Abstract
Background
The Reveal G4 antibody rapid test is FDA-approved for HIV-1 detection using the versions LAB S/P and POC in CLIA-moderate complexity settings with serum/plasma and whole blood, respectively. The same Reveal tests are CE-marked for HIV-1 and HIV-2 detection in laboratory and point-of-care (POC) settings.
Objective
We compared the performance of G4 LAB S/P with plasma and POC with whole blood (blood) for detecting early and established HIV-1/HIV-2 infections.
Study design
Matched well-characterized plasma and simulated blood were used to evaluate: sensitivity in 104 HIV-1 and 55 HIV-2 established infections, specificity in 49 HIV-negative, and reactivity in early HIV-1 infection in a performance panel (n=38) and 18 plasma panels from seroconverters (SCs, n=183). Median number of days after first RNA-positive was calculated for 13 SCs. Impact of viral suppression (VS) was evaluated in 3 SCs receiving early antiretroviral therapy (ART).
Results
Sensitivity was 100% for HIV-1 and 98.18% for HIV-2, while specificity was 100%. All 38 plasma and blood become reactive by Fiebig stage V. Of 18 SCs, 10 had similar reactivity in plasma/blood, 7 showed delayed reactivity in blood, and 1 was nonreactive in plasma/blood. The median days for a G4-reactive after first RNA-positive was 13 for plasma and 14 for blood. Long-term VS had no impact on G4 reactivity.
Conclusions
Overall reactivity in early HIV-1 infections is delayed by one day in blood compared to plasma. If FDA-approved for POC settings, the G4 POC is a fast sensitive screening tool for HIV-1/HIV-2-specific IgG even during VS.
Keywords: HIV diagnostics, Rapid test, HIV-1, HIV-2
1. Background
Globally, an estimated 9.4 million people living with HIV (PLHIV) were unaware of their status in 2017, highlighting the need for development and distribution of new testing strategies to increase the number of people aware of their HIV diagnosis [1]. Of the 1.1 million PLHIV in the US, 38,000 were newly diagnosed and 15% were unaware of their status in 2017 [2, 3]. HIV testing performed in point-of-care (POC) settings increases access for high risk individuals, and can be performed using oral fluid or fingerstick whole blood (FSB) [4]. Most rapid tests (RT) used for screening at POC detect HIV in 15–20 minutes to inform on the HIV status then refer to further testing or care [5, 6].
The Reveal G4 Rapid HIV-1 Antibody Test (G4; MedMira Laboratories, Inc., Halifax, Nova Scotia, Canada) was approved by the Food and Drug Administration (FDA) in 2015 for detection of HIV-1 in plasma, serum, and venipuncture and FSB samples in laboratory settings [7, 8]. The G4 uses patented Rapid Vertical Flow (RVF) technology that captures IgG anti-HIV-1/2 antibodies (Ab) through an immunoreactive test membrane, displaying results within 2 minutes [9]. In the U.S. market, the G4 is offered in three versions containing the same test components (cartridge, InstantGold cap and buffer solution) with different supplied materials based on type of specimen collected: the LAB S/P (LAB) for serum and plasma, the POC version for FSB, and the LAB+ for venipuncture whole blood, serum, and plasma. The POC version has not yet received the Clinical Laboratory Improvement Amendment (CLIA)-waiver. In addition to the U.S., the Reveal RT is also approved in Canada, China, and Europe for detection of both HIV-1 and HIV-2, and is the only HIV RT approved in all four markets [10–12]. Previous versions of Reveal including the G2 and G3 have been evaluated using serum and plasma showing high sensitivity (≥99%) in HIV-1 established infections, with a slight decrease in reactivity for early infections [13–16]. Evaluations of INSTI HIV-1/2 Ab and Determine HIV-1/2 Ag/Ab Combo assays have shown the performance in plasma and simulated whole blood in early infections [17, 18]. However, the performance of G4 in matched plasma and whole blood samples and its ability to detect acute infections and HIV-2 antibodies has not been reported.
