To the Editor:
There is a growing body of evidence that suggests that children have largely been spared of much of the morbidity associated with the ongoing SARS‐CoV‐2 pandemic 1 , 2 Over the last several weeks, however, there has been an increasing awareness and understanding of a hyperinflammatory shock syndrome that appears to mimic some aspects of Kawasaki disease in some pediatric patients. 3 , 4 , 5 It has been termed multisystem inflammatory syndrome in children (MIS‐C), and clinically this syndrome presents as a constellation that may include high‐spiking fevers, abdominal pain, rash, conjunctivitis, peripheral edema, hypotension, and cardiac dysfunction. 3 , 4 , 5 The pathophysiology that underlays this syndrome has yet to be elucidated; however, the overlap in clinical presentation with Kawasaki disease may suggest a commonality. Work to further clarify this relationship is ongoing. What is well described is an association between this syndrome and recent infection with SARS‐CoV‐2. 3 , 4 , 5 While polymerase chain reaction (PCR)‐based testing for SARS‐CoV‐2 from the nasopharynx is variably positive, serologies are frequently suggestive of recent infection. 4 Affected children show marked elevation in inflammatory markers, including C‐reactive protein (CRP), ferritin, and D‐dimer. 3 Boston, Massachusetts has been an epicenter of the coronavirus disease 2019 (COVID‐19) pandemic here in the United States, and there are a growing number of children with this syndrome being cared for at our institution over the last month.
Given the clinical overlap of MIS‐C with Kawasaki disease, there has been an effort to identify a common pathway that may be involved in the two entities. While the role that neutrophils play in the development of Kawasaki disease continues to be clarified, it is described in the literature that higher neutrophil to lymphocyte ratios (NLRs) may portend an increased risk of resistance to treatment with intravenous immunoglobulin as well as development of coronary aneurysms 6 , 7 Indeed, there is also evidence that suggests that higher NLRs in patients with COVID‐19 are associated with increased levels of inflammation and clinical severity. 8 Neutrophils in Kawasaki disease have been observed to exhibit morphologic changes, including vacuolization and toxic granulation, a phenomenon observed in inflammatory states and sepsis. 9 , 10 , 11
Here, we report our observations from peripheral blood smears for three children for whom the pediatric hematology service at our institution was consulted for assistance in management of COVID‐19‐related coagulopathy. During the course of their care, we reviewed the peripheral smears of these children and noted the changes described below. These children all met diagnostic criteria for MIS‐C as outlined by the Centers for Disease Control and Prevention. 12 All were less than 21 years of age and presented with fever and evidence of a severe inflammatory process, including elevation in CRP, ferritin, and D‐dimer (Table 1). All required admission for severe illness with multisystem organ involvement and all were found to have evidence of current or recent infection with SARS‐CoV‐2.
TABLE 1.
Clinical and laboratory parameters for patients A‐C with cell counts prior to receipt of systemic corticosteroids
| Patient | A | B | C |
|---|---|---|---|
| Age (years) | 9 | 8 | 6 |
| Sex | F | M | M |
| Ethnicity | African American | African American | Not reported |
| Clinical features | Fever, hypotension, coronary artery dilation, LV dysfunction, hypoxemia, rash, conjunctivitis | Fever, hypotension, abdominal pain, emesis, rash | Fever, hypotension, LV dysfunction, hypoxemia, abdominal pain, AKI, conjunctivitis |
| Hematologic parameters | |||
| WBC (1 × 103/μL) | 12.9 | 7.42 | 7.65 |
| Neutrophil/band (%) | 87.7 | 87 | 93 |
| Immature granulocytes (%) | 0.6 | 1.7 | 2.6 |
| Lymphocyte (%) | 7.4 | 8 | 2 |
| ANC (1 × 103/μL) | 11.3 | 6.46 | 7.11 |
| ALC (1 × 103/μL) | 0.95 | 0.58 | 0.15 |
| ANC/ALC ratio | 11.9 | 11.1 | 47.4 |
| Left shift | Present | Present | Present |
| Inflammatory markers (peak) | |||
| CRP a (mg/dL) | 17.4 | 35.2 | 25.8 |
| Ferritin (ng/mL) | 1500 | 1471 | 1203 |
| D‐dimer b (μg/mL) | 10.9 | 5.9 | 8.2 |
| SARS‐CoV‐2 RT‐PCR | Negative | Negative | Positive |
| SARS‐CoV‐2 serologies | Positive | Positive | Positive |
Note. Peak inflammatory markers are shown here along with results of SARS‐CoV‐2 RT‐PCR and serologic testing.
Abbreviations: AKI, acute kidney injury; ALC, absolute lymphocyte count; ANC, absolute neutrophil count; ANC/ALC ratio, absolute neutrophil count to absolute lymphocyte count ratio; CRP, C‐reactive protein; LV, left ventricular; RT‐PCR, reverse transcription polymerase chain reaction; WBC, white blood cell count.
Normal range < 0.5 mg/dL.
Normal range < 0.5 μg/mL.
Baseline characteristics, clinical features of illness, and hematologic parameters are depicted in Table 1. Cell counts shown in Table 1 represent peak counts prior to receipt of corticosteroids. NLRs were calculated using the ratio of absolute neutrophil count (ANC) to absolute lymphocyte count (ALC). Peak inflammatory markers represent maximal values resulted during the course of admission. The images shown in Figure 1 are from peripheral smears obtained prior to receipt of systemic corticosteroids.
FIGURE 1.
