Summary
Human Coronaviruses (HCoVs) have long been known as respiratory viruses. However, there are reports of neurological findings in HCoV infections, particularly in patients infected with the novel severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) amid Coronavirus disease 2019 (COVID‐19) pandemic. Therefore, it is essential to interpret the interaction of HCoVs and the nervous system and apply this understanding to the COVID‐19 pandemic. This review of the literature analyses how HCoVs, in general, and SARS‐CoV‐2, in particular, affect the nervous system, highlights the various underlying mechanisms, addresses the associated neurological and psychiatric manifestations, and identifies the neurological risk factors involved. This review of literature shows the magnitude of neurological conditions associated with HCoV infections, including SARS‐CoV‐2. This review emphasises, that, during HCoV outbreaks, such as COVID‐19, a focus on early detection of neurotropism, alertness for the resulting neurological complications, and the recognition of neurological risk factors are crucial to reduce the workload on hospitals, particularly intensive‐care units and neurological departments.
Keywords: autopsy, brain, COVID‐19, human coronavirus, nervous system, neuroinvasion, SARS‐CoV‐2
Abbreviations
- ACE‐2
angiotensin‐converting enzyme‐2
- ADEM
acute disseminated encephalomyelitis
- ARDS
acute respiratory distress syndrome
- BBB
blood‐brain barrier
- CIM
chronic illness myopathy
- CNS
central nervous system
- COVID‐19
coronavirus disease 2019
- CSF
cerebrospinal fluid
- DPP‐4
dipeptidyl peptidase‐4
- EEG
electroencephalogram
- HCoV
human coronavirus
- HCoV‐229E
human coronavirus‐229E
- HCoV‐HKU1
human coronavirus‐HKU1
- HCoV‐NL63
human voronavirus‐NL63
- HCoV‐OC43
human coronavirus‐OC43
- ICH
intracranial haemorrhages
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- NMS
neuromusculoskeletal system
- PNS
peripheral nervous system
- SARS‐CoV
severe acute respiratory syndrome coronavirus
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
1. INTRODUCTION
Human Coronaviruses (HCoVs) are enveloped RNA viruses of zoonotic nature including the strains SARS‐CoV, Middle East respiratory syndrome coronavirus (MERS‐CoV), HCoV‐229E, HCoV‐OC43, HCoV‐NL63, and HCoV‐HKU1. 1 The classic target tissue for HCoVs is the respiratory tract. However, the novel HCoV strain identified as severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2), along with recent reports recognising the nervous system as an unconventional tissue target for SARS‐CoV‐2, 2 , 3 , 4 , 5 emphasises the need to interpret the interaction of HCoVs and the nervous system, and apply this understanding to SARS‐CoV‐2 pandemic infection known as Coronavirus Disease 2019 (COVID‐19). 6
This review aims to analyse the possible underlying mechanisms of nervous system involvement in HCoV infections, emphasize the magnitude of HCoV infection‐associated neurological manifestations, including those related to the central nervous system (CNS), peripheral nervous system (PNS), and neuromusculoskeletal system (NMS), understand the relationships between HCoVs and neurological diseases, and address the psychological and psychiatric aspects of HCoV infections.
2. METHODS
2.1. Search strategy and selection criteria
References for this review were identified by searches of PubMed between 1980 and April 1, 2020, and references from relevant articles. The search terms “coronavirus,” “SARS‐CoV” “SARS‐CoV‐2,” “MERS‐CoV,” “COVID‐19,” “nervous system,” “neuroinvasion,” “brain,” “autopsy,” and “neurological disorders” were used. There were no language restrictions. The final reference list was created on the basis of relevance to the topics covered in this review
Twenty‐five articles, including a total of 334 cases, were found to be useful and relevant to the research topic, in particular, 13 case reports, five prospective studies, three autopsy studies, two retrospective studies, one retrospective multicentre case series, and one case series.
The 25 selected articles were chronologically tabulated to demonstrate the number of cases, HCoV strains, neurological presentations and findings, neurological diagnoses, and neurological outcomes.