2. Objectives
We evaluated the performance of the Reveal G4 LAB test using plasma and POC test using simulated whole blood from HIV-negative persons, persons in different stages of HIV-1 infection and persons with HIV-2 infection. We also compared the G4 results to other FDA-approved HIV tests including previous versions of Reveal (G2/G3) in early HIV-1 infections.
3. Study design
3.1. Plasma Sample Sets
The established HIV-1 plasma set consisted of 104 HIV-1-positive samples: 50 US, 48 Cameroonian [15] (non-B subtype) anonymous blood donors, and six from an AccuSet performance panel (SeraCare Diagnostics, Milford, MA). The established HIV-2 plasma set included 55 HIV-2 antibody-positive samples, with 15 from the U.S and 34 from Ivory Coast, (Boca Biolistic, Inc., Coconut Creek, FL) [19], and six from AccuSet performance panel. The HIV-negative plasma set included 49 HIV-uninfected samples from US anonymous donors purchased from the Research Sample Bank, Inc. and confirmed negative for HIV-1/2 using FDA-approved serological and molecular methods.
An early HIV-1 infection performance panel was created combining samples from the modified anti-HIV-1 low titer panel (n=15) and HIV-1 early infection panel (n=23) (SeraCare). Well-characterized HIV-1 plasma panels from seroconverters (SCs) (n=183) were purchased from Zeptometrix, Inc (Donor IDs 65522, 65661, 68106, 62216, 63753, 75018, 75062; Buffalo, NY) and BBI-SeraCare (Donor IDs PRB916, PRB917, PRB924, PRB925, PRB926, PRB931, PRB940, PRB944, LSS102, LSS103, LSS104; SeraCare) [14, 18–20]. Of the 18 SCs, 15 (n=144) were antiretroviral therapy (ART)-naïve and three (LSS102, LSS103, LSS104; n=39) were from individuals treated with ART within 120 days from estimated day of infection (early ART initiation). Most panels were collected in the US pre-ART introduction and a subset were genotyped as subtype B [21], so the remaining SCs are presumably subtype B which is the most prevalent subtype in the US.
Specimens used in this study were from commercial source or discarded anonymous blood donations unlinked from personal identifiers and determined by the CDC to be research not involving human subjects.
3.2. Simulated whole blood specimens
All 429 plasma specimens tested with G4 LAB were used to prepare simulated whole blood (blood) by mixing 60 μL plasma with 40 μL washed red blood cells (HIV-negative, blood group O-) to achieve a hematocrit of 40%. The blood was tested on G4 POC immediately after preparation.
3.3. HIV Testing
The G4 LAB and POC tests were performed as indicated in the package insert with the exception of using the plastic pipettes provided and instead utilizing 30 μL of plasma or blood for testing [9]. Plasma and blood specimens were tested blind on the same day by two different lab members.
In addition, at CDC, plasma specimens from the early infection performance panel and ART-naïve SCs (n=144) were tested with Determine HIV-1/2 Ag/Ab Combo (Determine; Alere Medical Co., Ltd. Scarborough, ME) and Geenius HIV-1/2 Supplemental Assay (Geenius; Bio-Rad Laboratories, Redmond, WA). Plasma specimens from SCs on ART (n=39) were tested with Aptima HIV-1 Quantitative assay (APT-Quant; Hologic Inc., San Diego, CA).
