Abstract
Coronavirus disease 2019 (COVID‐19) may be associated with worse outcome in solid organ transplant (SOT) recipients. We performed a prospective cohort study of hospitalized patients with confirmed diagnosis of COVID‐19, from March 15 to April 30, 2020, at two tertiary hospitals in Emilia‐Romagna Region. SOT recipients were compared with non‐SOT patients. Primary endpoint was all‐cause 30‐day mortality. Relationship between SOT status and mortality was investigated by univariable and multivariable Cox regression analysis. Patients were assessed from COVID‐19 diagnosis to death or 30‐day whichever occurred first. Study cohort consisted of 885 patients, of them 24 SOT recipients (n = 22, kidney, n = 2 liver). SOT recipients were younger, had lower BMI, but higher Charlson Index. At admission they presented less frequently with fever and respiratory failure. No difference in 30‐day mortality between the two groups (19% vs 22.1%) was found; however, there was a trend toward higher rate of respiratory failure (50% vs 33.1%, P = .07) in SOT recipients. Superinfections were more represented in SOT recipients, (50% vs 15.5%, P < .001). At multivariate analysis adjusted for main covariates, there was no association between SOT and 30‐day mortality HR 1.15 (95% CI 0.39‐3.35) P = .79. Our data suggest that mortality among COVID‐19 SOT recipients is similar to general population.
Keywords: COVID‐19, SARS‐CoV‐2, solid organ transplantation
Abbreviations
- CMV
cytomegalovirus
- CNI
calcineurin inhibitor
- COVID‐19
coronavirus disease 2019
- ICU
intensive care unit
- IL‐6
interleukin‐6
- KT
kidney transplant
- LOS
length of hospital stay
- LT
liver transplant
- MMF
mycophenolate mofetil
- mTOR
mammalian target of rapamycin
- qSOFA
quick sequential organ failure assessment
- RT‐PCR
real‐time polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- SOT
solid organ transplant
- SRF
severe respiratory failure
- WHO
World and Health Organization
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐associated coronavirus disease 2019 (COVID‐19) has gripped the world in a pandemic, challenging its culture, economy, and healthcare infrastructure. Available epidemiological data depict COVID‐19 as a polymorphic disease with several clinical presentations and severity degrees. Crude fatality rates are on average 5% with notable variation by age groups and settings. 1 , 2 Solid organ transplantation (SOT) is a challenging setting for several reasons. Symptoms and imaging findings can be confused with underlying conditions or initially mitigated by the immunosuppressive state. 3 Efficacy and safety of antiviral and immunomodulatory drugs used as COVID‐19 treatment are unknown in this setting. 4 Finally, published cases series of SOT patients diagnosed with COVID‐19 have reported high morbidity and mortality, with intensive care unit admission and mortality rates ranging from 0%‐39% to 0%‐50%, respectively. 4 , 5 , 6 , 7 , 8 , 9 , 10 However, data on differences in symptoms, clinical severity, treatment management and outcome between SOT and non‐SOT patients hospitalized with COVID‐19 have yet to be investigated. We performed a prospective multicenter cohort study of hospitalized patients with COVID‐19 diagnosis to compare clinical presentation, therapeutic management and outcome between SOT and non‐SOT recipients.
2. MATERIALS AND METHODS
2.1. Study design, population, and setting
Prospective cohort study of adult patients hospitalized at Sant'Orsola‐Malpighi Hospital and Rimini Hospital, from March 15 to April 30, 2020. Patients with detection of SARS‐CoV‐2 by RT‐PCR on respiratory specimens were included. Patients were followed up to death, or hospital discharge. Diagnostic testing for COVID‐19 and hospitalization was performed according to local policy and clinical judgment, and were not dictated by study protocol. Local microbiology databases were used to identify patients. Clinical charts and hospital electronic records were used as data sources. Data were collected de‐identified and managed using REDCap electronic data capture tools hosted at University of Bologna. 11 , 12 The study was approved by the Ethic Committee of the promoting center (Comitato Etico Indipendente di Area Vasta Emilia Centro, n. 283/2020/Oss/AOUBo).
