1. INTRODUCTION
‘Two roads diverged in a wood and I…
I took the one less travelled by,
And that has made all the difference’.
Robert Frost.
The COVID‐19 pandemic, caused by SARS‐CoV‐2, is an immense challenge for global healthcare. Diabetes mellitus, hypertension and obesity have been shown to portend poor prognosis in COVID‐19 despite no greater susceptibility to the infection. 1 Chronic hypertension is commonly associated with vasculopathy which can predispose to severe infection. In patients with diabetes, severity is attributable to impaired innate, adaptive immunity, upregulation of ACE2 (entry receptor for SARS‐CoV2) by acute hyperglycaemia and diabetic vasculopathy. The background of chronic low grade inflammation characterised by increased levels of IL‐6 and CRP in diabetes and obesity can also lead to an enhanced ‘cytokine storm’ in COVID‐19. 2 ACE2 expression on endothelial cells has been reported to cause viral‐mediated endothelitis and precipitate vascular dysfunction manifesting as acute respiratory distress syndrome as well as myocarditis, heart failure, arrhythmias, myocardial infarction and renal failure. 3 In patients with pre‐existing comorbidities like hypertension, diabetes, obesity and chronic kidney disease, this new‐onset organ dysfunction can have deleterious additive effects.
SGLT2 inhibitors (SGLT2i) are oral antidiabetic drugs that cause glycosuria, natriuresis and diuresis by inhibiting the sodium‐glucose co‐transporters in renal tubules. Substantial evidence supports their cardiovascular and renal benefits conferred by reduction in plasma glucose, blood pressure and weight, which in turn, outweigh their modest effect on glycaemia. Cumulative cardiorenal outcomes from large, randomised, placebo‐controlled trials (>30 000 participants) have shown reduction in major adverse cardiovascular events (MACE) (11%), hospitalisation for heart failure (31%) composite of cardiovascular death or hospitalisation for heart failure (23%) and renal disease (45%) with the use of SGLT2i empagliflozin, canagliflozin and dapagliflozin. 4 There is also a documented reduction in all‐cause mortality with the use of SGLT2i. 4 Importantly, these effects have been confirmed in a wide spectrum of clinical situations: prior cardiovascular disease (EMPA‐REG), cardiovascular risk factors without event (CANVAS, DECLARE‐TIMI 58) and even in persons without diabetes mellitus (DAPA‐HF).
Diabetes has profound effects on the pump (heart failure), pipes (atherosclerosis), and filter (renal disease) and pharmacological SGLT2 inhibition entails all these components. 5 Interestingly, severe COVID‐19 seems to manifest similar effects by causing myocarditis and heart failure, myocardial infarction and renal dysfunction. 6 Cardiovascular manifestations of COVID‐19 include cardiac troponin elevation, myocarditis, arrhythmias, new‐onset or worsening heart failure and myocardial infarction. These are attributed to endothelitis, cytokine storm and probably direct viral‐mediated myocardial dysfunction. This acute cardiac decompensation, irrespective of background cardiovascular disease, results in higher mortality compared to prior cardiovascular disease alone (37.5% vs 13.3%) due to increased risk of arrhythmias and heart failure. 7 Adverse renal outcomes in patients with COVID‐19 is also attributed to intrarenal inflammation and cardiomyopathy which can lead to cardiorenal syndrome type 1 mediated by endothelial injury. Further, there is a putative mechanism involving tubulopathy and tubulointerstitial damage by the virus itself. Although the incidence of acute kidney injury (AKI) is reportedly low (0.5%‐4.5%), evidence suggests a much higher rate of renal involvement in patients with ARDS. 8 Overall renal involvement (AKI, proteinuria and haematuria) has been reported in three‐fourths of patients with COVID‐19, and this is also associated with significantly higher mortality (11.2% vs 1.2%). In this context, it can certainly be hypothesised that SGLT2i could influence outcomes in COVID‐19, especially by attenuating the risk of severe infection conferred by risk factors like diabetes, hypertension and obesity.