3.4. Analysis
The sensitivity of each test version was calculated in 104 HIV-1 and 55 HIV-2 antibody positive samples (established infections) and the specificity in 49 HIV-negative samples. The early infection performance panel samples (n=38) were characterized by Fiebig stages of infection (I through V) based on the combination of biomarkers present after exposure to HIV (RNA detection, presence of p24 antigen, and anti-HIV antibody response), and compared to results from other FDA-approved tests[22]. Results from 15 ART-naïve SCs (n=144) were compared to results from other FDA-approved tests to evaluate performance. The HIV tests with previously published or commercially available results included: COBAS AmpliPrep/COBAS TaqMan HIV-1 Test, v2.0 (CAP/CTM), Amplicor Monitor HIV-1 RNA (Amplicor) (Roche Diagnostics, Indianapolis, IN), Aptima HIV-1 Qualitative Assay (APT-Qual; Hologic, Inc.), ARCHITECT HIV Ag/Ab Combo assay (Architect; Abbott Diagnostics, Chicago, IL), GS HIV-1/HIV-2 PLUS O EIA (GS+O; Bio-Rad Laboratories), Cambridge Biotech HIV-1 Western blot Kit (WB; Maxim Biomedical Inc., Rockville, MD) or Bio-Rad Genetic Systems HIV-1 Western blot Kit (WB; Bio-Rad Laboratories) HIV-2 Western blot (WB; MP Diagnostics, The Cavendish, Singapore), and Reveal G2/G3 HIV-1 Antibody RT (G2/G3; MedMira Laboratories) [14, 15, 17, 19].
Of 18 SCs, 13 ART-naïve SCs (n=129, range of 5–28 samples in each panel followed up for a median of 42 days) were available to calculate the days after first available RNA-positive (RNA+) test using APT-Qual. The impact of VS on G4 reactivity was evaluated using APT-Quant in three SCs with early ART initiation (n=39) with a mean of 158 days follow up (range:320–389 days). Data was analyzed with Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA) and Medcalc v18.2.1(MedCalc, Ostend, Belgium).
4. Results
4.1. Sensitivity and Specificity
G4 sensitivity in HIV-1/HIV-2 positive samples was 99.37% (158/159) in both LAB-plasma and POC-blood [95%CI: 96.55%−99.98%]: HIV-1 was 100% (104/104) [95%CI: 96.52%−100%] and HIV-2 was 98.18% (54/56) [95%CI: 90.28%−99.95%]. One HIV-2 sample (HIV-2 WB-positive, Geenius antibody-negative) was nonreactive in both plasma and blood.
For the 49 HIV-negative samples tested, one sample was invalid/invalid with blood and only 48 used for the analysis. G4 specificity was 100% (49/49) [95%CI: 92.75%- 100%] in LAB-plasma and 100% (48/48) [95%CI: 92.60%- 100%] in POC-blood. Overall reactivity is displayed in Table 1.
Table 1:
Overall Performance and Reactivity of sample sets in G4 LAB and POC Tests
| G4 LAB plasma |
G4 POC blood |
|||||
|---|---|---|---|---|---|---|
| R | NR | Reactivity | R | NR | Reactivity | |
| Established HIV infections | ||||||
| HIV-1 + | 104 | 0 | 100% | 104 | 0 | 100% |
| HIV-2 + | 54 | 1 | 98.2% | 54 | 1 | 98.2% |
| HIV-1 seroconversion panels | ||||||
| Before 1st RNA+ | 0 | 47 | 0% | 0 | 47 | 0% |
| After 1st RNA+ | 95 | 41 | 69.9% | 86 | 50 | 63.2% |
| HIV Negative Samples | 0 | 49 | 0% | 0 | 49 | 0% |
4.2. Performance in Early Infections
The results of the early HIV-1 performance panel for different HIV assays are displayed in Table 2. Of 13 samples in Fiebig stage IV (HIV-1 WB-indeterminate), seven were reactive in plasma and two in blood showing plasma detects IgG response earlier than blood. All six samples in Fiebig stage V (HIV-1 WB-positive minus-p31) were reactive in both plasma and blood.
