Abstract
Background
COVID‐19 infection varies in severity from minimal symptoms to critical illness associated with a hyperinflammatory response. Data on disease progression in immunosuppressed solid organ transplant (SOT) recipients are limited.
Methods
We examined the electronic medical records of all SOT recipients with COVID‐19 from 12 Massachusetts hospitals between February 1, and May 6, 2020. We analyzed the demographics, clinical parameters, course, and outcomes of illness in these patients.
Results
Of 52 COVID‐19‐positive SOT patients, 77% were hospitalized and 35% required ICU admission. Sixty‐nine percent of hospitalized patients had immunosuppression reduced, 6% developed suspected rejection. Co‐infections occurred in 45% in ICU vs 5% in non‐ICU patients (P = .037). A biphasic pattern of evolution of laboratory tests was observed. In the first 5 days of illness, inflammatory markers were moderately increased. Subsequently, WBC, CRP, ferritin, and D Dimer increased with increasing stay in the ICU, and lymphocyte counts were similar. Five patients (16%) died.
Conclusions
Our data indicate that SOT is associated with high rate of hospitalization, ICU admission, and death from COVID‐19 compared to data in the general population of patients with COVID‐19. Despite reduction in immunosuppression, suspected rejection was rare. The clinical course and trend of laboratory biomarkers is biphasic with a later, pronounced peak in inflammatory markers seen in those admitted to an ICU. CRP is a useful marker to monitor disease progression in SOT.
Keywords: COVID‐19, cytokines, hospitalization, immunosuppression, inflammation, solid organ transplantation
Abbreviations
- ACE
angiotensin‐converting enzyme
- ALT
alanine transaminase
- ARB
angiotensin II receptor blocker
- ARDS
adult respiratory distress syndrome
- ARF
acute renal failure
- AST
aspartate transaminase
- BMI
body mass index
- BWH
Brigham and Women's Hospital
- CK
creatine kinase
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- FIO2
fraction of inspired oxygen
- ICU
intensive care unit
- IL‐6
interleukin‐6
- LDH
lactate dehydrogenase
- LFT
liver function tests
- MGH
Massachusetts General Hospital
- RNA
ribonucleic acid
- SARS‐CoV‐2
severe adult respiratory syndrome‐coronavirus‐2
- SOT
solid organ transplantation
- WBC
white blood cell
1. INTRODUCTION
The presentation and progression of infections in solid organ transplant recipients (SOT) differ from immunocompetent hosts, frequently with greater severity. 1 The COVID‐19 pandemic due to SARS‐CoV‐2 has resulted in nearly 12 million documented cases worldwide. The severity of COVID‐19 in the general population ranges from minimally symptomatic to critical illness in a proportion of patients. 2 Several risk factors for admission and for development of severe and critical disease have emerged including older age, gender, and various common comorbid conditions. Based on experience with viral respiratory infections in SOT, it was speculated that the clinical phenotype of COVID‐19 would be more severe in solid organ transplant recipients. However, the natural history of infection is undefined, and published literature on the manifestations of COVID‐19 in solid organ transplant recipients remains limited. 3 , 4 , 5 , 6 , 7 To address some of these questions, we studied the progression of the disease in 52 solid organ transplant recipients by analyzing changes in their inflammatory markers and clinical progression over 3 weeks following the onset of symptoms.
2. METHODS
2.1. Data source and collection
We examined the medical records of all patients who tested positive at the Mass General Brigham healthcare system, a not‐for‐profit healthcare system affiliated with Harvard Medical School that includes 12 hospitals serving a third of the Massachusetts population. The Massachusetts General Hospital (MGH) and Brigham and Women's Hospital (BWH) are academic medical centers of the Mass General Brigham system in Boston, MA. Each centre performs solid organ transplantation of kidney, heart, lung, pancreas (MGH and BWH), and liver (MGH only). 446 transplants were performed in 2019 by the two programs. Over 8000 transplant recipients are followed in these centers.
We collected data from reporting functions of the integrated electronic health record used by all 12 hospitals of the Mass General Brigham network (Epic Systems). Data on all transplant recipients over 18 years of age that tested positive for COVID‐19 between February 1, 2020, and May 6, 2020, were included. Records were reviewed up to May 16, 2020, for follow‐up. This study was approved by the Mass General Brigham Institutional Review Board.
COVID‐19 was diagnosed if SARS‐CoV‐2 RNA was detected in clinical specimens (upper and/or lower respiratory tract specimens) by nucleic acid testing designated for emergency use authorization by the Food and Drug Administration and conducted in agreement with guidelines provided by the US Centers for Disease Control and Prevention.