3. DISCUSSION
3.1. Mechanisms of nervous system involvement by HCoVs
Since viral pathologies depend on host‐pathogen interaction, the nervous system demonstrates unique characteristics that invite direct injury by respiratory viruses like the HCoVs. The blood‐brain barrier (BBB) is susceptible to become more permeable to HCoVs after viremia leading to neuroinvasion via the haematogenous route, in the late stages of the viral infection. 1 Within the blood stream, the virus triggers a profound systemic inflammatory storm with a massive release of cytokines and chemokines, which compromise the BBB and hence the virus passes through the disrupted BBB, by a trans‐endothelial mechanism, resulting in neuroinvasion. Cytokines, chemokines, and other inflammatory signals also trigger a massive neuroinflammatory response. 2 More interestingly, the olfactory nerve communicates the nasal mucosa with the olfactory bulb, the gateway to the CNS, and the proximity of the olfactory nerve to the nasal inoculation of HCoV may promote neuroinvasion of HCoV via the transneural route, and therefore a direct early neural injury before disease progression is possible. 1 , 3 , 7 This route utilises axonal transport and synaptic transmission to allow the virus to travel across the nervous system in a retrograde fashion, 8 however, it is not well described if this neurotropic process can occur antegrade for HCoV. Neuroinvasion and subsequent neuropropagation lead to dissemination of the virus to several regions within the CNS, particularly the brain stem. 1 , 2 , 4 Neuronal cells possess trans‐membrane binding receptors required for the cellular entry of HCoV, enhanced by the presence of HCoV spike proteins and envelope proteins. 9 , 10 Whilst the binding affinity of SARS‐CoV to angiotensin‐converting enzyme‐2 (ACE‐2) receptor is evident in in vitro studies, 11 , 12 animal experiments show that MERS‐CoV has an affinity to dipeptidyl peptidase‐4 (DPP‐4) receptor. 13 Recently, a genomic resemblance of SARS‐CoV‐2 to SARS‐CoV has been identified 5 and expression of SARS‐CoV‐2 cell receptor gene ACE‐2 in a wide variety of human tissues, including the brain, has been reported. 6 Apart from viral replication‐induced direct neural damage (ie, virus‐induced neuropathology), it is also possible that HCoV infections, as result of the misdirected host autoimmune responses in susceptible patients, promote indirect neural damage (ie, virus‐induced neuro‐immunopathology) 14 , 15 , 16 (Figure 1). On the other hand, pre‐existing neurological disorders, particularly autoimmune diseases and the immunosuppressive or immunomodulatory therapies, may indirectly induce or exacerbate immune‐mediated neural damage by HCoVs. 17 , 18
FIGURE 1.
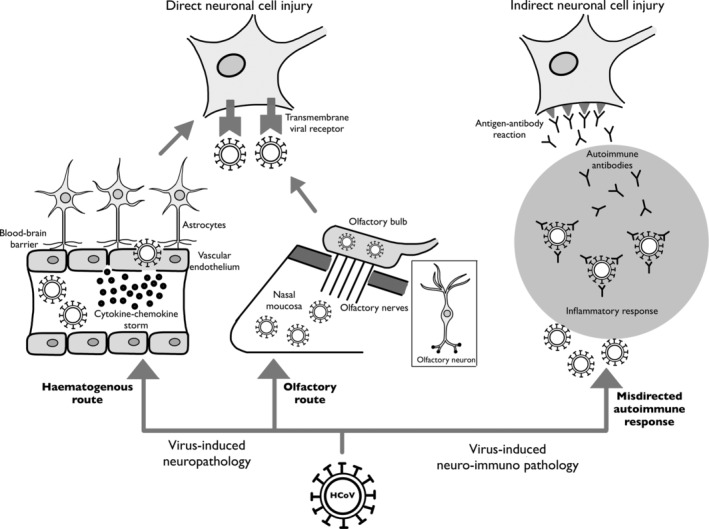
A diagram illustrating the various mechanisms of neuronal cell injury by human coronaviruses (HCoVs)
3.2. Neurological findings in HCoV infections
It is evident from the relatively large body of literature, shown in Table 1, that various strains of HCoVs are indeed associated with a wide range of neurological findings and conditions.
TABLE 1.
Literature review of human coronavirus infections with nervous system involvement
| No. | Study (authors, country) | Study design | No. of cases with nervous system involvement | HCoV strain | Neurological presentations and findings | Neurological diagnosis | Clinical outcome |
|---|---|---|---|---|---|---|---|
| 1 | Mao et al, 19 China | Retrospective multicentre case series | 78 out of total 214 cases | SARS‐CoV‐2 (COVID‐19) | CNS manifestations (24.8%): dizziness (16.8%), headache (13.1%), impaired consciousness (7.5%), acute cerebrovascular disease (2.8%), ischemic stroke (2.3%), cerebral haemorrhage (0.5%), epilepsy (0.5%), ataxia (0.5%). Brain CT scan showed new onset of ischemic stroke in one patient. Brain MRI was not performed. CSF analysis was not done. PNS manifestations (8.9%): hypogeusia (5.6%), hyposmia (5.1%), neuralgia (2.3%). Musculoskeletal manifestations (10.7%): myalgia, elevated serum creatine kinase level above 200 U/L. | Acute cerebrovascular disease, ischemic stroke, cerebral haemorrhage, neuropathy, neuromuscular disorder | Not reported |