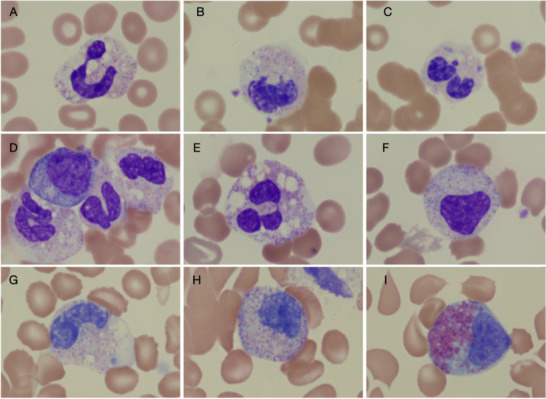
Images of myeloid forms seen in children with multisystem inflammatory syndrome (MIS‐C). A‐C, Images from patient A. A, Neutrophil with toxic granulation and heavy vacuolization. B, An early myeloid form with vacuolization. C, A neutrophil with presence of Dohle bodies. D‐F, Images from patient B. D, An early myelocyte, metamyelocyte, and neutrophils with vacuolization and toxic granulation. E, A neutrophil with significant vacuolization and granulation. F, An early myeloid form, likely metamyelocyte. G‐I, Images are from patient C. G, A late metamyelocyte with vacuolization. H, An early metamyelocyte. I, An eosinophilic myelocyte. Together these images suggest significant inflammation with vacuolated myeloid forms and the presence of toxic granulation. The early myeloid forms (bands, metamyelocytes, and myelocytes) are seen in high number in the peripheral smears of children with MIS‐C
Through review of hematologic parameters for these three patients with MIS‐C, we make two observations. The first is that these children exhibited high NLRs, a phenomenon previously described in adult patients with severe courses of COVID‐19 but also in children with Kawasaki disease. 6 , 7 This is notable, given considerable overlap between SARS‐CoV‐2‐associated MIS‐C and atypical Kawasaki disease. We additionally observed the consistent finding of a left‐shifted blood smear with prominent bandemia and presence of other early myeloid forms, including metamyelocytes and myelocytes. Prominent vacuolization and toxic granulation, along with the presence of Dohle bodies, was observed in the myeloid lineage. These findings have been described in severe inflammatory processes, sepsis, and, notably, Kawasaki disease. 9 , 10 , 11
Morphologic changes in peripheral blood smears have been reported recently in patients with COVID‐19, 13 , 14 however this has, to our knowledge, not been described in the pediatric population. These changes are interesting in relationship to the marked differences in phenotype of COVID‐19 infections in adults versus children and the evolving entity of SARS‐CoV‐2‐associated MIS‐C.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ACKNOWLEDGMENT
We thank Mursal Hassan for assistance in preparing the manuscript.
REFERENCES
- 1. Dong Y, Mo X, Hu Y, et al. Epidemiology of COVID‐19 among children in China. Pediatrics. 2020;145(6):e20200702. [DOI] [PubMed] [Google Scholar]
- 2. Shekerdemian LS, Mahmood NR, Wolfe KK, et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID‐19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020. 10.1001/jamapediatrics.2020.1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Riphagen S, Gomez X, Gonzalez‐Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID‐19 pandemic. Lancet. 2020;395(10237):1607‐1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet. 2020;395(10239):1771‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belhadjer Z, Méot M, Bajolle F, et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS‐C) in the context of global SARS‐CoV‐2 pandemic [published online ahead of print May 17, 2020]. Circulation. 2020. 10.1161/circulationaha.120.048360 [DOI] [PubMed] [Google Scholar]
- 6. Cho HJ, Bak SY, Kim SY, et al. High neutrophil: lymphocyte ratio is associated with refractory Kawasaki disease. Pediatr Int. 2017;59(6):669‐674. [DOI] [PubMed] [Google Scholar]
- 7. Ha KS, Lee J, Jang GY, et al. Value of neutrophil‐lymphocyte ratio in predicting outcomes in Kawasaki disease. Am J Cardiol. 2015;116(2):301‐306. [DOI] [PubMed] [Google Scholar]
- 8. Lagunas‐Rangel FA. Neutrophil‐to‐lymphocyte ratio and lymphocyte‐to‐C‐reactive protein ratio in patients with severe coronavirus disease 2019 (COVID‐19): a meta‐analysis [published online ahead of print April 3, 2020]. J Med Virol. 2020. 10.1002/jmv.25819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruggiero A, Delogu AB, Riccardi R, Mastrangelo S, Segni G. Early reactive neutrophil changes in an infant with Kawasaki syndrome. J Pediatr Hematol Oncol. 1999;21(4):303‐305. [DOI] [PubMed] [Google Scholar]
- 10. Rowe PC, Quinlan A, Luke BK. Value of degenerative change in neutrophils as a diagnostic test for Kawasaki syndrome. J Pediatr. 1991;119(3):370‐374. [DOI] [PubMed] [Google Scholar]
- 11. Liu CH, Lehan C, Speer ME, Fernbach DJ, Rudolph AJ. Degenerative changes in neutrophils: an indicator of bacterial infection. Pediatrics. 1984;74(5):823‐827. [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention . Multisystem Inflammatory Syndrome in Children (MIS‐C) Associated with Coronavirus Disease 2019 (COVID‐19) . Published May 14, 2020. https://emergency.cdc.gov/han/2020/han00432.asp
- 13. Zini G, Bellesi S, Ramundo F, d'Onofrio G. Morphological anomalies of circulating blood cells in COVID‐19. Am J Hematol. 2020;95(7):870‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun S, Cai X, Wang H, et al. Abnormalities of peripheral blood system in patients with COVID‐19 in Wenzhou, China. Clin Chim Acta. 2020;507:174‐180. [DOI] [PMC free article] [PubMed] [Google Scholar]