2.2. Covariates
We extracted baseline demographic data (age, gender, race, ethnicity, zip code), smoking status, comorbidities, medications at time of COVID‐19 diagnosis, SOT type and date, history of rejection within 3 months prior to COVID‐19 diagnosis. For patients admitted within the Mass General Brigham system, details of admission including presenting symptoms and vital signs, therapeutic drugs and strategies, clinical outcomes, serial clinical and laboratory parameters (complete blood count, CBC, creatinine, liver function tests, LFT, C‐reactive protein, procalcitonin, ferritin, D Dimer, IL‐6 level, hypersensitive troponin T, creatinine kinase, lactate dehydrogenase, LDH, and tacrolimus levels) through COVID‐19 illness.
2.3. Statistical analysis
Comparison is made between patients admitted and those managed as outpatients. In addition, among those admitted to the hospital, comparison is made between those admitted to ICU at any point in their admission (ICU patients) and those managed exclusively in a non‐ICU, general medical/surgical setting (Non‐ICU patients). Statistical analysis was performed using Prism 8 (Version 8.4.2, GraphPad Software LLC) and Microsoft Excel (Version 16.16.21, Microsoft). Variables were compared using Fisher's exact test for categorical variables, Mann‐Whitney U test for non‐parametric continuous variables, single‐factor ANOVA for parametric categorical variables. Daily mean CRP, ferritin, D Dimer, and albumin were determined by averaging individual patient values over the next 3 days from collection for the period in which values were available for each patient to account for intermittent test acquisition in individual patients. Daily mean value was then obtained for all patients with values on the day of illness. ICU and non‐ICU daily means were compared. Graphs were prepared using Prism 8 (Version 8.4.2, GraphPad Software LLC). P‐value <.05 was considered significant.
3. RESULTS
A total of 52 solid organ transplant recipients were identified as COVID‐19 positive with demographic data (Table 1). The median age was 58 years (IQR 50‐66), 34 (65%) were male. Median time from transplant was 43.5 months with a range of 4‐310 months, 12 (23%) were within 1 year of transplant. The majority were kidney transplant recipients (29, 56%) consistent with the population studied. Median body mass index (BMI) was 27.0 kg/m2. 25 (48%) recipients were identified as white, 10 (19%) as Black, and 17 (33%) as Hispanic. Most recipients were immunosuppressed with tacrolimus, mycophenolate, and low‐dose corticosteroids. Only 1 recipient had experienced an episode of rejection within 3 months prior to COVID‐19 diagnosis. Hypertension (43 subjects, 83%), diabetes (35%), and chronic renal insufficiency were common comorbid conditions. Comparison between those admitted to any hospital (n = 40) vs those managed at home (n = 12) is shown in Table 1. Those admitted had a higher median age (61 years vs 50 years, P = .002) and were more likely to have a diagnosis of ischemic heart disease (12 vs 0, P = .047). Pre‐admission medications included a statin in 29 (53%) and ACE‐inhibitor or angiotensin II receptor blocker (ARB) in 7 (13%) and were not associated with admission or ICU care. No other baseline factors were associated with admission.
TABLE 1.