Multiple mechanisms are implicated in causing the remarkably better cardiorenal outcomes with the use of SGLT2i. The major ones include reduction in preload and afterload by preferential loss of interstitial volume, improvement in cardiomyocyte bioenergetics by increased lipolysis leading to more ketone bodies which act as efficient energy substrates, increased haematocrit by increased erythropoietin and reduction in blood pressure and weight. 9 Emerging evidence suggests the inhibition of NHE (sodium hydrogen exchanger) as a pivotal unifying mechanism for improved cardiorenal outcomes noted with SGLT2i. NHE is an antiporter causing extracellular shift of H+ with intracellular shift of Na+. The H+ lactate symporter causes parallel shift of H+ and lactate. These transporters are involved in maintaining intracellular pH (Figure 1). In patients with COVID‐19, elevated levels of LDH are reported, possibly as markers of multiorgan dysfunction syndrome and cell destruction and are associated with ~6‐fold to 16‐fold increase in odds of severe disease and mortality. LDH is a bidirectional enzyme with lactate generation in hypoxic milieu and pyruvate production in normoxia. 10 Impaired tissue oxygenation in COVID‐19 results in anaerobic glycolysis and failure of conversion of lactate to pyruvate thereby causing accumulation of lactate. This lactate then enters the cell via the H+ lactate symporter along with H+. Increased H+ inside the cell activates the NHE, leading to Na+ accumulation intracellularly in exchange for H+, and consequent cell oedema. Simultaneously, the Na+ Ca2+ exchanger causes exchange of the accumulating Na+ for Ca2+, increasing cellular Ca2+ concentration and excitotoxicity (Figure 1). SGLT2i tilt this equation in favour of cell survival by inhibiting the NHE, preventing lactate generation (by improving tissue oxygenation as a result of improved haematocrit and reduced oxygen demand) and enhancing renal excretion of lactate. Though NHE inhibition is a class effect, reducing production and increasing excretion of lactate have been reported with dapagliflozin. 10 Insulin resistance, even without diabetes, has been reported to have adverse outcomes in COVID‐19. This may be aggravated by cytokines (IL‐6 and TNF‐α) in patients with COVID‐19, as evidenced by greatly increased insulin requirements and new‐onset hyperglycaemia reported in upto one‐fifth of cohorts. Reduced ectopic fat in the pericardium (with dapagliflozin) and favourable alteration in cytokine profile (reduction in IL‐6, TNF‐α with canagliflozin) are other putative mechanisms that could mediate benefits in COVID‐19. 11 Recent evidence also suggests significant reduction in new‐onset arrhythmias (HR 0.83, P = .002) 12 and benefit in heart failure with preserved ejection fraction (not uncommon in patients with obesity and diabetes) with the use of SGLT2i. Apart from established benefits in chronic heart failure, evidence from clinical studies using empagliflozin and rodent models using dapagliflozin suggests cardioprotection (reduced infarct size and improved LV function) during acute decompensated heart failure and ischaemia‐reperfusion injury. 13
Figure 1.