Table 2:
Results from HIV assays b in early infection samples characterized by Fiebig stage
| n with HIV test result: c | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fiebig Stage: | RNAa | Architect | Determine | GS+O | Geenius | HIV-1 WB | G4 LAB | G4 POC | |||||
| n | Pos | Ag/Ab-R | Ag-R | Ag/Ab-R | Ab-R | IgG/IgM R | Neg | Ind | Pos | Interpretation | IgG R | IgG R | |
| Eclipse | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | Neg | 0 | 0 |
| I | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | Neg | 0 | 0 |
| II | 4 | 4 | 4 | 3 | 0 | 0 | 0 | 4 | 0 | 0 | Neg | 0 | 0 |
| III | 6 | 6 | 6 | 4 | 2 | 3 | 6 | 4 | 2 | 0 | Neg | 1 | 0 |
| IV | 13 | 13 | 13 | 7 | 6 | 11 | 12 | 4 | 5 | 4 | HIV-1 Ind | 7 | 2 |
| V | 6 | 6 | 6 | 0 | 0 | 6 | 6 | 0 | 0 | 6 | HIV-1 Posd | 6 | 6 |
Plasma testing for HIV-1 RNA included results from either Roche CAP/CTM or Roche Amplicor Monitor.
Plasma testing: Architect HIV Ag/Ab Combo Assay, Determine HIV-1/2 Ag/Ab Combo, Geenius HIV-1/2 Supplemental Assay (IgG), HIV-1 WB: Cambridge Biotech HIV-1 Western blot Kit or Bio-Rad Genetic Systems HIV-1 (IgG), GS+O: GS HIV-1/HIV-2 PLUS O EIA (IgG/IgM), G4 LAB: MedMira Reveal G4 LAB S/P (IgG Rapid Test). Blood testing: G4 POC: MedMira Reveal G4 POC (IgG Rapid Test).
Neg: negative; Ind: indeterminate; Pos: positive; R: reactive; Ag: antigen; Ab: antibody. Geenius results: Neg: Ab-Neg; Ind: HIV-1 indeterminate; Pos: HIV-1 positive.
HIV-1 positive (p31-).
4.3. Performance in HIV-1 seroconversion panels
Of the 183 longitudinal samples from 18 SCs, 47 were collected before HIV-1 RNA was detected and 136 after first RNA+. All 47 samples collected before first RNA+ tested nonreactive in plasma and blood. Of the remaining 136 samples following the first RNA+ test, 95 were reactive in plasma (69.9%) and 86 in blood (63%) (Table 1). Of the 18 SCs, ten (55.6%, n=85) showed no difference in reactivity with plasma and blood, including three SCs that initiated early ART. One SC (5.5%, n=8) never became reactive in plasma and blood up to 14 days after follow-up and another one (5.5%, n=8) never became reactive in blood but reactive in plasma at 14 days. Seven SCs (38.9%, n=90) showed HIV-1 antibodies detected earlier in plasma compared to blood, with the observed reactivity delayed by 10–71 days in blood compared to plasma. Nine samples from seven SCs were discordant in plasma and blood, and two blood samples from SC#14 and SC#15 became nonreactive after first POC-reactive test (Table 3). Additionally, one sample became nonreactive after first reactive test in plasma (SC#15; 6 days after first LAB-reactive, 55 days after first sample collected) and one became nonreactive after first reactive test in both plasma and blood (SC#12; 15 days after first LAB/POC-reactive result, 47 days after first sample collected).