Demographic characteristics of SOT recipients diagnosed with COVID‐19, with comparison between those admitted and not admitted
| Characteristics | All (%) | Admitted (%) | Not admitted (%) | P‐value | |||
|---|---|---|---|---|---|---|---|
| Number | 52 (100%) | 40 (77%) | 12 (23%) | ||||
| Median Age (IQR) | 58 (50‐66) | 61 (52‐66) | 50 (36‐53) | .002 | |||
| Male | 34 (65%) | 28 (70%) | 6 (50%) | .202 | |||
| Organ | |||||||
| Kidney | 29 (56%) | 22 (55%) | 7 (58%) | .838 | |||
| Liver | 9 (17%) | 7 (18%) | 2 (17%) | >.99 | |||
| Heart | 6 (12%) | 5 (13%) | 1 (8%) | .655 | |||
| Lung | 6 (12%) | 5 (13%) | 1 (8%) | >.99 | |||
| Multi‐organ | 2 (4%) | 1 (13%) | 1 (8%) | .412 | |||
| Median time from transplant (months) | 43.5 | 45 | 33 | .962 | |||
| Range | 4‐310 | 4‐298 | 5‐310 | ||||
| 0‐3 mo | 0 | 0 | 0 | ||||
| 3‐12 mo | 12 (23%) | 9 (23%) | 3 (25%) | ||||
| Baseline immunosuppression | |||||||
| CNI | 44 (85%) | 34 (85%) | 10 (83%) | ||||
| Mycophenolate/Azathioprine | 38 (73%) | 28 (70%) | 10 (83%) | ||||
| Prednisone | 37 (71%) | 29 (73%) | 8 (67%) | ||||
| Belatacept | 5 (10%) | 3 (8%) | 2 (17%) | ||||
| Sirolimus/Everolimus | 5 (10%) | 4 (10%) | 1 (8%) | ||||
| Rejection in last 3 mo | 1 (2%) | 1 (3%) | 0 (0%) | ||||
| Taking >5 mg prednisone | 4 (8%) | 3 (8%) | 1 (8%) | ||||
| Race/Ethnicity | |||||||
| White | 25 (48%) | 21 (53%) | 4 (33%) | .329 | |||
| Black or African American | 10 (19%) | 9 (23%) | 1 (8%) | .42 | |||
| Hispanic | 17 (33%) | 10 (25%) | 7 (58%) | ||||
| Comorbidities | |||||||
| Hypertension | 43 (83%) | 33 (83%) | 10 (83%) | >.99 | |||
| Diabetes mellitus | 18 (35%) | 15 (38%) | 3 (25%) | .508 | |||
| Chronic renal failure | 39 (75%) | 30 (75%) | 9 (75%) | >.99 | |||
| Chronic lung disease | 9 (17%) | 8 (20%) | 1 (8%) | .666 | |||
| Asthma | 3 (6%) | 3 (8%) | 0 (0%) | >.99 | |||
| HIV | 1 (2%) | 1 (3%) | 0 (0%) | >.99 | |||
| Ischemic heart disease | 12 (23%) | 12 (30%) | 0 (0%) | .047 | |||
| Congestive cardiac failure | 10 (19%) | 9 (23%) | 1 (8%) | .42 | |||
| Hyperlipidemia | 16 (31%) | 14 (35%) | 2 (17%) | .301 | |||
| Active cancer | 1 (2%) | 1 (3%) | 0 (0%) | >.99 | |||
| Current/former smoker | 23 (44%) | 19 (48%) | 4 (33%) | .513 | |||
| BMI (median) (kg/ms) | 27 | 27.1 | 25.1 | .932 | |||
| Admission in Mass General Brigham System | 32 (80%) | ||||||
| Admission OUTSIDE Mass General Brigham | 8 (20%) | ||||||
Abbreviations: BMI, Body mass index; CNI, Calcineurin inhibitor (includes tacrolimus and cyclosporine).
Forty patients were hospitalized, 32 were admitted within the Mass General Brigham system allowing detailed analysis of their admission. Indications for admission are summarized in Table S1. Of 32 admitted patients, 11 patients received ICU care at any point in their admission (ICU patients) and 21 patients were managed exclusively in a non‐ICU setting (non‐ICU patients). Indications for admission are in Table S1. Demographic characteristics for these patients are shown in Table S2. Clinical characteristics at presentation are shown in Table 2. The need for supplemental oxygen on initial presentation was associated with admission to an ICU (11 vs 8, P = .001). Those admitted to an ICU had higher WBC (7.38 vs 5.09, P = .022), higher absolute neutrophil count (7.19 vs 4.3, P = .0056), higher aspartate transaminase (AST) (46 vs 26, P = .0028), higher alanine transaminase (ALT) (52 vs 22, P = .0024), higher lactate dehydrogenase (LDH) (383 vs 254, P = .024), higher C‐reactive protein (CRP) (120.4 vs 46.4, P = .0305), higher procalcitonin (0.51 vs 0.13, P = .0138), and higher creatinine kinase (CK)(159 vs 59.5, P = .0319) than those managed without ICU care.
TABLE 2.
