Abstract
Recent evidence has indicated that overweight/obese children may experience cognitive and immune dysfunction, but the underlying mechanisms responsible for the association between overweight/obesity, immune dysfunction, and cognition have yet to be established. The present study aimed to identify a novel link between obesity-induced immune system dysregulation and cognition in preadolescent children. A total of 27 male children (age: 8–10 years) were recruited and separated by body mass index (BMI) into healthy weight (HW: 5th–84.9th percentile, n = 16) and overweight/obese (OW: P85th percentile, n = 11) groups. Adiposity was assessed using dual energy X-ray absorptiometry (DXA), and aspects of executive function were assessed using the Woodcock-Johnson III Tests of Cognitive Abilities. Monocyte populations (CD14+CD16−, CD14+CD16+) with and without expression of chemokine receptor type 2 (CCR2), and circulating progenitor cells (CPCs: CD34+CD45dim), in peripheral blood were quantified by flow cytometry. CPCs were isolated by flow sorting and cultured for 24 h for collection of conditioned media (CM) that was applied to SH-SY5Y neuroblastomas to examine the paracrine effects of CPCs on neurogenesis. OW had significantly higher quantities of both populations of monocytes (CD14+CD16−: 57% increase; CD14+CD16+: 95% increase, both p < 0.01), monocytes expressing CCR2 (CD14+CD16−CCR2+: 66% increase; CD14+CD16+CCR2+: 168% increase, both p < 0.01), and CPCs (47% increase, p < 0.05) than HW. CPCs were positively correlated with abdominal adiposity in OW, and negatively correlated in HW with a significant difference between correlations (p < 0.05). CPC content was positively correlated with executive processes in OW, and negatively correlated in HW with a significant difference in the strength of the correlations between groups (p < 0.05 for correlation between OW and HW). Finally, CPC-CM from OW trended to increase neuroblast viability in vitro relative to HW (1.79 fold, p = 0.07). These novel findings indicate that increased content of CPCs among OW children may play a role in preventing decrements in cognitive function via paracrine mechanisms.
Keywords: Hematopoietic stem/progenitor cells, Inflammation, Executive function, Neurogenesis, Paracrine factor
1. Introduction
The nervous and immune systems have a collaborative relationship where cross talk between these two systems is important for their regulation. The influence of the immune system on cognitive functioning and neurogenesis is becoming better established in both pathological and steady-state conditions (Donzis and Tronson, 2014; Marin and Kipnis, 2013). One mechanism by which the immune system has been shown to influence the brain and cognition is via secretion of cytokines. These effects have primarily been evaluated under pathological, pro-inflammatory conditions, and secretion of pro-inflammatory cytokines, such as interleukins, interferons, and members of the tumor necrosis factor family by immune cells caused a decrease in hippocampal specific tasks (Nemni et al., 1992; Rachal Pugh et al., 2001; Tancredi et al., 1990; Valentine et al., 1998). Additionally, conditions associated with immunological stress and inflammation, such as infection, obesity, or genetic models of immune system dysregulation are also associated with decreased performance on executive functioning tasks (i.e., goal directed processes underlying perception, cognition, and action) and negative effects on the brain (Buckman et al., 2014; Cohen et al., 2006; Kipnis et al., 2008; Dinel et al., 2011; André et al., 2014). Recent work from our group has demonstrated that obese/overweight (OW) children have decreased performance on tasks of executive function compared to healthy weight (HW) controls Kamijo et al., 2012a,b. Additionally, obese adolescent rodents had decreased cognitive performance which was associated with impaired neurogenesis (Boitard et al., 2012). The role of specific immune cell populations on brain and cognition, and how chronic immune system dysregulation alters the relationship between the nervous and immune systems have not be well established.
Although the central nervous system (CNS) was believed to be impervious to peripheral immune cells, recent evidence has questioned this belief. The migration of circulating immune cells into the CNS is exacerbated following acute inflammatory conditions, such a stroke, and is facilitated by expression of the chemokine receptor type 2 (CCR2) Djukic et al., 2006. These findings suggest that under certain conditions, peripheral immune cells can enter the CNS. Immune cells are derived from hematopoietic stem/progenitor cells (HSPCs) primarily located within the bone marrow (King and Goodell, 2011). HSPCs can also be found in very small quantities in circulation, and these circulating progenitor cells (CPCs) Bellows et al., 2011 have been shown to contribute to tissue repair via secretion of paracrine factors (Palermo et al., 2005). Interestingly, inflammatory conditions, such as acute infection and obesity, increase the content of CPCs and enhance myeloid differentiation of HSPCs (Bellows et al., 2011; Liu et al., 2015; Zaretsky et al., 2014). The role of CPCs in the brain and cognition has not previously been explored in the context of overweight/obese children.