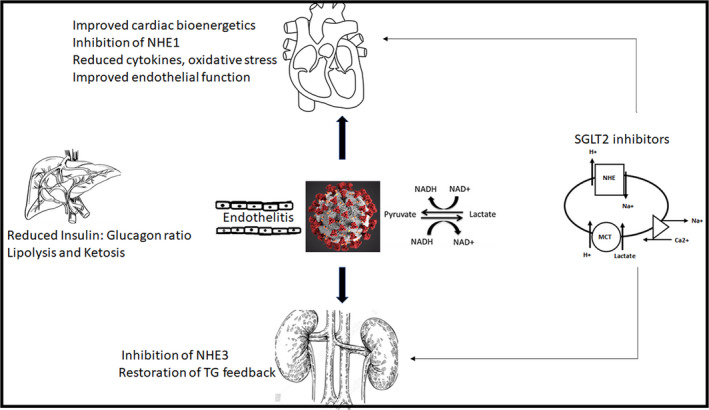
Schematic representation of the proposed pathophysiology of COVID‐19 induced cardiac and renal dysfunction induced by endothelitis and increased lactate production under conditions of impaired tissue oxygenation induced anaerobic glycolysis which leads to increased cellular entry of H + along with lactate, leading to activation of the sodium hydrogen exchanger (NHE), sodium accumulation and cell oedema and destruction. SGLT2 inhibitors, apart from having beneficial effects on multiple cardiovascular risk factors (diabetes, hypertension and obesity) which predispose to adverse outcomes in COVID‐19, may possibly have benefits in acute decompensated states by inhibiting the NHE, decreasing lactate and improving endothelial function. MCT, Monocarboxylate transporter (H + lactate symporter); NHE, sodium hydrogen exchanger; SGLT2, Sodium‐glucose co‐transporter; TG, Tubuloglomerular feedback
Renoprotection conferred by SGLT2i is again multifactorial. The major mechanism is through restoration of the tubuloglomerular feedback due to increased sodium delivery to the macula densa resulting in afferent arteriolar vasoconstriction and reduction in albuminuria. But benefits observed in normoalbuminuric type of kidney disease which involve reduction in inflammation, upregulation of anti‐inflammatory peptide Ang 1 , 2 , 3 , 4 , 5 , 6 , 7 and improvement in markers of tubular function and tubular cell hypoxia may be of use in patients with AKI due to cytokine storm, inflammation and tubulopathy.
Despite several putative benefits with the use of SGLT2i in COVID‐19, there are certain attendant risks. Firstly, cytokine storm characterised by elevations in IL‐6 and TNF‐α can cause peripheral lipolysis, leading to ketosis. This, coupled with a dehydration inherent to any febrile state, could lead to increased risk of euglycaemic DKA. Though potentially fatal, DKA with the use of SGLT2i is underwhelmingly low in patients with T2DM (0.5 per 1000 person years in CANVAS and 0.1% in DECLARE). Increased predisposition to euglycaemic DKA is seen in T1DM, prolonged duration of diabetes and surgical stress/intercurrent illness. In a study of 658 positive cases of COVID‐19, DKA was reported in only 3 cases. 14 On the other hand, in patients without diabetes, modest elevations in ketones may infact improve organ functioning owing to the fact that ketones are good metabolic substrates especially for the brain, heart and skeletal muscles. Apart from this, ketonemia can also promote resistance to oxidative stress by inhibiting histone deactylases. These benefits may be explored with the use of SGLT2i in COVID‐19. The other contentious issue with use of SGLT2i in COVID‐19 is ACE2 upregulation by these agents. This can theoretically increase risk of viral entry, but may not always hold true because of the limited availability of the serine protease TMPRSS2 which is required for viral binding. 2 Further, ACE2 levels are higher in females and young but available literature shows higher mortality in males and the elderly, suggesting the involvement of factors other than ACE2 upregulation in the pathogenesis of COVID‐19.
In conclusion, balancing risks versus benefits of SGLT2i therapy in individual patients with COVID‐19 seems to be a prudent approach (Table 1). Improvement in cardiovascular risk factors including blood pressure, ambient glycaemia, weight and cardiac function and a certain degree of anti‐inflammatory activity can address multiple risk factors that predispose to adverse outcomes in COVID‐19. This may possibly ameliorate the risk of severe COVID‐19. In acute phase of illness with dehydration especially in patients with long‐standing T2DM and T1DM of any duration, withholding SGLT2i is certainly advisable. At the same time, judicious use of these pleiotropic agents by identifying subsets of patients with multiple cardiovascular risk factors especially in those with cardiac injury, new‐onset or worsening heart failure and multiple risk factors can may possibly be helpful. In the LIFE trial, angiotensin receptor blockers were found to be superior to beta blockers in preventing cardiovascular morbidity and death. But combination of both is certainly better than either alone. Similarly, insulin is superior to SGLT2i but combination of both can prove to be better than either alone.
Table 1.