Presenting characteristics of those hospitalized with comparison between those admitted to ICU at any point in their admission vs those never admitted to ICU (non‐ICU)
| Characteristic | Admitted (%) | ICU (%) | Non‐ICU (%) | P‐value |
|---|---|---|---|---|
| N | 32 (100%) | 11 (35%) | 21 (65%) | |
| Symptoms | ||||
| Fever | 24 (75%) | 9 (82%) | 15 (71%) | .681 |
| Dyspnea | 18 (56%) | 8 (73%) | 10 (48%) | .266 |
| Cough | 20 (63%) | 7 (64%) | 13 (62%) | >.99 |
| Sore throat | 3 (9%) | 3 (27%) | 0 (0%) | .033 |
| Myalgias | 13 (41%) | 6 (55%) | 7 (33%) | .283 |
| Fatigue | 19 (59%) | 6 (55%) | 13 (62%) | .687 |
| Nausea/vomiting | 10 (31%) | 4 (36%) | 6 (29%) | .703 |
| Diarrhea | 9 (28%) | 3 (27%) | 6 (29%) | |
| Chest pain | 6 (19%) | 4 (36%) | 2 (10%) | .148 |
| Time course | ||||
| Symptom onset to test (median, days) (IQR) | 3.5 (6) | 2 (5) | 4 (6) | .775 |
| Symptom onset to admission (median, days) (IQR) | 7 (10) | 5 (6) | 7 (8) | .128 |
| Initial observations | ||||
| Febrile >37.9 | 4 (13%) | 2 (18%) | 2 10%) | .593 |
| Supplemental O2 requirement on admission | 12 (38%) | 11 (100%) | 8 (38%) | .001 |
| FiO2 range | 24%‐100% | 24%‐100% | 24%‐44% | |
| Chest x‐ray findings | ||||
| None | 10 (31%) | 2 (18%) | 8 (38%) | .425 |
| Unilateral | 5 (16%) | 1 (9%) | 4 (19%) | .637 |
| Bilateral | 17 (53%) | 8 (73%) | 9 (43%) | .148 |
| Median initial laboratory results on presentation (IQR) | ||||
| WBC (K/uL) | 5.86 (3.4) | 7.38 (7.3) | 5.09 (3.41) | .022 |
| ANC (K/uL) | 4.84 (3.5) | 7.19 (6.85) | 4.3 (2.92) | .006 |
| ALC (K/uL) | 0.56 (0.47) | 0.48 (0.58) | 0.61 (0.43) | .152 |
| Creatinine (mg/dL) | 1.45 (1.05) | 1.96 (1.16) | 1.43 (0.88) | .703 |
| AST (U/L) | 31 (22) | 46 (29.25) | 26 (15) | .003 |
| ALT (U/L) | 27 (19) | 52 (44) | 22 (12) | .002 |
| LDH (U/L) | 295 (147) | 383 (254) | 254 (111) | .024 |
| C‐reactive protein (mg/L) | 60.7 (89) | 120.4 (117) | 46.4 (82) | .031 |
| Procalcitonin (ng/mL) | 0.17 (0.35) | 0.51 (4.51) | 0.13 (0.13) | .014 |
| Ferritin (ug/L) | 702 (2754) | 1361 (2761) | 686 (1818) | .212 |
| D Dimer (ng/mL) | 1074 (1570) | 1121 (1470) | 978 (1547) | .470 |
| Troponin T (ng/L) | 23 (41) | 22.5 (41) | 22 (50) | >.99 |
| Creatinine kinase (U/L) | 71 (76) | 159 (199) | 59.5 (60) | .032 |
Abbreviations: ALC, absolute lymphocyte count; ALT, Alanine transaminase; ANC, absolute neutrophil count; AST, Aspartate transaminase; ICU, intensive care unit; LDH, Lactate dehydrogenase; WBC, White blood cell count.
3.1. Inpatient management
Inpatient management is shown in Table 3. All of those admitted to ICU were intubated and required vasopressors (11, 100%); 8 (73%) required prone positioning and 5 (45%) required dialysis. Median time to ICU admission was 7 days from symptom onset. Only 12 (57%) of the non‐ICU patients required supplemental oxygen at some point in their hospitalization with 8 (38%) of these requiring supplemental oxygen on admission.
TABLE 3.