Table 3:
Results of FDA-approved diagnostic assays a in nine discordant samples from seroconverters (SC) with G4 LAB-plasma Reactive and POC-blood Nonreactive results
| Days after first sample collected: | RNA: | Ag/Ab: | IgG/IgM: | IgG Suppl: | |||
|---|---|---|---|---|---|---|---|
| SC#: | APT-Qual | Architect | Determine | GS+O | Geenius | ||
| Ag | Ab | ||||||
| 9 | 10 | Pos | R | NR | R | R | HIV-1 Pos |
| 7 | 11 | Pos | R | R | NR | R | HIV Neg |
| 11 | 29 | Pos | R | NR | R | NR | HIV Neg |
| 10 | 35 | Pos | R | R | NR | R | HIV Neg |
| 4 | 44 | Pos | R | R | R | R | HIV-1 Ind |
| 14 | 57 | Pos | R | NR | R | R | HIV-1 Pos |
| 14 | 71b | Pos | R | NR | R | R | HIV-1 Pos |
| 15 | 49 | Pos | R | R | NR | R | HIV Neg |
| 15 | 64b | Pos | R | NR | R | R | HIV-1 Pos |
Plasma testing: HIV-1 RNA assay: Aptima HIV-1 Qualitative Assay (APT-Qual), Ag/Ab assays: Architect HIV Ag/Ab Combo Assay, Determine HIV-1/2 Ag/Ab Combo, IgG/IgM assay: GS HIV-1/HIV-2 PLUS O EIA (GS+O), IgG supplemental assay (IgG Suppl.): Geenius HIV-1/2 Assay.
Samples that became nonreactive in blood after a first POC-reactive test result, potentially due to a second negative diagnostic phase during seroconversion. Sample from SC#14 became nonreactive 12 days after first POC-reactive result, and SC#15 became nonreactive 2 days after first POC-reactive result.
Of 15 ART-naïve SCs (n=144) with G2/G3 data, discordant results between G4-LAB were seen in eight plasma samples, with six G4-reactive and G2/G3-nonreactive and two G4-nonreactive and G2/G3-reactive.
Thirteen ART-naive SCs (n=129) were available for calculating the overall median number of days for a G4-reactive result following the first RNA+ result, which was 13 days for plasma and 14 days for blood; two SCs never became reactive with blood and were therefore excluded. For the six SCs with discordant results, the median was 12.5 days for plasma and 16.5 days for blood. The median number of days for a G2/G3-reactive test with plasma was 16 days after the first RNA+ test.
4.4. Effect of Viral Suppression on Assay Performance
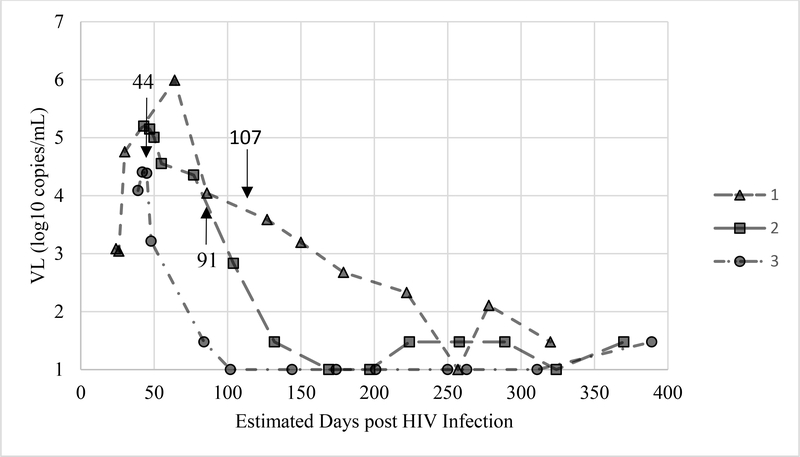
Three SCs (n=39) were reactive in plasma and blood before early ART initiation (44, 91, and 107 estimated days post-HIV infection) and remained reactive after ART initiation, and VS was achieved for a median of 238 days. VS was confirmed using APT-Quant with a limit of quantification of 1.48 log10 copies/mL (30 cop/mL) and shown compared to amount of days post-HIV infection in Figure 1.