2.2. Definitions and variables
Primary endpoint was all‐cause 30‐day mortality. Secondary endpoints included severe respiratory failure and length of hospital stay. Severe respiratory failure was assessed from diagnosis to hospital discharge and defined according to World and Health Organization (WHO) criteria as: SpO2 <93% with 100% FiO2 (reservoir mask or continuous positive airway pressure ventilation or other non‐invasive ventilation), respiratory rate >30 bpm, or respiratory distress (https://www.who.int/publications‐detail/clinical‐management‐of‐severe‐acute‐respiratory‐infection‐when‐novel‐coronavirus‐(ncov)‐infection‐is‐suspected, accessed 5 April 2020).
Exposure variables included age, sex, and body mass index. Underlying conditions were assessed according to Charlson comorbidity index. For SOT patients, baseline immunosuppression regimen and management of immunosuppressive drugs after COVID‐19 diagnosis were recorded. As for SARS‐CoV‐2 infection, we collected (a) date of symptoms onset; (b) date and symptoms at hospitalization; (c) vital signs, laboratory tests, and imaging findings at hospitalization; (d) clinical severity at hospitalization classified according to qSOFA, SOFA, and CURB‐65; and (e) all administered antiviral and immunomodulatory treatments. Finally, occurrence of viral, bacterial, and/or fungal superinfection was assessed from COVID‐19 diagnosis to hospital discharge. Cytomegalovirus (CMV) infection was diagnosed by evidence of CMV replication regardless of symptoms in any body fluid or tissue specimen. 13 CMV‐DNAemia was detected by quantitative nucleic acid testing on whole blood. Diagnostic testing for CMV infection was requested by attending physicians and was not dictated by study protocol; generally, antiviral treatment was recommended for high levels CMV‐DNAemia >100 000 copies/mL. Bacterial infection was assessed according to CDC criteria. 14 Invasive fungal infections were defined according to European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) criteria. 15 Diagnostic testing for infection and its etiology was performed by attending physicians and was not dictated by study protocol.
2.3. Statistical analysis
Categorical variables were expressed as absolute numbers and their relative frequencies. Continuous variables were expressed as mean ± standard deviation (SD) if normally distributed, or as median and interquartile range (IQR) if non‐normally distributed. For comparison of SOT and non‐SOT patients, Pearson chi‐square or Fisher's exact test were used for categorical variables. Continuous variables were compared using Student's t or Mann‐Whitney U test according to their distribution. Univariate and multivariate analysis of risk factors for all‐cause 30‐day mortality was done. After verifying for proportional hazard and collinearity, all variables with a P value <.1 were introduced in a Cox regression model adjusted for the SOT condition. Patients were considered from COVID‐19 diagnosis to death or 30‐days. Statistical significance was considered for P <05. All analysis was performed with SPSS 21.0.
3. RESULTS
Study cohort consisted of 885 hospitalized patients with COVID‐19 diagnosis: median age was 69 (IQR 57‐80) years, 66.9% were male (see Table 1). Overall, 24 SOT patients were included: 22 (91.7%) kidney transplant (KT), and 2 (8.3%) liver transplant (LT) recipients. Median time from SOT to COVID‐19 diagnosis was 3.8 (0.15‐14.3) years; with 4 (16.7%) patients diagnosed of COVID‐19 within 1 month after SOT, 5 (20.8%) between 2 and 6 months, and 15 (62.5%) over 6 months. Among KT recipients, 10 (41.7%) presented with renal failure at COVID‐19 diagnosis, of them 8 had chronic renal failure and 2 developed an acute worsening of graft function. Baseline immunosuppressive regimens consisted of calcineurin inhibitors (CNI) plus mycophenolate mofetil (MMF) (n = 14), CNI plus mammalian target of rapamycin (mTOR) inhibitors (n = 4), CNI alone (n = 3), mTOR alone (n = 1), low dose prednisone alone (n = 1), and none (n = 1). Low dose prednisone was concomitantly administered to all except three patients. Upon COVID‐19 diagnosis, immunosuppressive regimens were modified in all patients. Briefly, mTOR was always discontinued, MMF was discontinued in all but 1 case, CNI was discontinued in 19 out of 21 patients.
TABLE 1.