Features of inpatient management for hospitalized patients with comparison between those admitted to ICU at any point in their admission vs those never admitted to ICU (non‐ICU)
| All | ICU | Non‐ICU | P‐value | |
|---|---|---|---|---|
| N | 32 (100%) | 11(35%) | 21 (65%) | |
| Immunosuppression changes | ||||
| Proportion any IS changes | 22 (69%) | 8 (73%) | 14 (67%) | >.999 |
| Pre‐admission Aza/MMF | 24 | 9 | 16 | |
| Aza/MMF held | 12 (50%) | 6 (67%) | 6 (38%) | |
| Aza/MMF halved | 7 (29%) | 2 (22%) | 5 (31%) | |
| Pre‐admission CNI | 26 | 9 | 17 | |
| CNI held | 1 (4%) | 1 (11%) | 0 (0%) | |
| CNI started | 1 (3%) | 0 (0%) | 1 (5%) | |
| Pre‐admission MTOR | 3 | 0 | 3 (14%) | |
| MTOR held | 3 (100%) | 0 (0%) | 3 (100%) | |
| Pre‐admission Belatacept | 3 | 2 | 1 (5%) | |
| Belatacept held | 2 (67%) | 2 (100%) | 0 (0%) | |
| Pre‐admission steroids | 25 | 9 | 16 (76%) | |
| Steroids held | 0 (0%) | 0 (0%) | 0 (0%) | |
| Steroids increased | 4 (16%) | 4 (44%) | 0 (0%) | |
| Other drugs | ||||
| Antibiotics | 20 (63%) | 11 (100%) | 9 (43%) | .002 |
| Hydroxychloroquine | 11 (34%) | 6 (55%) | 5 (24%) | .123 |
| Remdesivir | 1 (3%) | 0 (0%) | 1 (5%) | >.999 |
| Statin | 22 (69%) | 9 (82%) | 13 (62%) | .425 |
| Tocilizumab | 1 (3%) | 0 (0%) | 1 (5%) | >.999 |
| Trial enrollment | 9 (28%) | 3 (27%) | 6 (29%) | .387 |
| Other management | ||||
| Supplemental O2 required during admission | 23 (72%) | 11 (100%) | 12 (57%) | .013 |
| Prone positioning | 9 (28%) | 8 (73%) | 1 (5%) | <.001 |
| Vasopressors required | 11 (34%) | 11 (100%) | ‐ | <.001 |
| Dialysis | 5 (16%) | 5 (45%) | 1 (5%) | .011 |
Abbreviations: Aza, Azathioprine; CNI, calcineurin inhibitor; ICU, intensive care unit; IS, Immunosuppression; MMF, mycophenolate; MTOR, Mammalian target of rapamycin inhibitor.
Antibiotics were more frequently given to ICU patients than non‐ICU patients (11 vs 9, P = .0021). Hydroxychloroquine was given to 11 patients (35%) including 6 (55%) admitted to ICU. Statins were given or continued in 21 (68%), off‐label tocilizumab was used in one patient, and nine patients (28%) were enrolled in therapeutic drug trials. Immunosuppression was changed in 22 (69%) of those admitted including 8 (73%) of the ICU patients and 14 (67%) of the non‐ICU patients. Of those on an antimetabolite (MMF or azathioprine), 19 (71%) had these drugs held (50%) or reduced by half (29%). Only one patient admitted to ICU on mycophenolate had no adjustment. Calcineurin inhibitors were held in one patient (4%), and mTOR inhibitors were held in 3 (100%) patients. BelataceptTM was deferred in two of three patients on this agent. Steroids were not withdrawn in any patients; 4 (44%) patients in the ICU group had stress dose steroids, usually in conjunction with reduction in other agents. Median tacrolimus levels pre‐COVID diagnosis and during inpatient admission were not different between ICU and non‐ICU patients (Figure S1).
3.2. Clinical outcomes
Co‐infections were more frequent in those admitted to ICU compared to non‐ICU patients (5 vs 1, P = .037) (Table 4). The most common proven co‐infections were pulmonary infections including ventilator‐associated pneumonia (2, 18%) and fungal infection (1 Aspergillus versicolor, one patient had severe cutaneous candidiasis). Suspected co‐infection occurred in 4 (36%) of ICU patients and 3 (15%) non‐ICU patients. There were only two episodes of suspected rejection, both occurring in liver transplant recipients who had immunosuppression reduced during severe COVID‐19 in the ICU; tacrolimus levels in these patients were between 2 and 4 ug/mL at the time of suspected rejection. Deranged LFTs resolved with increased tacrolimus to baseline levels and re‐addition of mycophenolate mofetil.
TABLE 4.
