Abbreviations
- ALC
absolute lymphocyte count
- ANC
absolute neutrophil count
- CRP
C‐reactive protein
- Hb
hemoglobin
- WBC
white blood cell
To the Editor,
Severe acute respiratory syndrome coronavirus 2 has emerged in December 2019 causing the ongoing coronavirus disease 2019 (COVID‐19) pandemic. 1
Evidence about COVID‐19 in children is evolving, but many questions are still unanswered. In systematic reviews, children represent 1% to 5% of all confirmed cases, often occurring in family clusters. 2
Sickle cell anemia (SCA) patients are immunocompromised mainly because of the loss of splenic functions. 3 Viral infections are triggers of vaso‐occlusive crises (VOC) and acute chest syndrome (ACS). 4 , 5 At present, COVID‐19 presentation and outcomes in SCA have not been reported in the Kingdom of Saudi Arabia (KSA). We are reporting three cases of SCA with COVID‐19 in a mother and two of her children.
Case 1: A 14‐year‐old Saudi girl, known to have SCA, presented to the Emergency Department in April 2020 with multifocal body pain with no respiratory symptoms or fever or other symptoms. She had a history of recurrent multiple VOC and was receiving hydroxyurea 30 mg/kg daily.
On physical examination, she was in moderate to severe pain without respiratory distress, oxygen saturation 99% at room air, heart rate 95/minute, temperature 38.5°C, respiratory rate 20 breath/minute, and blood pressure 121/61 mmHg. A systemic examination showed generalized mild tenderness in both thighs and arms, and she was otherwise normal.
Her complete blood count was normal except anemia (hemoglobin) 88 g/L and leukocytosis (white blood cells 16.2 × 109/L). C‐reactive protein (CRP) was normal at 3.2 mg/L As the patient is living in Al‐Madinah, an endemic city for COVID‐19 since late March 2020, SARS‐CoV‐2 real‐time polymerase chain reaction (RT‐PCR) was requested and was found positive. However, there was no history of contact with COVID‐19 patients.
She was managed as having VOC, and to rule out sepsis, received intravenous (i.v.) fluid, i.v. morphine and ceftriaxone until negative blood cultures were confirmed. She was monitored for 10 days, and remained afebrile. Her chest X‐ray was normal with no evidence of infiltrates and symptoms resolved on day 6 of admission. A second nasopharyngeal swab for PCR remained positive at 10 days; a third swab was negative at 2 weeks.
Case 2: A 12‐year‐old boy, a younger brother of case 1 and known to have SCA, presented 6 days after case 1 with back pain. There was no fever on presentation and no other complaint. He had had a splenectomy a year earlier due to recurrent sequestration crises and was receiving hydroxyurea 35 mg/kg daily.
His examination showed temperature 36.6°C with other vital signs stable and normal examination.
His complete blood count was normal except anemia (hemoglobin 8.8 g/dL) and leukocytosis (white blood cells 23.4 × 109/L). CRP was 17.7 mg/L. The chest X‐ray was normal initially.
He was admitted as having VOC with suspected COVID‐19. On day 2 of admission, he developed fever (39.3°C) with hypoxia; SpO2 was 88% on room air. A repeat chest X‐ray showed new infiltration with a suspicion of ground glass opacities (Figure 1). Ceftriaxone and azithromycin were administered due to suspected ACS, with oxygen 2 L/min by nasal cannula. The inflammatory markers were worsening (Supporting Information Table S1). SARS‐CoV‐2 RT‐PCR was positive, and a respiratory virus multiplex PCR was negative for other viruses. Antibiotics were upgraded to meropenem and vancomycin. He received a blood transfusion on day 3 and dexamethasone 0.3 mg/kg/dose every 12 hours for 3 days along with hydroxychloroquine as recommended by the Saudi Ministry of Health guideline, at a dose of 6.5 mg/kg the first day, then 3.5 mg/kg for 5 days.
FIGURE 1.
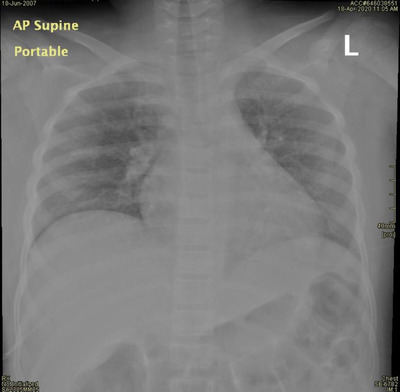
Anteroposterior supine portable chest X‐ray shows bilateral ground‐glass opacification of both lung fields, no collapse or consolidation, no pneumothorax/pleural effusion (all are possible COVID‐19 characteristics)
He improved gradually, and the oxygen supply was discontinued on day 4. Repeated SARS‐CoV‐2 PCR remained positive for 12 days.