Comparison of SOT and non‐SOT hospitalized patients with COVID‐19 diagnosis
|
Total N = 885 (%) |
SOT N = 24 (%) |
Non‐SOT N = 861 (%) |
P | |
|---|---|---|---|---|
| Demographic and comorbidities | ||||
| Age (years) [median (IQR)] | 69 (57‐80) | 62 (48‐67) | 70 (57‐80) | 0.001 |
| Sex, male | 592 (66.9) | 15 (62.5) | 577 (67) | 0.6 |
| BMI [median (IQR)] | 26 (24‐29) | 24.8 (23.5‐26) | 26 (24‐29) | 0.03 |
| Charlson Index [median (IQR)] | 4 (2‐6) | 5 (4‐7) | 3 (1‐6) | 0.002 |
| Signs and symptoms at hospitalization | ||||
| Time from symptoms onset to hospitalization (days) [median (IQR)] | 6 (3‐9) | 3 (0‐7) | 6 (3‐9) | 0.017 |
| Fever >38°C | 329 (37.5) | 5 (20.8) | 324 (37.9) | 0.02 |
| Cough | 473 (54.1) | 12 (50) | 461 (54.2) | 0.7 |
| Dyspnea | 384 (43.8) | 9 (37.5) | 375 (44) | 0.5 |
| Diarrhea | 73 (8.3) | 2 (8.3) | 71 (8.3) | 0.9 |
| MAP [median (IQR)] | 90 (83‐97) | 93 (88‐105) | 90 (83‐97) | 0.03 |
| Pulse rate [median (IQR)] | 86 (76‐97) | 80 (75‐88) | 86 (76‐97) | 0.09 |
| Respiratory rate [median (IQR)] | 20 (18‐24) | 19 (15‐21) | 20 (18‐24) | 0.02 |
| PaO2/FiO2 [median (IQR)] | 314 (254‐368) | 352 (314‐410) | 314 (252‐367) | 0.028 |
| Laboratory parameters at hospitalization | ||||
| WBC (109/L) | 5.9 (4.4‐8.1) | 5.6 (4.4‐7.3) | 5.9 (4.4‐8.1) | 0.7 |
| Lymphocytes count (109/L) | 0.95 (0.7‐1.3) | 0.97 (0.6‐1.3) | 0.95 (0.7‐1.3) | 0.4 |
| Creatinine (mg/dL) | 0.99 (0.8‐1.3) | 2.1 (1.5‐4.9) | 0.98 (0.8‐1.2) | <0.001 |
| LDH (U/L) | 295 (233‐397) | 218 (199‐351) | 297 (237‐398) | 0.01 |
| CRP (mg/dL) | 6.4 (2.4‐11.7) | 2.1 (0.4‐10.2) | 6.1 (2.5‐11.7) | 0.009 |
| PCT (ng/mL) | 0.1 (0.1‐0.4) | 0.5 (0.1‐1.9) | 0.1 (0.1‐0.3) | 0.03 |
| Disease severity at admission | ||||
| qSOFA | 0 (0‐1) | 0 (0‐1) | 1 (0‐1) | 0.04 |
| SOFA | 2 (1‐3) | 3 (2‐5) | 2 (1‐3) | 0.001 |
| CURB65 | 1 (0‐1) | 1 (0‐1) | 1 (0‐1) | 0.6 |
| Needing of oxygen support | 462 (52.2) | 9 (37.5) | 453 (52.6) | 0.14 |
| Radiological findings | ||||
| Positive chest‐Rx | 638 (76.1) | 13 (59.1) | 625 (76.6) | 0.55 |
| HRCT lung consolidation | 249 (60.9) | 3 (16.7) | 246 (62.9) | <0.001 |
| HRCT ground glass | 359 (87.6) | 17 (94.4) | 342 (87.2) | 0.3 |
| HRCT crazy paving | 79 (20.1) | 2 (11.1) | 77 (20.5) | 0.3 |
| COVID‐19 treatment | ||||
| Hydroxychloroquine | 811 (91.6) | 23 (95.8) | 788 (91.5) | 0.4 |
| Azithromycin | 150 (16.9) | 7 (29.2) | 143 (16.6) | 0.1 |
| HCQ + AZT | 141 (15.9) | 6 (25) | 135 (15.7) | 0.2 |
| DRV/cobi | 76 (8.6) | 6 (25) | 70 (8.1) | 0.004 |
| Tocilizumab | 177 (20) | 12 (50) | 165 (19.2) | <0.001 |
| High dose steroids a | 369 (41.7) | 15 (62.5) | 349 (40.5) | 0.03 |
| Superinfections during hospitalization | ||||
| CMV infection | 84 (9.5) | 9 (37.5) | 75 (8.7) | <0.001 |
| Bacterial infection | 142 (16.4) | 12 (50) | 130 (15.5) | <0.001 |
| Fungal infection b | 15 (1.7) | 2 (8.3) | 13 (1.5) | 0.01 |
| Outcome | ||||
| Severe respiratory failure | 293 (33.5) | 12 (50) | 281 (33.1) | 0.07 |
| Days from diagnosis to severe respiratory failure [median (IQR)] | 2, 0‐6 | 6, 2‐9 | 2, 0‐5 | <0.001 |
| ICU admission | 290 (19.9) | 13 (52) | 277 (19.3) | <0.001 |
