) Parasitic (malaria, leishmaniasis, babesiosis) |
| Tissue injury |
Trauma Burns Hyperthermia Rhabdomyolysis Surgery Frostbite |
| Malignancy |
Hematological malignancy (acute promyelocytic leukemia, acute lymphoblastic leukemia) Solid tumor (prostate carcinoma, gastric carcinoma, pancreatic carcinoma, colon carcinoma, breast carcinoma) Metastases |
| Obstetrical complications |
Abruptio placentae Pre‐eclampsia Eclampsia Acute fatty liver of pregnancy Amniotic fluid embolism Septic abortion Intrauterine fetal demise |
| Coagulation disorders |
Protein C deficiency Antithrombin III deficiency |
| Other |
Hypoxia Hypoxemia Liver disease/hepatic failure Toxins (viper venom, insect bites) Pancreatitis Giant hemangioma Severe inflammatory bowel disease |
Abbreviations: CMV, cytomegalovirus; COVID‐19, Coronovirus‐2019; HIV, human immunodeficiency virus; HSV, human herpes virus.
4. DIAGNOSIS
A cutaneous biopsy specimen may be beneficial in establishing the diagnosis, but the diagnosis is mainly clinical (Table 2). DIC is characterized by fibrin thrombi within capillary walls. 8 , 9 These fibrin thrombi lack the associated inflammation that is seen in vasculitis. 9 Many histochemical stains can aid in the identification of vascular occlusion. 32 Fibrin is strongly periodic acid–Schiff stain positive. It also shows a deep blue color with phosphotungstic acid hematoxylin. Fibrin colors bright red on Carstairs' stain, which is a picric acid‐acid fuchsin‐based stain. Platelets are gray‐blue using this stain. Immunofluorescent staining with antifibrin serum yields positive results. After vascular occlusion, endothelial cells become edematous, resulting in capillary congestion. Endothelial cell nuclei first become hyperchromatic and then pyknotic. The junctions between endothelial cells separate, resulting in extravasation of blood elements. Rupture of capillaries results in formation of petechiae. Continued vascular damage causes hemorrhage into the dermis, resulting information of ecchymoses, which can eventually lead to hemorrhagic bullae. Necrosis and gangrene occur when obstruction of venules distal to the dermovascular loop results in coagulative necrosis. Rotational thromboelastometry may be of value in reflecting a hypocoagulable or hypercoagulable profile to help delineate DIC. 1
TABLE 2.
Diagnostic criteria for disseminated intravascular coagulation from three different organizations
| International Society on Thrombosis and Hemostasis | Japanese Association of Acute Medicine | Japanese Ministry of Health and Welfare | |
|---|---|---|---|
| Underlying disease | 0 points, but patient must have an underlying disorder known to be associated with DIC | 1 point | 0 points, but patient must have an underlying disorder known to be associated with DIC |
| Clinical symptoms | 0 points |
Bleeding: 1 point Organ failure: 1 point |
SIRS score ≥ 3: 1 point |
| Platelet count (×103/μL) |
>100—0 points <100 but >50—1 point <50—2 points |
>120—0 points <120 but >80—1 point <80 but >50—2 points <50—3 points |
<120 but >80 or >30% drop—1 point <80 or >50% drop—3 points |
| Fibrin‐related marker |
Elevated fibrin monomers/degradation products: No increase: 0 Moderate increase: 2 Strong increase: 3 |
Fibrin degradation products μg/mL >10 but <20—1 point >20 but <40—2 points >40—3 points |
Fibrin degradation products μg/mL >10 but <25—1 point >25—3 points |
| Prothrombin time (PT) |
Prothrombin time: <3 seconds—0 >3 but <6—1 >6 seconds—2 |
PT ratio: >1.25 but <1.67—1 point >1.67—2 points |
PT ratio: >1.2—1 point |
| Fibrinogen (g/L) |
>1 g: 0 <1 g: 1 |
>1 but <1.5—1 point <1—2 points |
None |
| Total score to diagnose DIC | ≥5 | ≥7 points | ≥4 points |
There is no definitive blood test to diagnose DIC. 1 Most patients show depletion of coagulation proteases and thrombocytopenia. 1 , 8 , 9 Depression of coagulation factor levels is due to consumption, as well as decreased production. Low platelet count, although characteristic of DIC, is not a specific finding. 1 More than 80% of critically ill, post‐operative patients have less than 100 000 platelets/μl. 1 , 33 10% to 15% of patients with DIC will have platelet counts below 50 000. 1 Compared to those with normal platelet levels, these individuals have an enhanced risk of developing hemorrhagic complications. 1 , 34 The differential for thrombocytopenia due to DIC includes sepsis, impaired production, blood loss, and hypersplenism. 1