Figure 1:
Viral load of three seroconverters before and after initiation of ART
Viral load (VL) in HIV-1 RNA log10 copies/ml of three plasma panels from seroconverters (LSS102, LSS103, LSS104) from individuals with early ART initiation. Panel 1 consisted of 12 samples and began treatment around day 107, panel 2 with 14 samples that began treatment around day 91 and panel 3 with 13 samples that began treatment around day 44. Arrows indicate estimated day of ART treatment initiation in patients. All samples remained G4-Reactive in both LAB-plasma and POC-blood tests.
Discussion
This study provides a direct comparison of the MedMira Reveal G4 antibody RT using matched plasma and blood specimens from acute HIV-1 infections and HIV-2 positive samples. In this evaluation, there was no difference between G4 LAB-plasma and POC-blood performance in established HIV infections. The sensitivity and specificity of both HIV-1 and HIV-2 was very good and comparable to values reported by the manufacturers. The G4 package insert reports HIV-1 sensitivity above 99% for all specimen types and specificity 98.6% in plasma and above 99% for blood, but does not directly compare test versions [9]. HIV-2 sensitivity for Reveal HIV in Canadian and CE package inserts using plasma/serum was 99.6% and 100%, respectively, [10, 11] which is equivalent to what we observed in our sample set with overlapping confidence intervals.
Overall reactivity in early HIV-1 infections is delayed by one day in blood compared to plasma. Nevertheless, the test performs accurately based on the stage of infection and presence of IgG antibodies and is not influenced by VS. The G4 detects IgG antibodies in 100% of samples by Fiebig stage V, which is when IgG antibody response is strong around 1–2 months after infection [22]. Also, in a limited number of samples G4-reactivity appears to be more sensitive than previous G2/G3 versions. One SC that never became G4-reactive remained Geenius HIV-negative and only became Determine Ag-reactive, showing that antibodies were not produced at a detectable level. Another SC was G4-reactive in LAB-plasma 14 days after the first RNA+ but nonreactive in POC-blood and Geenius HIV-indeterminate. Although G4-reactivity is not as sensitive as other HIV assays such as Ag/Ab and IgG/IgM in detecting early infections, IgG RTs are important screening tools and G4 can provide HIV status in less than 2 minutes while most RTs are interpreted between 15–20 minutes. [6, 23, 24]. Short waiting times for results at POC-settings would be preferable. In the US there are no RTs with a portable reader, thus, visual interpretation cannot be avoided and may lead to misinterpretation of test results [25]. On the other hand, RTs with instant results are more appealing to POC settings without the requirement of timers. Development of smartphone-based apps for the interpretation of vertical flow RT results similar to ones that have already been designed could prevent errors in interpreting results [12, 26]. Due to the nature of the test and antibodies targeted, selection must be done after considering the circumstances of populations and their level of risk and exposure to HIV [25]. Some studies have shown decreased sensitivity in RTs in individuals with VS due to long-term ART use [13]. However, this study shows that even in samples with long-term VS the G4 was able to remain reactive up to one year after ART initiation and VS. A limitation of this study is the lack of performance evaluation in persons taking pre-exposure prophylaxis (PrEP) and false-positive results have been reported in this population [27, 28].
Other limitations are the use of simulated blood due to the lack of access to PLHIV to obtain fresh FSB, requiring the use of frozen plasma specimens that were stored long-term at −80°C; this could explain some of the unexpected results, such as the HIV-2 sample that was nonreactive for both plasma and blood or the SCP samples that became nonreactive after first reactive sample. However, the latter also could be due to a second negative diagnostic phase during seroconversion when immunocomplexes are formed and analytical sensitivity is challenged early in the infection. Another limitation involved the provided pipettes that were not optimal for simulated blood in the laboratory. Using the plastic pipette provided in the POC version, we estimated a volume of 30 μL in each drop, although the pipette provided in the LAB version may hold up to 50 μL. Therefore, extrapolation to real word performance may not be accurate due to our use of 30 μL of samples during this evaluation.