| Days from diagnosis to ICU admission [median (IQR)] | 2.5 (0.25‐6.75) | 7.5 (4.5‐10.75) | 2 (0‐6) | 0.005 |
| Length of hospital stay (days) (median, IQR) | 10 (6‐17) | 22 (8‐36) | 10 (6‐17) | 0.047 |
| All‐cause 30‐d mortality | 190 (22) | 4 (19) | 186 (22.1) | 0.5 |
| Time to death (days) [median (IQR)] | 10, 6‐18 | 20, 7‐30 | 10,6‐17 | <0.001 |
Abbreviations: BMI, Body Mass Index; CMV, Cytomegalovirus; COVID‐19, Coronavirus Disease 2019; CRP, C‐Reactive Protein; CURB65, Confusion, Urea, Respiratory rate, Blood pressure and age ≥ 65; FiO2, Fraction of inspired oxygen; HRCT, High‐resolution computed tomography; ICU, Intensive Care Unit; IL‐6, Interleukin‐6; IQR, Interquartile Range; LDH, Lactate dehydrogenase; MAP, Mean Arterial Pressure; PaO2, Partial pressure of arterial oxygen; PCT, Procalcitonin; qSOFA, quick sequential organ failure assessment; Rx, Radiography; SOT, Solid Organ Transplant; WBC, White Blood Cell.
Defined as 0.5‐1 mg/Kg/daily of prednisone equivalents.
Included three invasive candidiasis and 12 pulmonary aspergillosis.
Comparison of SOT and non‐SOT patients showed several differences (Table 1). SOT recipients showed lower median age (62 vs 70 years, P < .001), lower median BMI (24.8 vs 26, P = .03), but higher median Charlson Index (5 vs 3, P = .002) than non‐SOT patients. The median time from symptom onset to hospitalization was significantly lower (3 vs 6 days, P = .017) in SOT patients. At hospital admission, significant difference between SOT and non‐SOT patients was found for fever (20.8% vs 37.9%, P = .02), PaO2/FiO2 ratio (352 vs 314, P = .028), median LDH (218 vs 297, P = .01) and lung consolidations (16.7% vs 72.9%, P < .001). As for therapeutic management, SOT patients received more frequently darunavir/cobicistat (25% vs 8.1%, P = .004), tocilizumab (50% vs 19.2%, P < .001), and high doses steroids (62.5% vs 40.5%, P = .03). CMV infection was diagnosed more frequently in SOT recipients (37.5% vs 8.7%, P < .001), as well as bacterial (50% vs 15.5%, P < .001) and fungal (8.3% vs 1.5%, P = .01) infections.
All‐cause 30‐day mortality rates were similar in the two groups (19% vs 22.1%, P = .5). Among KT recipients, mortality rate was 20%, whereas none of LT recipients died. During hospitalization, SOT recipients developed more frequently severe respiratory failure (50% vs 33.1%, P = .07), and were more frequently admitted to ICU (52% vs 19.3%, P < .001), length of hospital stay was also longer (22 vs 10 days, P = .047).
Univariate analysis for 30‐day mortality showed significant differences between survivors and non‐survivors for age, sex, and Charlson Index. Needing of oxygen support at hospital admission, as well as higher qSOFA and SOFA scores, was associated to death within 30 days from COVID‐19 diagnosis (see Table S1). At multivariate analysis, independent predictors of 30‐day mortality were age, Charlson Index, increased respiratory rate, LDH, elevated C‐reactive protein, and SOFA score. Female sex was found to be protective (Table 2). Therefore, SOT was not associated with increased risk of death (Figure 1).