Outcomes of hospitalized patients with comparison between those admitted to ICU at any point in their admission vs those never admitted to ICU (non‐ICU)
| All | ICU | Non‐ICU | P‐value | |
|---|---|---|---|---|
| N | 32 (100%) | 11 (35%) | 21 (65%) | |
| Co‐infections | ||||
| Proven co‐infection | 6 (19%) | 5 (45%) | 1 (5%) | .037 |
| VAP | 2 (6%) | 2 (18%) | 0 (0%) | |
| UTI | 1 (3%) | 1 (9%) | 1 (5%) | |
| Fungal infection | 2 (6%) | 2 (18%) | 0 (0%) | |
| Suspected co‐infection | 7 (22%) | 4 (36%) | 3 (14%) | .197 |
| Pneumonia | 5 (16%) | 2 (18%) | 3 (14%) | |
| Bacteraemia | 2 (6%) | 2 (18%) | 0 (0%) | |
| Graft outcomes | ||||
| Episode of rejection | 2 (6%) | 2 (18%) | 0 (0%) | .1109 |
| Suspected | 2 (6%) | 2 (18%) | 0 (0%) | |
| Proven | 0 (0%) | 0 (0%) | 0 (0%) | |
| Patient outcomes | ||||
| Discharged | 23 (72%) | 3 (27%) | 20 (95%) | <.001 |
| Remain inpatient | 4 (13%) | 4 (36%) | 0 (0%) | |
| Remain in ICU, intubated | 2 (6%) | 2 (18%) | 0 (0%) | |
| Died | 5 (16%) | 4 (36%) | 1 (5%) | .037 |
Abbreviations: ICU, intensive care unit; UTI, urinary tract infection; VAP, ventilator‐associated pneumonia.
Supplemental oxygen requirements increased for both ICU and non‐ICU patients after admission, peaking at day 6 of symptoms with mean FiO2 of 56% among ICU patients (Figure S2). Inpatient dialysis was required in five patients; one was on dialysis prior to admission, two were kidney transplant recipients.
Mean laboratory values during hospitalization are shown in Figure 1 from the day from symptom onset; each daily value is presented as an average over 3 days to account for alternate day testing in some patients. Both the ICU and non‐ICU patients have similar admission WBC, ALC, ferritin, CRP, D Dimer and albumin. Over time, WBC, ANC, CRP, ferritin, and D Dimer trended higher in those admitted to the ICU compared to the non‐ICU group, while albumin trended lower in those admitted to ICU compared to the non‐ICU group. Comparison of mean daily WBC, CRP, Ferritin D Dimer demonstrates higher levels overall in ICU vs non‐ICU patients (P < .0001) but not ALC (P = .991), while mean daily albumin is lower in ICU vs non‐ICU patients (P < .0001).
FIGURE 1.
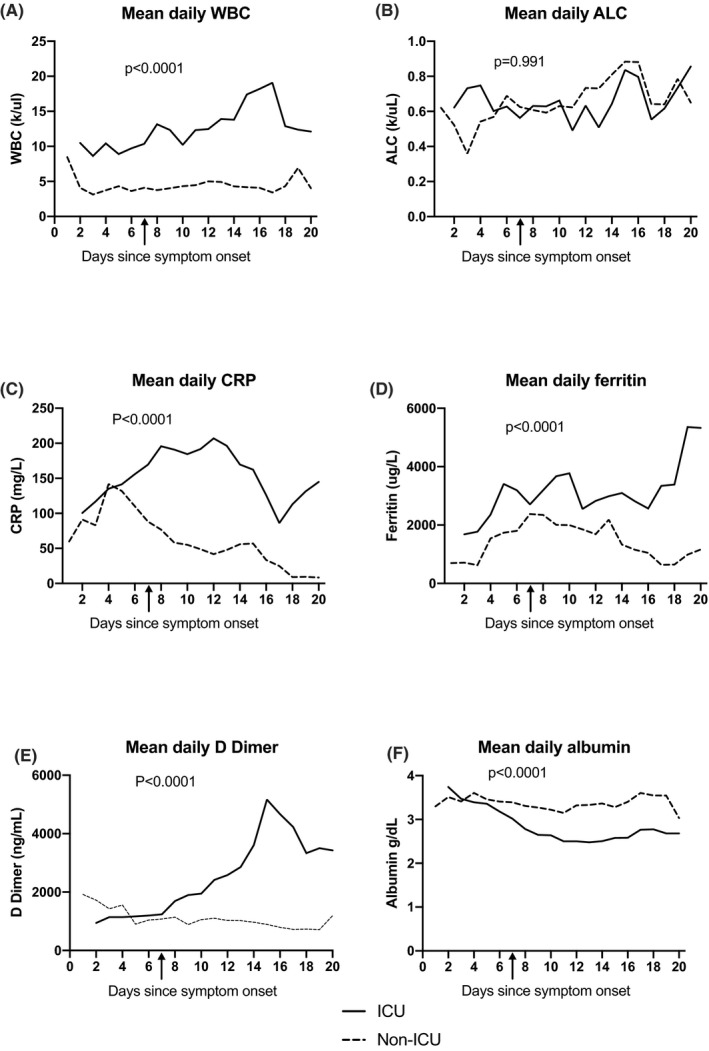
Mean daily laboratory results for first 20 d of illness since symptom onset. Mean daily WBC (panel A), ALC (panel B), CRP (panel C), Ferritin (panel D), D Dimer (panel E) and albumin (panel E) in ICU (black line) vs non‐ICU (dashed line) group, with mean day of ICU admission demonstrated by black arrow (7 d). For each group, daily values of CRP, ferritin, D Dimer, and albumin are averaged over 3 d for each individual patient to account for alternate day testing. ICU, intensive care unit, WBC, white blood cell count, ALC, absolute lymphocyte count, CRP, C‐reactive protein. Mean daily WBC, CRP, ferritin and D Dimer are higher in the ICU group than Non‐ICU group (P < .0001). Mean daily albumin is lower in the ICu group vs the non‐ICU group (P < .001). There is no difference in mean daily ALC between the ICU and non‐ICU group (P = .991). ICU, intensive care unit; WBC, white blood cell count; ALC, absolute lymphocyte count; CRP, C‐reactive protein