| 2 | Filatov et al, 20 USA | Case report | One case | SARS‐CoV‐2 (COVID‐19) | Headache, severely altered mental status, encephalopathic, nonverbal and unable to follow any commands; however, able to move all extremities and reacts to noxious stimuli. No nuchal rigidity. Brain CT scan showed no acute abnormalities, except for a left temporal encephalomalacic area, consistent with history of old ischemic stroke. Brain MRI was not done. EEG showed bilateral slowing and focal slowing in the left temporal region with sharply countered waves. CSF (via lumbar puncture) analysis did not reveal any evidence of infection. | Encephalopathy | Deteriorated and became critically ill and hence intubated in the ICU with poor prognosis |
| 3 | Zhao et al, 21 China | Case report | One case | SARS‐CoV‐2 (COVID‐19) | Acute flaccid paraplegia, bilateral lower limbs and trunk hypoesthesia with a sensory level at T10, and bilateral lower limbs hyporeflexia. Urinary and bowel incontinence. Normal cranial nerve examination. Brain CT scan showed brain atrophy and bilateral basal ganglia and paraventricular lacunar infarctions. Brain MRI was not performed. | Post infectious acute myelitis | Recovered and transferred to rehabilitation therapy |
| 4 | Poyiadji et al, 22 USA | Case report | One case | SARS‐CoV‐2 (COVID‐19) | Altered mental status. CSF analyses were negative for bacteria or viruses. Non‐contrast brain CT scan demonstrated symmetric hypoattenuation within the bilateral medial thalami. CT angiogram and venogram were normal. Brain MRI showed haemorrhagic ring enhancing lesions within the bilateral thalami, medial temporal lobes and the subinsular regions. | Acute necrotizing haemorrhagic encephalopathy | Not reported |
| 5 | Nilsson et al, 23 Sweden | Case report | One case | HCoV‐OC43 | Altered behaviour, myoclonic seizures involving abdominal wall. Brain MRI showed slightly increased T2 signal in periventricular white matter, thalami and pons. Spectroscopy showed low levels of lactate. EEG was inconclusive. CSF analyses were negative for infectious causes of meningoencephalitis. Brain biopsy showed positive PCR for HCoV‐OC43 RNA. | Encephalitis | Progressive neurological deterioration, comatose and intubated in ICU, then died (following consented discontinuation of all intensive care to the patient) |
| 6 | Schattner et al, 24 Israel | Case report | One case | HCoV‐NL63 | Altered behaviour, apathy, slow speech, disorientation, somnolence, refusal to feed, altered consciousness, symmetric four‐limb spasticity, catatonia, incontinence.EEG demonstrated temporal epileptiform activity and generalized slowing. Neuroimaging were unremarkable. CSF analyses were twice negative for multiple infectious agents. | Acute encephalitis | Improved completely and regained full previous functional status |
| 7 | Al‐Hameed, 25 Saudi Arabia | Case report | One case | MERS‐CoV (MERS) | Sudden onset diabetes insipidus followed by unresponsiveness with GCS 3/15 and the pupils were 3 mm wide with sluggish reaction. Then rapid progression to loss of all brain stem reflex and the pupils became dilated and fixed. Urgent CT brain showed right frontal haematoma, subarachnoid haemorrhage extending to the ventricles, causing midline shift and subfalcine herniation. Follow up CT brain showed complete loss of grey and white matter differentiation of both cerebral hemispheres with large frontal haematoma and complete effacement of the lateral ventricles and extra‐axial CSF spaces, including basal cisterns. Brain CT angiography revealed no visualization of MCA, PCA, AComA, and no flow in posterior circulation. Brain MRI was not done. | Spontaneous intracranial haemorrhage | Brain death, cardiac arrest, and death |
| 8 | Kim et al, 26 South Korea | Retrospective | Four out of total 23 cases | MERS‐CoV (MERS) | Case 1: Hypersomnolence, bilateral complete external ophthalmoplegia, weakness and hyporeflexia in all four limbs. Cerebrospinal fluid analysis and brain MRI were normal. Case 2: Tingling and pain in four distal limbs, bilateral lower limbs proximal weakness and mild hyporeflexia. Normal sensory examinations. Case 3: Bilateral tingling and hypesthesia in distal upper and lower limbs, hyporeflexia of both lower limbs. Case 4: Tingling in both hands. | Case 1: Bickerstaff's encephalopathy overlapping with Guillain‐Barré syndrome. Case 2: ICU‐acquired weakness or Guillain‐Barré syndrome. Case 3: Infectious or toxic polyneuropathy. Case 4: Infectious or toxic acute sensory neuropathy. | Case 1: Fully recovered after 2 months. Case 2: Improved over 2–7 months. Case 3: Gradually improved over 6 months. Case 4: Patient lost to follow up. |