The UNAIDS 90–90-90 targets and the US Ending the HIV Epidemic initiatives focus on using diagnostic tools to increase awareness and the global demand for sensitive, user-friendly and affordable HIV tests [2, 29]. CLIA-waived RTs using unprocessed specimens such as FSB and produce results within a few minutes are advantageous to busy health-care environments [5]. Also, the RVF technology in the Reveal supports multiplex RTs to detect HIV, HBV and HCV on one single membrane [12]. Receiving the CLIA-waiver and a claim for HIV-2 by the FDA for the Reveal G4 could provide the US market with increased fast options for POC testing to reach more high-risk individuals.
Acknowledgements
Thank you to Neeraj Vats and Hermes Chan for insightful discussion during the study design.
Conflicts of Interest and sources of funding: The authors declare no conflicts of interest. This work was funded by the Centers for Disease Control and Prevention.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the U.S. Centers for Disease Control and Prevention (CDC). Use of trade names is for identification purposes only and does not constitute endorsement by the CDC or the US Department of Health and Human Services.
References
- [1].UNAIDS . Global HIV & AIDS statistics-2018 fact sheet. UNAIDS; Geneva, Switzerland; 2018. [Google Scholar]
- [2].Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV Epidemic: A Plan for the United StatesEnding the HIV EpidemicEditorial. JAMA. 2019;321:844–5. [DOI] [PubMed] [Google Scholar]
- [3].Harris NS JA, Huang YA, Kern D, Fulton P, Smith DK, Valleroy LA, Hall HI. Vital Signs: Status of Human Immunodeficiency Virus Testing, Viral Suppression, and HIV Preexposure Prophylaxis — United States, 2013–2018.. MMWR Morb Mortal Wkly Rep. 2019;68:1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Drain PK, Rousseau C. Point-of-care diagnostics: extending the laboratory network to reach the last mile. Curr Opin HIV AIDS. 2017;12:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Minichiello A, Swab M, Chongo M, Marshall Z, Gahagan J, Maybank A, et al. HIV Point-of-Care Testing in Canadian Settings: A Scoping Review. Frontiers in Public Health. 2017;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Arbelaez C, Block B, Losina E, Wright EA, Reichmann WM, Mikulinsky R, et al. Rapid HIV testing program implementation: lessons from the emergency department. Int J Emerg Med. 2009;2:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].US Food and Drug Administration. Premarket Approval: Reveal G4 Rapid HIV-1 Antibody Test (Reveal G4). 2015.
- [8].Centers for Disease Control and Prevention. Advantages and Disadvantages of Different Types of HIV tests. https://www.cdc.gov/hiv/pdf/testing/hiv-tests-advantages-disadvantages_1.pdf2018.
- [9].MedMira Laboratories Inc. Reveal G4 Rapid HIV-1 Antibody Test [package insert]. 2015.
- [10].MedMira Laboratories Inc. Reveal Rapid HIV Antibody Test Package Insert [Canada]. 2015.
- [11].MedMira Laboratories Inc. Reveal Rapid HIV Test Package Insert [CE-Marked]. 2015.