WBC increased in ICU patients by day 2 and peaked by day 17 with a mean of 19.06 c/ul ± 16.67 before decreasing (Figure S2). In contrast, most patients remained lymphopenic throughout their hospital stay; absolute lymphocyte counts gradually increased throughout hospitalization (Figure S2). CRP levels started increasing early in the disease course peaking by day 13 in ICU patients (mean 196.3 mg/L ± 54.3) and at day 4 in non‐ICU patients (mean 141.9 mg/L ± 89) (Figure S2). Ferritin and D Dimer levels continuously increased throughout admission and peaked by days 19 and 15 (peak mean ferritin 5356 ± SD 3564, peak mean D Dimer 5169 ± SD 3055 (Figure S2).
With 10‐28 days of follow‐up, all but 4 (13%) patients have been discharged or died. 23 (72%) patients have been discharged without subsequent readmissions for COVID‐19 or other complications. Five (16%) patients have died, 4 (36%) of the ICU group and 1(5%) of the non‐ICU group who requested comfort measures. Two ICU patients remain intubated in the ICU, 2 ICU patients have improved and transferred to general medical care. All non‐ICU patients have been discharged or died.
4. DISCUSSION
The impact of COVID‐19 infections in immunosuppressed hosts remains to be defined. Our series of solid organ transplant recipients with documented COVID‐19 demonstrates a hospitalization rate of 77% and an ICU admission rate of 35%. Such rates are higher than the general population of COVID‐19 patients in our hospitals where admission rates are 28.6% and ICU admission is 9.4% (manuscript under consideration). These rates are also higher than the reported rates of hospitalizations and ICU admission in other published studies. 8 , 9 These heightened hospitalization and ICU admission rates suggest a potential adverse impact of immunosuppression. In support of this possibility, mechanical ventilatory support is required in about 12.2% of the general population admitted with COVID‐19, 8 while our SOT cohort has almost 3 times this rate (35%). Furthermore, ICU level care is associated with death in 36% in our cohort, while a further 18% remain intubated in the ICU at time of last follow‐up. Overall mortality in our cohort was 16%, much higher than the overall mortality rate for all COVID‐19 patients in our system (4.3%, manuscript under consideration) and higher than the reported rates in the general population of 1%‐5%. 10 Similar cohorts of SOT recipients have reported mortality rates between 24%‐28% overall and 52%‐64% in ICU patients. 4 , 5 , 6 Our mortality rate is likely lower than these cohorts as we did not face resource limitations, and our hospitals had more time to prepare for COVID‐related admissions than other sites and the presence of demographic differences. Consistent with prior observations of risk factors for greater disease severity,, 2 , 8 those admitted in our cohort were more likely to be older and have ischemic heart disease. No association was found with other previously implicated associated comorbidities in our cohort, such as hypertension and diabetes, potentially due to small sample size and overall very high frequency of comorbidities in the transplant population.