In the present report, we evaluate the relationship between CPCs, monocyte populations, executive function, and neurogenesis in healthy weight (HW) and overweight/obese (OW) children using a combined in vivo and in vitro approach. OW children have a compromised immune system (Inzaugarat et al., 2014), decreased performance on tests of executive function (Kamijo et al., 2014; Khan et al., 2015), and decreased hippocampal volume (Bauer et al., 2015; Moreno-López et al., 2012). Thus, comparing HW to OW children allowed us to evaluate the link between immune system dysregulation on cognition. We hypothesized that compared to HW children, OW children would have higher quantities of CPCs and monocyte populations expressing CCR2 in peripheral blood compared to HW, and that CPC and monocyte content would be negatively associated with executive function in OW children. Furthermore, we hypothesized that paracrine factors released from hematopoietic cells isolated from OW children would inhibit neurogenesis in vitro.
2. Methods
2.1. Participants
Twenty-seven preadolescent (age: 8–10-years; 16 HW and 11 OW) male children who previously underwent cognitive testing as part of a larger study (Hillman et al., 2014) participated in the present study. HW and OW were matched for age, fat free VO2 max, and general intelligence (IQ) as determined by Woodcock Johnson III Brief Intellectual Ability tests. Participants provided informed written assent with parental/legal guardian consent to participate in the study and were compensated for their time. This project was approved by the Institutional Review Board of the University of Illinois at Urbana-Champaign.
2.2. Anthropometric measures, body composition, and fitness
Participants were separated into healthy weight (HW) and overweight/obese (OW) based on body mass index (BMI) with those having a BMI of ≥85th percentile of their age-matched peers considered OW (Kuczmarski et al., 2000). Whole body and regional soft tissue composition was measured by dual-energy X-ray absorptiometry (DXA) using a Hologic Discovery A bone densitometer (software version 12.7.3; Hologic, Bedford, MA). DXA measures of interest included whole body fat mass (%) and abdominal fat mass (%). Participants completed a maximal exercise test to assess aerobic fitness on a motorized treadmill using a modified Balke protocol (Armstrong, 2006).
2.3. Whole blood analysis
Participants reported to the lab between the hours of 6:00–10:00AM CST after at least an 8-hour fast. Blood was collected from the antecubital vein into EDTA-anticoagulant tubes. Plasma was aliquoted after Ficoll-Paque separation on EDTA-collected blood prior to isolation of peripheral blood mononuclear cells (PBMCs) used for CD34+ isolation by flow sorting. Lineage negative PBMCs were magnetically separated, incubated with PE-conjugated CD34 antibody (1:100 dilution), and sorted using either an iCyt Reflection flow sorter (iCyt; Champaign, IL) or a FACS ARIA II fluo-rescence activated sorter (BD; Franklin Lakes, NJ). All isolated CPCs from each participant were plated in 250 lL of Serum Free Expansion Media (StemCell Technologies; Vancouver, Canada) for 3 h. The average concentration of plated cells was 25,000 cells/well. Conditioned media was collected and frozen at −80°C until further analysis.
Quantification of CPCs and monocytes with and without CCR2 expression was conducted from whole blood (200 μL/population) collected in EDTA tubes. Samples were incubated with PE conjugated CD34 (1:40; Invitrogen; Grand Island, NY), FITC conjugated CD45 (1:200; Invitrogen; Grand Island, NY), PE conjugated CD14 (1:100; Invitrogen; Grand Island, NY), FITC conjugated CD16 (1:100; Invitrogen; Grand Island, NY), or CCR2 (CD192; 1:100; Biolegend; San Diego, CA). Gating for CPCs was conducted as previously described (Bellows et al., 2011). Flow cytometric analysis was performed within 3 h of blood collection using an Attune Focusing Flow Cytometer (Life Technologies; San Diego, CA).
2.4. Cognitive tasks
A subset of participants completed a subtest of the Woodcock-Johnson III Tests of Executive Processing (WJ III) to assess cognitive performance as described previously (McGrew and Woodcock, 2001) as part of a larger on-going study (Hillman et al., 2014). The WJ III test of Executive Processing is a test of fluid reasoning and decision-making, which involves making logical and novel decisions (Ferrer et al., 2009; Taub and McGrew, 2004) that requires input from a variety of brain regions (Diamond, 2013).