The prothrombin time (PT) is prolonged in DIC. 8 , 9 Due to its standardization, many centers are beginning to use INR instead of PT time. An abnormal PT or aPTT is found in most patients with DIC. The differential for an elevated PT includes hepatic failure, blood loss, and vitamin K deficiency. The differential diagnosis for an elevated PTT includes hepatic failure, blood loss, and heparin therapy. The levels of factor 8 are increased in DIC because it, along with von Willebrand factor, is released from injured vascular endothelial cells. 1 , 6 Measurement of fibrinogen level has no proven utility in the diagnosis of DIC. 1 , 35 Fibrin degradation products are increased in DIC. 1 , 8 , 9 They are elevated in 40% of critically ill patients, 80% of trauma patients, and nearly 100% of DIC patients. 1 , 36 As such, the specificity of fibrin split products is not high. The differential includes hematoma, post‐op status, systemic inflammation, deep vein thrombosis, and pulmonary embolism. 1 Inhibitors of coagulation, such as protein C, protein S, and anti‐thrombin, are decreased in 90% of patients with DIC. 1 The reason for their decrease is consumption, decreased production, and increased processing by neutrophil elastase. 1 , 37 Other causes include hepatic failure and capillary leakage. 1
The International Society on Thrombosis and Hemostasis has created a composite scoring system to assess the likelihood of coagulopathy. 38 This scoring system is based on platelet count, PT, D‐dimer, and fibrinogen levels. If the score is greater than or equal to 5 out of 8, then it is considered compatible with overt DIC. Other scoring systems that rely on INR instead of PT are pending widespread validation. The higher the composite score, the lower the survival rates. 1 The scoring should be repeated daily if it is greater than or equal to 5. 38 Prolonged elevation of PT and platelets has been affiliated with poorer outcomes. 1 , 39 A gold standard does not exist for the ideal diagnostic criteria, but three are primarily used throughout the world. 38 , 40 , 41
Patients with DIC can show signs of organ failure. 8 , 9 Blood cultures may show growth of a causative organ, such as Staphylococcus aureus, especially its community acquired methicillin‐resistant form, Group A streptococci, Neisseria meningitidis, Vibrio species, or Varicella virus. 5 , 8 , 11 , 13 , 15 , 28 , 42 One study reported N. meningitidis as the most common causative organism. 8 If other laboratory tests are equivocal, a punch biopsy specimen of cutaneous lesions may reveal fibrin thrombi. 8 , 9
5. TREATMENT
Treatment of DIC usually requires a multidisciplinary approach in an intensive care unit. 24 Therapy of DIC is not directed at the skin, but rather at the management of the causative condition. 1 , 24 , 43 , 44 , 45 , 46 COVID‐19‐associated DIC therapy should follow accepted strategy employing thromboembolic prophylaxis for critically ill hospitalized patients and standard supportive care measures. 47 However, a lack of thrombocytopenia may mean that COVID‐19 DIC is not always a typical consumptive coagulopathy with additional strategies possibly necessary. 45 Transfusion of platelets, plasma, cryoprecipitate or fibrinogen concentrate should be considered for hemorrhaging patients. 1 , 5 , 11 , 43
Patients with DIC due to infection should have it treated, depending on the causative organism. 15 Leukopenia is a poor prognostic factor. 21 Resection of necrotic tissue, including escharotomies, may be required. 26 Skin grafting should be performed, if possible. 5 , 28 Amputation of gangrenous areas may be compulsory. 5 , 8 , 15 While patients are being optimized for treatment, padding or vascular boots may be applied to the affected parts. 21 Improvement of the dermatologic findings is a useful measure in determining response to therapy. 9 With COVID‐19 producing florid DIC in some hospitalized patients and acral cyanosis in a variety of settings, DIC has risen to the forefront with no clear therapy yet of proven value for the COVID‐19 itself 1 , 4 , 5 , 7 , 48 Preliminary data suggests that low‐dose dexamethasone may reduce deaths in hospitalized COVID‐19 patients on ventilation, although its effects on DIC in this setting are yet to be determined. 49 One must also watch for co‐infections, as the COVID‐19 pandemic may merge with the Candida auris global epidemic in the intensive care unit with DIC being produced by either and possibly both of them. 50
CONFLICT OF INTEREST
The authors have no conflicts of interest.