Proposed clinical guidance for the use of SGLT2i in patients with COVID‐19
| Mild COVID‐19 | Moderate COVID‐19 | |
|---|---|---|
| Previously taking SGLT2 inhibitors |

|

|
| Newly initiated SGLT2 inhibitors |

|

|
| Long duration of disease with severe COVID‐19 | Insulin alone or Insulin (multiple subcutaneous insulin or continuous intravenous infusion) with SGLT2i | |
In patients with mild to moderate COVID‐19 without significant organ injury, continuation of SGLT2i seems to be prudent to possibly reduce severity of infection by favourably regulating multiple cardiovascular risk factors. In patients with mild to moderate COVID‐19, initiating SGLT2i needs to be a case‐based approach balancing the benefits in new‐onset hyperglycaemia, heart failure, myocarditis, arrhythmias, cytokine storm induced myocarditis and acute tubular necrosis versus risks of euglycaemic diabetic ketoacidosis. In patients with long‐standing diabetes (poor β‐cell reserve) especially with severe COVID‐19, insulin remains the choice of therapy. Adjunctive usage of SGLT2i may be beneficial in certain patients with close clinical surveillance for ketoacidosis.
The role of SGLT2i in COVID‐19 is therefore, conjectural. Though proposed, 2 , 10 till date, there are only anecdotal reports with the use of empagliflozin (n = 3) which failed to demonstrate reduced duration of hospitalisation. 15 However, large scale studies are required to assess the efficacy or lack of it in patients with COVID‐19 irrespective of diabetes. DARE‐19, a multicentric collaborative effort to investigate the use of dapagliflozin in patients with COVID‐19, is currently underway and is expected to resolve this clinical dilemma.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Das L, Dutta P. SGLT2 inhibition and COVID‐19: The road not taken. Eur J Clin Invest. 2020;50:e13339. 10.1111/eci.13339
REFERENCES
- 1. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Int Med. 2020;180(7):934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ceriello A, Standl E, Catrinoiu D, et al. Issues of cardiovascular risk management in people with diabetes in the COVID‐19 era. Diabet Care. 2020;43(7):1427‐1432. [DOI] [PubMed] [Google Scholar]
- 3. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta‐analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31‐39. [DOI] [PubMed] [Google Scholar]
- 5. Verma S, Jüni P, Mazer CD. Pump, pipes, and filter: do SGLT2 inhibitors cover it all? Lancet. 2019;393(10166):3‐5. [DOI] [PubMed] [Google Scholar]
- 6. Kang Y, Chen T, Mui D, et al. Cardiovascular manifestations and treatment considerations in COVID‐19. Heart. 2020;106:1132‐1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(7):811‐818. 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ronco C, Reis T. Kidney involvement in COVID‐19 and rationale for extracorporeal therapies. Nat Rev Nephrol. 2020;9:308‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verma S, McMurray JJ. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state‐of‐the‐art review. Diabetologia. 2018;61(10):2108‐2117. [DOI] [PubMed] [Google Scholar]
- 10. Cure E, Cure MC. Can dapagliflozin have a protective effect against COVID‐19 infection? A hypothesis. Diabet Metab Synd. 2020;14(4):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garvey WT, Van Gaal L, Leiter LA, et al. Effects of canagliflozin versus glimepiride on adipokines and inflammatory biomarkers in type 2 diabetes. Metabolism. 2018;1(85):32‐37. [DOI] [PubMed] [Google Scholar]
- 12. Chen HY, Huang JY, Siao WZ, Jong GP. The association between SGLT2 inhibitors and new‐onset arrhythmias: a nationwide population‐based longitudinal cohort study. Cardiovasc Diabetol. 2020;19(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lahnwong S, Palee S, Apaijai N, et al. Acute dapagliflozin administration exerts cardioprotective effects in rats with cardiac ischemia/reperfusion injury. Cardiovasc Diabetol. 2020;19(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID‐19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metabol. 2020;22:1935‐1941. 10.1111/dom.14057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bossi AC, Forloni F, Colombelli PL. Lack of efficacy of SGLT2‐i in severe pneumonia related to novel coronavirus (nCOV) infection: no little help from our friends. Diabetes Ther. 2020;23:1. [DOI] [PMC free article] [PubMed] [Google Scholar]