| 9 | Li et al, 27 China | Prospective | 22 out of total 183 cases | Various HCoVs: SARS‐CoV, MERS‐CoV, HCoV‐229E, HCoV‐OC43, HCoV‐NL63, HCoV‐HKU1 | Headache (45.5%), vomiting (36.4%), seizures (22.7%), neck stiffness (31.8%). Kernig sign (9.1%), Brudzinski sign (4.5%), Babiniski sign (9.1%). Brain CT and MRI showed abnormalities in temporal lobe, periventricular region, basal ganglia and thalamus. CSF analyses were positive for anti‐CoV IgM antibodies and showed high expression profiles for multiple cytokines (GM‐CSF, IL‐6, IL‐8, MCP‐1). EEG studies were normal. | Acute encephalitis | Full recovery of all 22 cases |
| 10 | Morfopoulou et al, 28 UK | Case report | One case | HCoV‐OC43 | Irritability alternating with sleepiness and abnormal posturing movements. Brain MRI showed loss of volume and abnormal signal in grey matter consistent with viral encephalitis. CSF analysis did not identify any pathogen. Post mortem brain biopsy showed the presence of the RNA sequence of HCoV‐OC43, which was subsequently confirmed on real‐time PCR and brain immunohistochemical analysis. | Encephalitis | Deterioration and death |
| 11 | Algahtani et al, 29 Saudi Arabia | Case report | Two cases | MERS‐CoV (MERS) | Case 1: Headache, nausea, vomiting, coma. Brain CT scan showed right frontal intracerebral haemorrhage with massive brain oedema and midline shift. Brain MRI was not performed. Case 2: Bilateral lower limb weakness and stocking distribution hypoesthesia. Nerve conduction studies showed axonal polyneuropathy. Spine MRI was normal. CSF analysis was normal. | Case 1: Intracerebralhaemorrhage. Case 2: Critical illness polyneuropathy complicating long ICU stay. | Case 1: Multiple organ failure, irreversible brain stem dysfunction and death. Case 2: Recovered |
| 12 | Arabi et al, 30 Saudi Arabia | Case report | Three cases | MERS‐CoV (MERS) | Case 1: Vomiting, confusion, ataxia, dysmetria, left sided motor weakness, coma. Brain CT scan showed numerous patchy hypodensities in periventricular deep white matter and subcortical regions, basal ganglia, thalami, pons, cerebellum, and corpus callosum. Brain MRI showed multiple bilateral patchy high T2/FLAIR signal abnormalities in periventricular deep white matter and subcortical regions, corpus callosum, midbrain, brachium pontis, cerebellum, and upper cervical cord. CSF analysis was not performed. Case 2: Left sided facial paralysis, coma. CT brain revealed multiple bilateral patchy hypodensities in periventricular deep white matter, basal ganglia, and corpus callosum. CT angiography showed complete occlusion of both ICAs and narrow M1 segment of left MCA. Brain MRI showed signal abnormality bilaterally in deep watershed, parasagittal region, cortical and subcortical regions of temporal, parietal, and occipital lobes, with restriction on diffusion study. CSF analysis was not performed. Case 3: Coma. Brain CT scan showed no acute abnormality. Brain MRI showed hyperintensity within the white matter of both cerebral hemispheres and along the corticospinal tract. CSF analysis was not performed. | Case 1: Acute disseminated encephalomyelitis. Case 2: Acute ischemic stroke. Case 3: Encephalitis | Case 1: Deterioration and death. Case 2: Severe shock, multiple organ failure and death. Case 3: Recovered. |
| 13 | Stainsby et al, 31 Canada | Case series | Three cases | SARS‐CoV (SARS) | Neuropathic pain and distal limbs paraesthesias. Muscular pain, contracture and weakness. Joint dysfunction, difficult walking, and abnormal gait. Neuroimaging studies were not performed. | Neuromusculoskeletal disorders | Recovered |
| 14 | Gu et al, 32 China, USA, Australia | Autopsy | Eight out of total 18 cases | SARS‐CoV (SARS) | SARS genome sequences were detected, with light microscopy, electron microscopy, and real‐time PCR, in the cytoplasm of numerous neurons of the hypothalamus and cortex of the brains of all eight SARS autopsies. | Severe acute respiratory syndrome (SARS) | N/A |
| 15 | Xu et al, 33 China | Case report | One case | SARS‐CoV (SARS) | Headache, dizziness, myalgia, obscured monocular vision, macula exudation, dysphoria, vomiting, deliria, coma. Brain CT revealed broad encephalic pathological changes of brain ischemia, necrosis and oedema. Brain MRI was not done. CSF analysis was not performed. Post mortem examinations of brain tissue obtained at autopsy identified SARS‐CoV morphology genome and antigen. | Chronic progressive viral cerebritis | Brain herniation and death |