Singh P, Schwartz RA. Disseminated intravascular coagulation: A devastating systemic disorder of special concern with COVID‐19. Dermatologic Therapy. 2020;33:e14053. 10.1111/dth.14053
REFERENCES
- 1. Mortus JR, Manek SE, Brubaker LS, et al. Thromboelastographic results and hypercoagulability syndrome in patients with coronavirus disease 2019 who are critically ill. JAMA Netw Open. 2020;3(6):e2011192. 10.1001/jamanetworkopen.2020.11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Müller MCA, Meijers JC, van Meenen DM, Thachil J, Juffermans NP. Thromboelastometry in critically ill patients with disseminated intravascular coagulation. Blood Coagul Fibrinolysis. 2019;30(5):181‐187. [DOI] [PubMed] [Google Scholar]
- 3. Han H, Yang L, Liu R, et al. Prominent changes in blood coagulations of patients with SARS‐CoC‐2 infection. Clin Chem Lab Med. 2020;58:1116‐1120. [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID‐2019 pneumonia and acro‐ischemia. Zhonghua Xue Ye Xue Za Zhi. 2020;41(0):E006. 10.3760/cma.j.issn.0253-2727.2020.0006 [DOI] [PubMed] [Google Scholar]
- 5. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1‐13. 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Andina D, Noguera‐Morel L, Bascuas‐Arribas M, et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol. 2020;37(3):406‐411. 10.1111/pde.14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almutairi N, Schwartz RA. COVID‐19 with dermatologic manifestations and implications: an unfolding conundrum. Dermatol Ther. 2020:e13544. 10.1111/dth.13544 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Robboy SJ, Mihm MC, Colman RW, Minna JD, et al. The skin in disseminated intravascular coagulation: Prospective analysis of thirty‐six cases. Br J Dermatol. 1973;88(3):221‐229. [DOI] [PubMed] [Google Scholar]
- 9. Colman RW, Minna JD, Robboy SJ. Disseminated intravascular coagulation: a dermatologic disease. Int J Dermatol. 1977;16(1):47‐51. [DOI] [PubMed] [Google Scholar]
- 10. Guldbakke KK, Schanbacher CF. Disseminated intravascular coagulation unmasked by Mohs micrographic surgery. Dermatol Surg. 2006;32(5):760‐764. [DOI] [PubMed] [Google Scholar]
- 11. Konda S, Zell D, Milikowski C, Alonso‐Llamazares J. Purpura fulminans associated with Streptococcus pneumoniae septicemia in an Asplenic pediatric patient. Actas Dermosifiliogr. 2013;104(07):623‐627. [DOI] [PubMed] [Google Scholar]
- 12. Miladi A, Thomas BC, Beasley K, Meyerle J. Angioimmunoblastic t‐cell lymphoma presenting as purpura fulminans. Cutis. 2015;95(2):113‐115. [PubMed] [Google Scholar]
- 13. Campanelli A, Kaya G, Ozsahin AH, et al. Purpura fulminans in a child as a complication of chickenpox infection. Dermatology. 2004;208(3):262‐264. [DOI] [PubMed] [Google Scholar]
- 14. Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69(3):450‐462. [DOI] [PubMed] [Google Scholar]
- 15. Davis MP, Byrd J, Lior T, Rooke TW. Symmetrical peripheral gangrene due to disseminated intravascular coagulation. Arch Dermatol. 2001;137(2):139‐140. [PubMed] [Google Scholar]
- 16. Schwartz RA, Moore HC 3rd, Lambert WC. Linear localized coumarin necrosis. Dermatologica. 1984;168(1):31‐34. [DOI] [PubMed] [Google Scholar]
- 17. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part I. Clinical perspectives. J Am Acad Dermatol. 2013;68(5):693.e614‐693.e691. [DOI] [PubMed] [Google Scholar]