TABLE 2.
Multivariate analysis of risk factors for 30‐d mortality
| HR (95% CI) | P | |
|---|---|---|
| Age | 1.07 (1.05‐1.09) | <.001 |
| Female | 0.65 (0.46‐0.91) | .01 |
| Charlson Index | 1.08 (1.01‐1.15) | .02 |
| BMI | 1.05 (1.01‐1.09) | .004 |
| RR | 1.04 (1.02‐1.06) | <.001 |
| LDH | 1.002 (1.001‐1.003) | <.001 |
| CRP | 1.02 (1.01‐1.03) | <.001 |
| SOFA | 1.32 (1.21‐1.43) | <.001 |
Abbreviations: BMI, body mass index; CRP, C‐reactive protein; HR, hazard ratio; LDH, lactate dehydrogenase; RR, respiratory rate; SOFA, sequential organ failure assessment.
FIGURE 1.
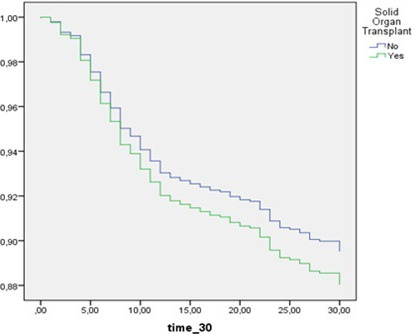
Survival between SOT and non‐SOT hospitalized patients with COVID‐19. Comparison adjusted for independent covariates by Cox regression analysis—SOT HR 1.15 (95% CI 0.39‐3.35) P = .79
4. DISCUSSION
Management of SOT recipients diagnosed of COVID‐19 is challenging. Although initial clinical presentation is milder compared to general population, progression toward severe respiratory failure is more frequent than in non‐SOT recipients. However, there was no significant difference in 30‐day survival between SOT recipients and general population, also when adjusted for potential confounders. SOT recipients showed higher rates of superinfection than non‐SOT patients with longer duration of hospital stay, confirming the high impact of COVID‐19 on morbidity of SOT patients.
In our study, the median time from transplant to COVID‐19 diagnosis was shorter compared to other series. 5 , 6 , 8 , 10 The timeline of transplantation seems to play an important role, as we observed higher rates of severe respiratory failure among patients who undergone transplantation within one year from COVID‐19 diagnosis compared to late transplant recipients (66.7% vs 16.7%, respectively—data not showed). This finding suggests that intensity of immunosuppression seems to be strictly related to the risk of developing a more severe disease.
In our cohort, typical symptoms of COVID‐19 were uniformly represented in both groups except fever. A recent case series of 36 kidney transplant recipients showed similar results. 6 In addition, SOT recipients presented with lower respiratory rate, better gas exchanges, and lower pulmonary consolidations at diagnosis than non‐SOT patients. These findings may reflect a milder disease at presentation in SOT patients, even if this assumption could be influenced by a more immediate clinical evaluation and diagnosis compared to general population. Indeed, the median time from symptoms onset to hospitalization was of 3 vs 6 days in SOT and non‐SOT groups, respectively.
Anyway, clinical evaluation alone could be unfruitful in identifying patients at high risk for complicated course. Indeed, we noted a discrepancy between qSOFA and SOFA score assessment at hospital admission, the median value of the latter was higher among SOT recipients differently for qSOFA. Although in our SOT population SOFA score was influenced by high rates of chronic renal failure, this observation can suggest that upon COVID‐19 diagnosis in a SOT patient, laboratory tests along with clinical evaluation are strongly recommended.
Previous case series 5 , 6 , 7 , 8 , 9 of SOT recipients showed high in‐hospital mortality rates ranging from 24% to 32%. In our study, mortality rates in SOT and non‐SOT patients were similar around 20%. Considering the high incidence of severe respiratory failure among SOT recipients, these relatively low mortality rates may reflect a massive use of healthcare resources. This assumption is supported by early hospitalization, a significant major use of novel immunosuppressive drugs, such as IL‐6 inhibitors, and a greater ease of access to ICU.