The optimal management of immunosuppression in COVID‐19 infection is complicated by the intensity of the hyperinflammatory state that appears to contribute to the development of acute respiratory distress syndrome (ARDS), multi‐organ failure, and death. Our data suggest immunosuppressed SOT recipients are not protected from this phenomenon and may be more prone to systemic injury. In this series, immunosuppression was frequently reduced in the setting of documented COVID‐19 infection (69%), yet episodes of suspected rejection were rare. There were two episodes of suspected rejection in liver transplant recipients during the recovery phase of COVID‐19; LFTs normalized with re‐established immunosuppression. However, neither patient had a biopsy to confirm this. This data are also limited by the difficulty in recognizing rejection in heart and lung recipients and the potential that acute kidney injury in renal transplant recipients was not recognized as rejection. The overall rate of co‐infection was low; this observation may allow the judicious use of immunomodulatory therapies for systemic inflammation in transplant recipients; larger, prospective series are needed.
Our analysis demonstrates several trends in laboratory parameters for this group of patients. A biphasic pattern for inflammatory markers distinguished patients at risk for critical illness. At presentation, SOT recipients appear little different from other hosts with moderately elevated WBC, CRP, Ferritin, and D Dimer values. The requirement for supplemental oxygen, higher WBC and ANC, greater elevation of liver function tests, LDH, CRP, procalcitonin, and creatinine kinase (CK) are associated with the need for ICU care. Patients ultimately admitted to the ICU after day 5 developed continued elevation in WBC, CRP, and ferritin possibly reflecting the inflammatory state of severe COVID‐19 like that of non‐immunocompromised patients. It remains unknown, however, whether reductions in immunosuppression are protective or detrimental. Additional studies are needed.
In addition to the strengths discussed above and the large detailed sample with serial laboratory results and outcomes, our series has some limitations. These include the retrospective nature of the study, reliance on medical records and the relative under‐representation of heart and lung recipients in our cohort. Similarly, we do not have a direct comparison of non‐immunocompromised cohorts with which to compare out data to determine if immunosuppression is the true cause of heightened admission, ICU admission, and mortality rates. Subsequent studies will define the duration of viral shedding in SOT, the incidence and duration of SARS‐CoV‐2 viremia, as well as the biologic basis of the observed biphasic inflammatory response. The roles of immunosuppression, age, comorbidities, and other factors in the progression of COVID‐19 infection require additional studies.
Our data show that a significant proportion of admitted SOT patients require ICU care with high mortality. Close follow‐up and a relatively low threshold for hospitalization may be beneficial in SOT given the lack of predictors for deterioration at admission.
CONFLICTS OF INTEREST
The authors have nothing to disclose.
AUTHOR CONTRIBUTION
MBR and JEK conceived the study, MBR, SI, ZT, and AAJ collected the data. MBR, JAF, and JEK analyzed and interpreted the data. MBR, JAF, and JEK wrote the manuscript.
Supporting information
App S1
ACKNOWLEDGEMENTS
The authors thank the staff of Mass General Brigham Hospitals who cared for the patients included in this study.
Roberts MB, Izzy S, Tahir Z, Al Jarrah A, Fishman JA, El Khoury J. COVID-19 in solid organ transplant recipients: Dynamics of disease progression and inflammatory markers in ICU and non-ICU admitted patients. Transpl Infect Dis. 2020;22:e13407. 10.1111/tid.13407
REFERENCES
- 1. Fishman JA. Infection in organ transplantation. Am J Transplant. 2017;17(4):856‐879. [DOI] [PubMed] [Google Scholar]
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banerjee D, Popoola J, Shah S, Ster IC, Quan V, Phanish M. COVID‐19 infection in kidney transplant recipients. Kidney Int. 2020;97(6):1076‐1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernandez‐Ruiz M, Andres A, Loinaz C, et al. COVID‐19 in solid organ transplant recipients: a single‐center case series from Spain. Am J Transplant. 2020;20(7):1849‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pereira MR, Mohan S, Cohen DJ, et al. COVID‐19 in solid organ transplant recipients: initial report from the US Epicenter. Am J Transplant. 2020;20(7):1800‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Akalin E, Azzi Y, Bartash R, et al. Covid‐19 and kidney transplantation. N Engl J Med. 2020;382(25):2475‐2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tschopp J, L'Huillier AG, Mombelli M, et al. First experience of SARS‐CoV‐2 infections in solid organ transplant recipients in the Swiss transplant cohort study. Am J Transplant. 2020. 10.1111/ajt.16062 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized With COVID‐19 in the New York City Area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Price‐Haywood EG, Burton J, Fort D, Seoane L. Hospitalization and mortality among black patients and white patients with Covid‐19. N Engl J Med. 2020;382(26):2534‐2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johns Hopkins University . Coronavirus Resource Centre. 2020; https://coronavirus.jhu.edu/map.html. Accessed June 20, 2020, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1