- 18. Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management and therapeutics. J Am Acad Dermatol. 2013;68(5):709.e701‐709.e709. [DOI] [PubMed] [Google Scholar]
- 19. Schwartz RA, McDonough PH, Lee BW. Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifestations, etiology, and immunopathogenesis. J Am Acad Dermatol. 2013;69(2):173.e113‐173.e171. [DOI] [PubMed] [Google Scholar]
- 20. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020;183:71‐77. 10.1111/bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanitakis J, Lesort C, Danset M, Jullien D. Childblain‐like acral lesions during the COVID‐19 pandemic (“COVID toes”): histologic, immunofluorescence and immunohistochemical study of 17 cases. J Am Acad Dermatol. 2020. 10.1016/j.jaad.2020.05.145 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patial T, Sharma K, Jaswal AS, Thakur V, Negi S. Symmetrical peripheral gangrene and tuberculosis: A rare kinship. Int J Mycobacteriol. 2017;6:407‐409. [DOI] [PubMed] [Google Scholar]
- 23. Pedraz J, Delgado‐Jiménez Y, González‐De Arriba A, Ríos‐Buceta L, Fernández‐Herrera J, García‐Diez A. Peripheral symmetrical gangrene. Clin Exp Dermatol. 2009;34(4):552‐554. [DOI] [PubMed] [Google Scholar]
- 24. Davis MDP, Dy KM, Nelson S. Presentation and outcome of purpura fulminans associated with peripheral gangrene in 12 patients at Mayo Clinic. J Am Acad Dermatol. 2007;57(6):944‐956. [DOI] [PubMed] [Google Scholar]
- 25. Rintala E, Kauppila M, Sepala OP, et al. Protein C substition in sepsis‐associated purpura fulminans. Crit Care Med. 2000;28(7):2373‐2378. [DOI] [PubMed] [Google Scholar]
- 26. Talwar A, Kumar S, Gopal MG, Nandini AS. Spectrum of purpura fulminans: report of three classical prototypes and review of management strategies. Indian J Dermatol Venereol Leprol. 2012;78(2):228. [DOI] [PubMed] [Google Scholar]
- 27. Darmstadt GL. Acute infectious purpura fulminans: pathogenesis and medical management. Pediatr Dermatol. 1998;15(3):169‐183. [DOI] [PubMed] [Google Scholar]
- 28. Demma LJ, Faraoni D, Winkler AM, Iba T, Levy JH. Predicting mortality in patients with disseminated intravascular coagulation after cardiopulmonary bypass surgery by utilizing two scoring systems. Blood Coagul Fibrinolysis. 2019;30(1):11‐16. [DOI] [PubMed] [Google Scholar]
- 29. Bone RC. Gram‐positive organisms and sepsis. Arch Intern Med. 1994;154(1):26‐34. [PubMed] [Google Scholar]
- 30. Contrino J, Hair G, Kreutzer DL, Rickles FR. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2(2):209‐215. [DOI] [PubMed] [Google Scholar]
- 31. Weiner CP. The obstetric patient and disseminated intravascular coagulation. Clin Perinatol. 1986;13(4):705‐717. [PubMed] [Google Scholar]
- 32. Adcock DM, Hicks MJ. Dermatopathology of skin necrosis associated with purpura fulminans. Semin Thromb Hemost. 1990;16(4):283‐292. [DOI] [PubMed] [Google Scholar]
- 33. Stéphan FO, Hollande J, Richard O, Cheffi A, Maier‐Redelsperger M, Flahault A. Thrombocytopenia in a surgical ICU. Chest. 1999;115(5):1363‐1370. [DOI] [PubMed] [Google Scholar]
- 34. Vanderschueren S, De Weerdt A, Malbrain M, et al. Thrombocytopenia prognosis in intensive care. Crit Care Med. 2000;28(6):1871‐1876. [DOI] [PubMed] [Google Scholar]