Management of chronic immunosuppressive regimen is another important issue. Reductions in immunosuppression are usually applied by many clinicians in order to improve viral clearance; however, this approach could increase the risk of immune reconstitution and rejection. Indeed, a Chinese case report showed an acute rejection in a liver transplant recipient following discontinuation of immunosuppressants and administration of low dose of methylprednisolone. 4 In our cohort, CNI, MMF, and mTOR inhibitors were mostly discontinued at diagnosis, especially if the patient was eligible to receive an IL‐6 inhibitor or high doses of methylprednisolone. No worsening of graft function was observed. Nevertheless, the wide use of immunosuppressive agents in an attempt to contrast the evolving course of COVID‐19 illness may lead to other infections. Indeed, half of SOT recipients in our cohort developed at least one bacterial infection, and a quarter of them developed CMV reactivation needing use of antiviral agents. However, CMV infection occurred also in non‐SOT patients, suggesting that high doses of steroids and others immunosuppressive drugs used for COVID‐19 could play a role in CMV reactivation even in general population.
Our study has some limitations, despite we analyzed a large cohort of hospitalized patients with COVID‐19; the number of SOT recipients was low limiting to assess predictors of poor outcome in this setting. The relatively low size of SOT recipients did not allowed further risk stratification analysis. Moreover, kidney transplants were an absolute majority, not allowing a homogeneous distribution of different SOT. Therefore, a 30‐day follow‐up from diagnosis is useful to depict a short outcome of patients affected by COVID‐19; however, it could not consider any late complication of the disease.
To conclude, our study underlines difficulties in the management of SOT patients with COVID‐19. Careful assessment seems to be important for recognizing patients at risk of complicated course. Risk‐benefit balance of using novel immunosuppressive drugs in this setting should be investigated in larger studies.
CONFLICT OF INTEREST
Authors declare no conflict of interest related to the content of the present manuscript.
AUTHORS' CONTRIBUTIONS
MR involved in data collection, data analysis, and drafting the manuscript. MB involved in data collection and data analysis. LB involved in data collection. LP involved in data collection. RP involved in data collection. GC involved in study design. MM involved in study design. MR involved in support in study design. MC involved in support in study design. FC involved in support in study design. PV involved in study design and manuscript revision. MG involved in study design, data analysis, and manuscript revision.
Supporting information
Tab S1
ACKNOWLEDGEMENT
No funding was received for this project.
Rinaldi M, Bartoletti M, Bussini L, et al. COVID-19 in solid organ transplant recipients: No difference in survival compared to general population. Transpl Infect Dis. 2021;23:e13421. 10.1111/tid.13421
REFERENCES
- 1. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;323(18):1775‐1776. [DOI] [PubMed] [Google Scholar]
- 3. Fishman JA, Grossi PA. Novel coronavirus‐19 (COVID‐19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transplant. 2020;20(7):1765‐1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhong Z, Zhang Q, Xia H, et al. Clinical characteristics and immunosuppressant management of coronavirus disease 2019 in solid organ transplant recipients. Am J Transplant. 2020;20(7):1916‐1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernández‐Ruiz M, Andrés A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020;20(7):1849‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020;382(25):2475‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short‐term outcome of 20 kidney transplant patients admitted for SARS‐CoV2 pneumonia. Kidney Int. 2020;97(6):1083‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Latif F, Farr MA, Clerkin KJ, et al. Characteristics and outcomes of recipients of heart transplant with coronavirus disease 2019. JAMA Cardiol 2020;5(10):1165–1169. 10.1001/jamacardio.2020.2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: Initial report from the US epicenter. Am J Transplant. 2020;20(7):1800‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gandolfini I, Delsante M, Fiaccadori E, et al. COVID‐19 in kidney transplant recipients. Am J Transplant. 2020;20(7):1941‐1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ljungman P, Boeckh M, Hirsch HH, et al. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis. 2017;64(1):87‐91. [DOI] [PubMed] [Google Scholar]
- 14. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care‐associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309‐332. [DOI] [PubMed] [Google Scholar]
- 15. De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European organization for research and treatment of cancer/invasive fungal infections cooperative group and the national institute of allergy and infectious diseases mycoses study group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008;46(12):1813‐1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tab S1