| 16 | Tsai et al, 34 Taiwan | Prospective | Four out of total 76 cases | SARS‐CoV (SARS) | Limb weakness, numbness, hypesthesia, hyporeflexia. Nerve conduction velocities and electromyography demonstrated axonopathic sensorimotor polyneuropathy (two cases), myopathy (one case), and combined polyneuropathy and myopathy (one case). Neuroimaging studies were not done. | Neuromuscular disorders | Improved |
| 17 | Lau et al, 35 China | Case report | One case | SARS‐CoV (SARS) | Generalized tonic–clonic convulsion with loss of consciousness. CSF tested positive for SARS‐CoV by PCR. Neuroimaging and EEG were not performed. | Central nervous system infection | Recovered |
| 18 | Yeh et al, 36 USA | Case report | One case | HCoV‐OC43 | Irritability, difficulty walking, clumsy right hand, numbness in lower extremities. Mild distal weakness in right hand and foot, patchy loss of vibration and temperature sensation below T10, mild dysmetria, poor heel‐to‐toe walking, antalgic gait. Cerebrospinal fluid tested positive for HCoV‐OC43 by PCR. Brain MRI revealed multiple bilateral T2 and FLAIR high signal lesions in the centrum semiovale and cerebellum. Some lesions showed enhancement. Spine MRI demonstrated lesions on T2‐weighted imaging at C4‐C5 and at T7‐T8 cord levels. The cord lesions were non‐enhancing. | Acute disseminated encephalomyelitis | Improved over several weeks |
| 19 | Hung et al, 37 Hong Kong | Case report | One case | SARS‐CoV (SARS) | Vomiting, status epilepticus, confusion, disorientation. CSF tested positive for SARS‐CoV genome by real‐time PCR assay. Neuroimaging and EEG were not performed. | Severe acute neurologic syndrome | Recovered |
| 20 | Arbour et al, 38 Canada and England | Autopsy | 65 out of total 90 cases | HCoV‐OC43 HCoV‐229E | PCR testing was positive for HCoV‐229E and HCoV‐OC43 genomes in 44% and 23% of human brain autopsy samples, respectively. A statistically significant higher prevalence of HCoV‐OC43 genome in multiple sclerosis cases (35.9%) than in controls (13.7%). In situ hybridization confirmed the presence of HCoV RNA in brain parenchyma, outside blood vessels. | Multiple sclerosis (39 cases). Other neurological diseases (26 cases) | N/A |
| 21 | Dessau et al, 39 Denmark | Retrospective | Four out of total 37 cases | HCoV‐229E | Loss of vision, retrobulbar pain, dyschromatopsia. CSF specimens tested positive, on real‐time PCR, for HCoV‐OC43 in only four (11%) cases. Neuroimaging studies were not performed. | Acute monosymptomatic optic neuritis | Not reported |
| 22 | Cristallo et al, 40 Italy | Prospective | 30 cases | HCoV‐OC43 HCoV‐229E | Cerebrospinal fluid specimens were positive for HCoV‐OC43RNA in 10 of 20 patients with multiple sclerosis and in 9 of 10 patients with other neurological diseases. CSF specimens were positive for HCoV‐229E RNA in 7 of 20 patients with multiple sclerosis and in 2 of 10 patients with other neurological diseases. Neuroimaging studies were not performed. | Multiple sclerosis (20 cases). Other neurological diseases (Parkinson's disease, senile dementia, medullary atrophy, polyneuropathy, headache) (10 cases) | N/A |
| 23 | Fazzini et al, 41 USA | Prospective | 49 cases | HCoV‐OC43 HCoV‐229E | Antibodies to HCoV antigens detected in CSF specimens of patients, with higher concentrations of antibodies to HCoV‐OC43 antigens in patients with Parkinson's disease. Neuroimaging studies were not performed. | Parkinson's disease (20 cases). Other neurological diseases (29 cases) | N/A |
| 24 | Salmi et al, 42 Finland | Prospective | 49 cases | HCoV‐OC43 HCoV‐229E | Antibodies to HCoV‐OC43 and HCoV‐229E were detected in 41% and 26% of CSF specimens, respectively. Neuroimaging studies were not performed. | Multiple sclerosis | N/A |
| 25 | Burks et al, 43 USA | Autopsy | Two cases | HCoV‐229E | HCoV isolated from brain material obtained at autopsy. High concentrations of antibodies to HCoV were found in the CSF specimens. | Multiple sclerosis | N/A |
Abbreviations: AComA, anterior communicating artery; CNS, central nervous system; COVID‐19, coronavirus disease 2019; CSF, cerebrospinal fluid; CT, computerized tomography; EEG, electroencephalography; GCS, Glasgow Coma Score; HCoV, human coronavirus; ICAs, internal carotid arteries; IgM, immunoglobulin M; MCA, middle cerebral artery; MERS, middle east respiratory syndrome; MRI, magnetic resonance imaging; N/A, not applicable; PCA, posterior cerebral artery; PCR: polymerase chain reaction; PNS, peripheral nervous system; SARS, severe acute respiratory syndrome.