- 35. Bakhtiari K, Meijers JCM, de Jonge E, Levi M. Prospective validation of the Internatinal Society of Thrombosis and Haemostasis scoring system for disseminated intravascular coagulation. Crit Care Med. 2004;32(12):2416‐2421. [DOI] [PubMed] [Google Scholar]
- 36. Shorr AF, Thomas SJ, Alkins SA, Fitzpatrick TM, Ling GS. D‐dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest. 2002;121(4):1262‐1268. [DOI] [PubMed] [Google Scholar]
- 37. Seitz R, Wolf M, Egbring R, Havemann K. The disturbance of hemostasis in septic shock: role of neutrophil elastase and thrombin, effects of antithrombin III and plasma substitution. Eur J Haematol. 1989;43(1):22‐28. [DOI] [PubMed] [Google Scholar]
- 38. Ding R, Wang Z, Lin Y, Liu B, Zhang Z, Ma X. Comparison of a new criteria for sepsis‐induced coagulopathy and International Society on Thrombosis and Haemostasis disseminated intravascular coagulation score in critically ill patients with sepsis 3.0: a retrospective study. Blood Coagul Fibrinolysis. 2018;29(6):551‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kinasewitz GT, Zein JG, Lee GL, Nazir SA, Taylor FB Jr. Prognostic value of a simple evolving disseminated intravascular coagulation score in patients with severe sepsis. Crit Care Med. 2005;33(10):2214‐2221. [DOI] [PubMed] [Google Scholar]
- 40. Wada H, Thachil J, Di Nisio M, et al. Guidance for diagnosis and treatment of disseminated intravascular coagulation from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013;11(4):761‐767. [Google Scholar]
- 41. Kaneko T, Wada H. Diagnostic criteria and laboratory tests for disseminated intravascular coagulation. J Clin Exp Hematop. 2011;51(2):67‐76. [DOI] [PubMed] [Google Scholar]
- 42. Handler MZ, Schwartz RA. Staphlococcal scalded skin syndrome: diagnosis and management in children and adults. J Eur Acad Dermatol Venereol. 2014;28:1418‐1423. [DOI] [PubMed] [Google Scholar]
- 43. Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. Br J Haematol. 2009;145(1):24‐33. [DOI] [PubMed] [Google Scholar]
- 44. Gando S, Levi M, Toh CH. Disseminated intravascular coagulation. Nat Rev Dis Primers. 2016;2:16037. 10.1038/nrdp.2016.37. [DOI] [PubMed] [Google Scholar]
- 45. Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease. Crit Care Med. 2020. 10.1097/CCM.0000000000004458 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wiedermann CJ. Anticoagulant therapy for septic coagulopathy and disseminated intravascular coagulation: where do KyberSept and SCARLET leave us? Acute Med Surg. 2020;7(1):e477. 10.1002/ams2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Connors JM, Levy JH. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033‐2040. 10.1182/blood.2020006000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med. 2020;383:120‐128. 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mahase E. Covid‐19: low dose steroid cuts death in ventilated patients by one third, trial finds. BMJ. 2020;369:m2422. 10.1136/bmj.m2422. [DOI] [PubMed] [Google Scholar]
- 50. Schwartz RA, Kapila R. Cutaneous manifestations of a 21st century worldwide fungal epidemic possibly complicating the COVID‐19 pandemic to jointly menace mankind. Dermatol Ther. 2020:e13481. 10.1111/dth.13481 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]