- [12].Jiang N, Ahmed R, Damayantharan M, Ünal B, Butt H, Yetisen AK. Lateral and Vertical Flow Assays for Point-of-Care Diagnostics. 2019;0:1900244. [DOI] [PubMed] [Google Scholar]
- [13].Delaney KP, Branson BM, Uniyal A, Phillips S, Candal D, Owen SM, et al. Evaluation of the Performance Characteristics of 6 Rapid HIV Antibody Tests. Clinical Infectious Diseases. 2011;52:257–63. [DOI] [PubMed] [Google Scholar]
- [14].Masciotra S, McDougal JS, Feldman J, Sprinkle P, Wesolowski L, Owen SM. Evaluation of an alternative HIV diagnostic algorithm using specimens from seroconversion panels and persons with established HIV infections. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2011;52 Suppl 1:S17–22. [DOI] [PubMed] [Google Scholar]
- [15].Owen SM, Yang C, Spira T, Ou CY, Pau CP, Parekh BS, et al. Alternative algorithms for human immunodeficiency virus infection diagnosis using tests that are licensed in the United States. J Clin Microbiol. 2008;46:1588–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Delaney KP, Hanson DL, Masciotra S, Ethridge SF, Wesolowski L, Owen SM. Time Until Emergence of HIV Test Reactivity Following Infection With HIV-1: Implications for Interpreting Test Results and Retesting After Exposure. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017;64:53–9. [DOI] [PubMed] [Google Scholar]
- [17].Adams S, Luo W, Wesolowski L, Cohen SE, Peters PJ, Owen SM, et al. Performance evaluation of the point-of-care INSTI™ HIV-1/2 antibody test in early and established HIV infections. Journal of Clinical Virology. 2017;91:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Masciotra S, Luo W, Westheimer E, Cohen SE, Gay CL, Hall L, et al. Performance evaluation of the FDA-approved Determine™ HIV-1/2 Ag/Ab Combo assay using plasma and whole blood specimens. Journal of Clinical Virology. 2017;91:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Masciotra S, Luo W, Youngpairoj AS, Kennedy MS, Wells S, Ambrose K, et al. Performance of the Alere Determine HIV-1/2 Ag/Ab Combo Rapid Test with specimens from HIV-1 seroconverters from the US and HIV-2 infected individuals from Ivory Coast. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2013;58 Suppl 1:e54–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nasrullah M, Wesolowski LG, Meyer WA, 3rd, Owen SM, Masciotra S, Vorwald C, et al. Performance of a fourth-generation HIV screening assay and an alternative HIV diagnostic testing algorithm. Aids. 2013;27:731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lipscomb JT, Switzer WM, Li JF, Masciotra S, Owen SM, Johnson JA. HIV reverse-transcriptase drug resistance mutations during early infection reveal greater transmission diversity than in envelope sequences. The Journal of infectious diseases. 2014;210:1827–37. [DOI] [PubMed] [Google Scholar]
- [22].Fiebig E, J, Wright D, Rawal B, Garrett P, Schumacher R, Peddada L, et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection 2003. [DOI] [PubMed] [Google Scholar]
- [23].Greenwald JL, Burstein GR, Pincus J, Branson B. A rapid review of rapid HIV antibody tests. Current Infectious Disease Reports. 2006;8:125–31. [DOI] [PubMed] [Google Scholar]
- [24].Nkeze JN, Constantine NT, Zhao RY. Laboratory Testing for HIV Infection: Advances After 28 Years In: Highsmith JWE, editor. Molecular Diagnostics: 12 Tests That Changed Everything. New York, NY: Springer New York; 2014. p. 81–106. [Google Scholar]
- [25].Branson BM. Point-of-Care Rapid Tests for HIV Antibodies. Laboratoriums Medizin. 2003;27:288–95. [Google Scholar]
- [26].Mak WC, Beni V, Turner APF. Lateral-flow technology: From visual to instrumental. TrAC Trends in Analytical Chemistry. 2016;79:297–305. [Google Scholar]
- [27].Stekler JD, Violette LR, Niemann L, McMahan VM, Katz DA, Baeten JM, et al. Repeated False-Positive HIV Test Results in a Patient Taking HIV Pre-Exposure Prophylaxis. Open Forum Infectious Diseases. 2018;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Ndase P, Celum C, Kidoguchi L, Ronald A, Fife KH, Bukusi E, et al. Frequency of False Positive Rapid HIV Serologic Tests in African Men and Women Receiving PrEP for HIV Prevention: Implications for Programmatic Roll-Out of Biomedical Interventions. PLOS ONE. 2015;10:e0123005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].UNAIDS. Ending AIDS: progress towards the 90–90–90 targets. UNAIDS; Geneva, Switzerland; 2017. [Google Scholar]