The post‐infectious phenomena in HCoV patients exhibiting late onset neurological manifestations seem to be more commonly reported. 19 , 21 , 22 , 23 , 25 , 26 , 29 It has been recently reported that more than one‐third of patients with COVID‐19 had neurological manifestations related to involvement of the CNS, PNS, and NMS. 19 Intracranial haemorrhages (ICH) or strokes, due to the systemic spread of HCoVs, are very serious post‐infectious complications of HCoVs and these patients may require neurosurgical interventions. 19 , 25 , 29 , 30 There is no explicit evidence to indicate if hypoxia from the impending acute respiratory distress syndrome (ARDS) in severe HCoV cases can induce brain ischemia, this is a reasonable assumption. 25 The polyneuropathies might be explained by prolonged hospitalisations hinting at the neurotoxins from the proceeding respiratory distress. 26 , 29 Reports of neuromusculoskeletal disorders, complicating HCoV infections and COVID‐19, postulate that myopathies and neuropathies are a result of the significantly elevated inflammatory cytokines in patients' sera leading to virus‐induced immune damage. 19 , 31 , 34 This is further supported by evidence of immune‐mediated neurological conditions due to cytokine and chemokine release after HCoV infection as the disease progresses. 21 , 24 , 44 , 45 Another explanation could be the development of chronic illness myopathy (CIM), particularly in patients requiring mechanical ventilation for long periods. 31 It is important to recognise the post‐infectious neurological sequalae and complications of HCoVs in order to design appropriate management strategies.
On the other hand, if the patient with HCoV infection presents with an initial onset of neurological symptoms, with or without fever, before any respiratory involvement, this suggests that the nervous system is the primary focus of HCoV, which directly triggers neuronal injury. 20 , 24 , 32 , 33 This primary neurotropism could be further supported by the recent reports of the occurrence of olfactory and/or taste disorders, as early atypical manifestations of SARS‐CoV‐2, which may precede the onset of full‐blown clinical COVID‐19 in more than one‐third of patients. 19 , 46 The convenient access of SARS‐CoV‐2 to the brainstem via the olfactory nerve may cause neuronal injury to the cardiorespiratory centre, leading to central respiratory failure. This could explain why some patients have a respiratory failure in the early stages of COVID‐19, as opposed to ARDS manifesting during the later stages of the same disease from local respiratory aetiologies. 2 , 3 Hence, it is of clinical significance to emphasise early detection of neurological symptoms of HCoVs, with a high index of suspicion to identify and isolate patients.
The detection of HCoV RNA in cerebrospinal fluid (CSF) samples of patients has been diagnostic for SARS‐CoV 35 , 37 and HCoV‐OC43. 36 , 40 However, it is not always necessary to depend upon the detection of HCoV RNA in the CSF to diagnose nervous system involvement. 20 , 24 , 28 Reports on deep sequencing of brain biopsy samples show the presence of HCoV‐OC43 in neuronal tissue of patients with fatal encephalitis and whose CSF analyses were negative for virus RNA. 23 , 28 This could mean that HCoV RNA in CSF may only be detected in the early stages of the infection. Moreover, creatine kinase identified in CSF, has been utilized as a marker to recognise neuromuscular disorders in COVID‐19 patients. 19 The misdirected immune response in neurological pathologies caused by HCoVs may have cytokine and interleukin levels detectable in CSF. 27 The detection of anti‐HCoV antibodies in high titres in CSF samples of patients with underlying neurological diseases, such as encephalitis, Parkinson's disease, and multiple sclerosis, suggests that HCoVs may play an etiologic or pathogenic role in these diseases. 27 , 41 , 42
Despite the excellent overview of neurological aspects of HCoV infections displayed in Table 1, this review has limitations. Some of the reported symptoms, such as nausea, myalgia, headache, dizziness, are common to any other viral infection. Many of the presented research articles were missing essential diagnostic studies, such as CSF analysis, MRI examination, electroencephalogram study, and polymerase chain reaction testing of CSF samples and nasopharyngeal swabs. Moreover, the study designs of many identified research reports were lacking the inclusion of control groups.
3.3. Neurological outcomes of HCoV infections
The neurological outcomes in patients with HCoV infections vary according to the part of nervous system involved. Fatalities and poor prognoses are more prominent when the brain is involved as indicated by COVID‐19 encephalopathy, 20 encephalitis caused by HCoV‐OC43, 23 , 28 ICH after MERS, 25 , 29 and cerebritis due to SARS. 33 Nevertheless, sometimes HCoV‐associated encephalitis can have a good prognosis. 24 , 27 , 30 Also, post‐infectious acute myelitis in a COVID‐19 patient had ameliorated and the patient was transferred to rehabilitation therapy. 21 MERS‐induced acute disseminated encephalomyelitis (ADEM) was fatal, 30 whereas the patient with ADEM afterHCoV‐OC43 infection had survived and showed improvement over several weeks. 36 Incontrast, MERS‐induced neuropathies 26 , 29 and SARS‐associated neuromusculoskeletal disorders 31 , 34 have less severe outcomes with at least an improvement of their neurological conditions, as expected for PNS involvement. Furthermore, these findings and outcomes give the impression that MERS neurological complications are more likely to be fatal than those with other HCoV infections, although, it is hard to apply the same for SARS or COVID‐19 patients and whether these deaths were due to irreversible brain damage.