Case 3: A 50‐year‐old female with sickle cell trait, the mother of cases 1 and 2, was tested for SARS‐CoV‐2 based on the history of exposure and proved positive. She had no significant symptoms except for headache and fatigability prior to testing. Her examination, laboratory investigation, and chest X‐ray were normal, and she remained asymptomatic.
To the best of our knowledge, this is the first report of SCA patients infected with COVID‐19 in KSA. VOC in the older sister was treated according to standard practice while the second case presented with VOC and developed symptoms of COVID‐19 pneumonia which coincided with the ACS symptoms and was treated according to the national guidelines for COVID19 and as ACS. 6
Hydroxychloroquine displays in vitro antiviral and immunomodulatory activity. 7 A recently published observational study with a cohort of 166 patients, hospitalized due to COVID‐19, showed increased mean survival by 1.4 to 1.8 times. 8 However, better evidence from an adequately powered randomized control trial is still not available.
It is not recommended to use corticosteroids for COVID‐19, based on an early observational study from China. 9 Dexamethasone was started for case 2 based on suspicion of ACS. Though the benefit of corticosteroid in ACS is controversial, it may shorten the duration and severity of mild and moderated ACS. 10
The pathogenesis of both ACS and COVID‐19 pneumonia is characterized by severe inflammatory response. 11 A 45‐year‐old male who presented with VOC associated with COVID‐19 progressed to severe ACS; treatment with hydroxychloroquine and tocilizumab improved the patient's respiratory and general condition. 12
CONFLICTS OF INTEREST
All the authors declare that they have no conflict of interest.
ETHICAL ISSUES
Ethical and scientific approval was received from the Institutional Review Board of King Abdullah International Medical Research Center, Riyadh, Saudi Arabia.
Supporting information
TableS1
REFERENCES
- 1. Wu P, Hao X, Lau EHY, et al. Real‐time tentative assessment of the epidemiological characteristics of novel coronavirus infections in Wuhan, China, as at 22 January 2020. Euro Surveill. 2020;25:2000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ludvigsson JF. Systematic review of COVID‐19 in children show milder cases and a better prognosis than adults. Acta Paediatrica. 2020;109(6):1088‐1095. https://onlinelibrary.wiley.com/doi/abs/10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brousse V, Buffet P, Rees D. The spleen and sickle cell disease: the sick (led) spleen. Br J Haematol. 2014;166:165‐176 [DOI] [PubMed] [Google Scholar]
- 4. Hebbel RP, Visser MR, Goodman JL, Jacob HS, Vercellotti GM. Potentiated adherence of sickle erythrocytes to endothelium infected by virus. J Clin Invest. 1987;80(5):1503‐1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vichinsky EP, Neumayr LD, Earles AN, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. N Engl J Med. 2000;342(25):1855‐1865. [DOI] [PubMed] [Google Scholar]
- 6.Saudi MoH protocol for patients suspected of/confirmed with COVID‐19 supportive care and antiviral treatment of suspected or confirmed COVID‐19 infection (version 1.4), April 12th. Saudi Arabia: Ministry of Health; 2020.
- 7. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020. URL: https://pubmed.ncbi.nlm.nih.gov/32150618/. DOI: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Membrillo de Novales FJ, Ramírez‐Olivencia G, Estébanez M, et al. Early hydroxychloroquine is associated with an increase of survival in Covid‐19 patients: an observational study. Preprints. 2020, 2020050057 https://doi.org.10.20944/preprints202005.0057.v2. [Google Scholar]
- 9. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet North Am Ed. 2020;395(10223):473‐475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernini JC, Rogers ZR, Sandler ES, Reisch JS, Quinn CT, Buchanan GR. Beneficial effect of intravenous dexamethasone in children with mild to moderately severe acute chest syndrome complicating sickle cell disease. Blood. 1998;92(9):3082‐3089. [PubMed] [Google Scholar]
- 11. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Luna G, Habibi A, Deux JF, et al. Rapid and severe Covid‐19 pneumonia with severe acute chest syndrome in a sickle cell patient successfully treated with tocilizumab. Am J Hematol. 2020;95(7):876‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TableS1