3.4. Risk factors for HCoV infections
Whilst immunosuppressive disorders and immunosuppressive drugs are known to provoke infections in general, this is also true for HCoV infections in cases of leukaemia 23 and severe combined immunodeficiency. 28 Old age, underlying comorbidities, and critical illness are identified as risk factors that are capable of producing serious neurological manifestations in COVID‐19 patients. 19 HCoV infections, in more vulnerable patients, can induce an exacerbation of underlying neurological conditions via virus‐induced neuro‐immunopathology. 14 , 15 , 16 , 44 , 45 It has been recognised that patients with pre‐existing neurological diseases, such as myasthenia gravis, 18 cerebrovascular stroke, 17 , 19 , 20 Parkinson's disease, 17 , 40 , 41 multiple sclerosis, 17 , 40 , 42 and other demyelinating disorders, 17 , 40 , 41 may be at a higher risk of contracting infection with SARS‐CoV‐2 or experiencing severe manifestations of COVID‐19. The reverse is also possible that the body's response to HCoVs infections, including COVID‐19, may cause worsening of symptoms of the patients' underlying neurological diseases. This could be attributed to certain risk factors associated with these patients, such as old age, significantly restricted mobility, intake of immunosuppressive or immunomodulatory drugs, and presence of chronic medical conditions, including diabetes mellitus, lung diseases, cardiovascular diseases, renal diseases, liver diseases, and cancer. 17 , 18
3.5. Psychological sequalae of HCoV infections
It is noteworthy to mention, in addition to the neurological impact, the psychological and the psychiatric impact of HCoV infections, not only on patients 2 but also on the health care workers providing treatment and nursing care to patients with HCoV infections, particularly during the pandemic of COVID‐19. 47 SARS‐CoV‐2 induced neuroinflammation together with prolonged hypoxia may promote the development of acute and chronic cognitive and psychiatric impairments in COVID‐19 patients. 2 Health care workers, particularly those providing medical care to COVID‐19 patients in overwhelmed hospitals, frequently reported symptoms of depression (50.4%), anxiety (44.6%), insomnia (34%), and stress (71.5%). 47 Such mental health problems have to be recognised in order to provide the appropriate psychological support and psychiatric treatments or interventions.
4. CONCLUSIONS
HCoVs attack the nervous system, both directly and indirectly, through a wide range of mechanisms, providing varied neurological outcomes in infected patients. This literature review shows the magnitude and breadth of neurological conditions associated with HCoV infections, including COVID‐19. The number of neurological cases from previous HCoV infections imply that nervous system involvement in the COVID‐19 pandemic seems inevitable, even if only a few cases are reported as of yet. Through this review, it can be derived that amid HCoV outbreaks, such as COVID‐19, an emphasis on early detection due to neurotropism, an alert for the aftermath influx of neurological complications, and the recognition of neurological risk factors is crucial in order to reduce the load on the hospitals, particularly the intensive‐care units and the neurological departments. The design of treatment and rehabilitation paradigms for patients and their health care providers during and after HCoV infections, in general, and COVID‐19, in particular, should take in consideration both the neurological and psychiatric sequalae of the disease.
CONFLICT OF INTEREST
The authors have no competing interest.
Abdelaziz OS, Waffa Z. Neuropathogenic human coronaviruses: A review. Rev Med Virol. 2020;30:e2118. 10.1002/rmv.2118
REFERENCES
- 1. Bohmwald K, Gálves NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Steardo L, Steardo L Jr, Zorec R, Verkhratsky A. Neuroinfection may potentially contribute to pathophysiology and clinical manifestations of COVID‐19. Acta Phys. 2020;e13473. 10.1111/alpha.13463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Desforges M, Le Coupanec A, Brison E, Meessen‐Pinard M, Talbot PJ. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol. 2014;807:75‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;1‐4. 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID‐19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Nerosci. 2020;11:995‐998. [DOI] [PubMed] [Google Scholar]
- 6. Li MY, Li L, Zhang Y, Wang XS. Expression of SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wheeler DL, Athmer J, Meyerholz DK, Perlman S. Murine olfactory bulb interneurons survive infection with a neurotropic coronavirus. J Virol. 2017;91(22):e01099‐e01017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dubé M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuron‐to‐neuron propagation of human coronavirus OC43. J Virol. 2018;92(17):e00404‐e00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Le Coupanec A, Desforges M, Meessen‐Pinard M, et al. Cleavage of a neuroinvasive human respiratory virus spike glycoprotein by proprotein convertases modulates neurovirulence and virus spread within the central nervous system. PLoS Pathog. 2015;11(11):e1005261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stodola JK, Dubois G, Le Coupanec A, Desforges M, Talbot PJ. The OC43 human coronavirus envelope protein is critical for infectious virus production and propagation in neuronal cells and is a determinant of neurovirulence and CNS pathology. Virology. 2018;515:134‐149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008;82(15):7264‐7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li K, Wohlford‐Lenane C, Perlman S, et al. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis. 2016;213(5):712‐722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bergmann CC, Lane TE, Stohlman SA. Coronavirus infection of the central nervous system: host–virus stand‐off. Nat Rev Microbiol. 2006;4(2):121‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Skinner D, Marro BS, Lane TE. Chemokine CXCL10 and coronavirus‐induced neurologic disease. Viral Immunol. 2019;32(1):25‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. 2019;12(1):E14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jin H, Hong C, Chen S, et al. Consensus for prevention and management of coronavirus disease 2019 (COVID‐19) for neurologists. Stroke Vasc Neurol. 2020; svn‐2020‐000382. 10.1136/svn-2020-000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jacob S, Muppidi S, Guidon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert‐Eaton Myasthenic Syndrome (LEMS) during the COVID‐19 pandemic. J Neurol Sci. 2020;412:116803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 19 in Wuhan, China. JAMA Neurol. 2020:e201127. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID‐19): encephalopathy. Cureus. 2020;12(3):e7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Acute myelitis after SARS‐CoV‐2 infection: a case report. MedRxiv. 2020. https://www.medrxiv.org/content/10.1101/2020.03.16.20035105v1. Accessed April 1, 2020. [Google Scholar]
- 22. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: CT and MRI Features. Radiology. 2020;30:e201187. 10.1148/radiol.2020201187. Accessed March 31, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nilsson A, Edner N, Albert J, Ternhag A. Fatal encephalitis associated with coronavirus OC43 in an immunocompromised child. Infect Dis. 2020;18:1‐4. [DOI] [PubMed] [Google Scholar]
- 24. Schattner A, Hadar S, Dubin I. Human coronavirus NL63 and acute encephalitis. Ann Infect Dis Epidemiol. 2018;3(2):1032. [Google Scholar]
- 25. Al‐Hameed FM. Spontaneous intracranial hemorrhage in a patient with middle east respiratory syndrome corona virus. Saudi Med J. 2017;38(2):196‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim JE, Heo JH, Kim HO, et al. Neurological complications during treatment of middle east respiratory syndrome. J Clin Neurol. 2017;13(3):227‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li Y, Li H, Fan R, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016;59:163‐169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Morfopoulou S, Brown JR, Davies EG, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016;375(5):497‐498. [DOI] [PubMed] [Google Scholar]
- 29. Algahtani H, Subahi A, Shirah B. Neurological complications of middle east respiratory syndrome coronavirus: a report of two cases and review of the literature. Case Rep Neurol Med. 2016;2016:3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS‐CoV). Infection. 2015;43:495‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stainsby B, Howitt S, Porr J. Neuromusculoskeletal disorders following SARS: a case series. J Can Chiropr Assoc. 2011;55(1):32‐39. [PMC free article] [PubMed] [Google Scholar]
- 32. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu J, Zhong S, Liu J, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005;41:1089‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tsai LK, Hsieh ST, Chao CC, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004;61:1669‐1673. [DOI] [PubMed] [Google Scholar]
- 35. Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004;10(2):342‐344. 10.3201/eid1002.030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yeh EA, Collins A, Cohen ME, Duffner PK, Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004;113(1):e73‐e76. [DOI] [PubMed] [Google Scholar]
- 37. Hung EC, Chim SS, Chan PK, et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. 2003;49(12):2108‐2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000;74(19):8913‐8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dessau RB, Lisby G, Frederiksen JL. Coronaviruses in spinal fluid of patients with acute monosymptomatic optic neuritis. Acta Neurol Scand. 1999;100:88‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cristallo A, Gambaro F, Biamonti G, Ferrante P, Battaglia M, Cereda PM. Human coronavirus polyadenylated RNA sequences in cerebrospinal fluid from multiple sclerosis patients. New Microbiol. 1997;20(2):105‐114. [PubMed] [Google Scholar]
- 41. Fazzini E, Fleming J, Fahn S. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson's disease. Mov Disord. 1992;7(2):153‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salmi A, Ziola B, Hovi T, Reunanen M. Antibodies to coronaviruses OC43 and 229E in multiple sclerosis patients. Neurology. 1982;32(3):292‐295. [DOI] [PubMed] [Google Scholar]
- 43. Burks JS, DeVald BL, Jankovsky LD, Gerdes JC. Two coronaviruses isolated from central nervous system tissue of two multiple sclerosis patients. Science. 1980;209(4459):933‐934. [DOI] [PubMed] [Google Scholar]
- 44. Metha P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Savarin C, Bergmann CC. Fine tuning the cytokine storm by IFN and IL‐10 following neurotropic coronavirus encephalomyelitis. Front Immunol. 2018;9:3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Giacomelli A, Pezzati L, Conti F, et al. Self‐reported olfactory and taste disorders in SARS‐CoV‐2 patients: a cross‐sectional study. Clin Infect Dis. 2020;ciaa330. 10.1093/cid/ciaa330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. [DOI] [PMC free article] [PubMed] [Google Scholar]