2.5. Neuroblastoma cell culture and conditioned media experiments
SH-SY5Y cells (CRL-2266; ATCC; Manassas, VA) were plated in growth media at 50,000 cells/well for 24 h prior to addition of conditioned media (CM). CM was derived from sorted CPCs as described above. All CM derived from CPCs sorted from healthy weight participants was pooled (HW-CM), while all CM derived from CPCs sorted from overweight/obese participants was pooled (OW-CM). To ensure that differences in CM experiments were not due to different numbers of CPCs contributing to CM, we added serum-free media to the pooled samples to equalize the number of input CPCs to CM between weight classes. In this way, the concentration (plated CPCs/mL of CM) was equal between HW-CM and OW-CM. As such, we could compare differences between groups caused by alterations in the secretome on an individual cell basis rather than a population or cell quantity basis. Neuroblastomas were then incubated with either CM from CD34+ cells isolated from OW or HW, or plasma from OW or HW for 24 h prior to viability analysis.
2.6. MTT assay
Neuroblastoma viability was assessed after 24 h in CM via MTT Assay (Sigma Aldrich; St. Louis, MO) as previously described (De Lisio et al., 2014) with minor modifications to account for partially adherent, partially floating growth of neuroblastomas. Briefly, after addition and incubation of MTT solution, media was centrifuged to collect floating cells. Half the volume of MTT solvent was added to the adhered cells, and the other half of the solvent was used to resuspend the centrifuged floating cells, which were then added back into the original well for analysis.
2.7. Statistical analyses
Group differences were analyzed by t-test in Microsoft Excel. Correlations were conducted in SPSS software (IBM Statistics: Version 22; Armonk, NY) using Spearman’s rho nonparametric analysis. Partial correlations were conducted controlling for abdominal body fat between CPCs and cognitive task standard scores in HW and OW groups using Spearman’s Rho nonparametric analysis. Comparisons between within group correlations were conducted by comparing Spearman’s Rho values using a Fisher’s R to Z transformation and converting the correlation coefficient into a Z-score for comparison. Data are presented as mean ± SEM with p < 0.05 considered significant.
3. Results
3.1. OW children have greater content of circulating CCR2-expressing inflammatory monocytes and CPCs compared to HW
HW and OW did not differ in age (HW: 8.7 ± 0.8 years; OW: 9 ± 0.8 years), fat free VO2 max (HW: 62.8 ± 9.8 ml/kgLBM/min; OW: 62.1 ± 7.9 ml/kgLBM/min), general intelligence (HW: 118.8 ± 12.1; OW: 106.7 ± 118.5). OW children had significantly higher BMI (HW: 16.2 ± 1.2 kg/m Marin and Kipnis, 2013; OW:24.00 ± 4.6 kg/m2; p < 0.05), BMI percentile (HW: 51 ± 22.7; OW:95.7 ± 3.7; p < 0.05), body fat percentage (HW: 24.8 ± 3.2%; OW:39.00 ± 5.9%; p < 0.001), and abdominal fat percentage (HW:17.4 ± 2.2%; OW: 32.9 ± 8.3%; p < 0.01). The quantity of classical (CD14+/CD16−) Shantsila et al., 2011 and non-classical (CD14+/CD16+) Ziegler-Heitbrock, 2007 monocytes in the OW group was significantly higher than that of HW (CD14+/CD16−: HW: 9128 ± 519 cells/mL vs. OW: 14351 ± 1831 cells/mL; p < 0.01; Fig. 1A and CD14+/CD16+: HW: 1327 ± 172 cells/mL vs. OW: 2598 ± 462 cells/mL; p < 0.01, Fig. 1B). The quantity of classical and non-classical monocytes expressing CCR2 (CD14+/CD16+/−/CCR2+), a phenotypic marker associated with increased migratory capacity (Krinninger et al., 2014) in OW children was significantly higher than HW (CD14+/CD16−/CCR2+: HW: 8382 ± 626 cells/mL vs. OW: 13947 ± 1838 cells/mL; p < 0.01, Fig. 1C; CD14+/CD16+/CCR2+: HW: 496 ± 101 cells/mL vs. OW: 1331 ± 300 cells/mL; P < 0.01, Fig. 1D, respectively).
Fig. 1.
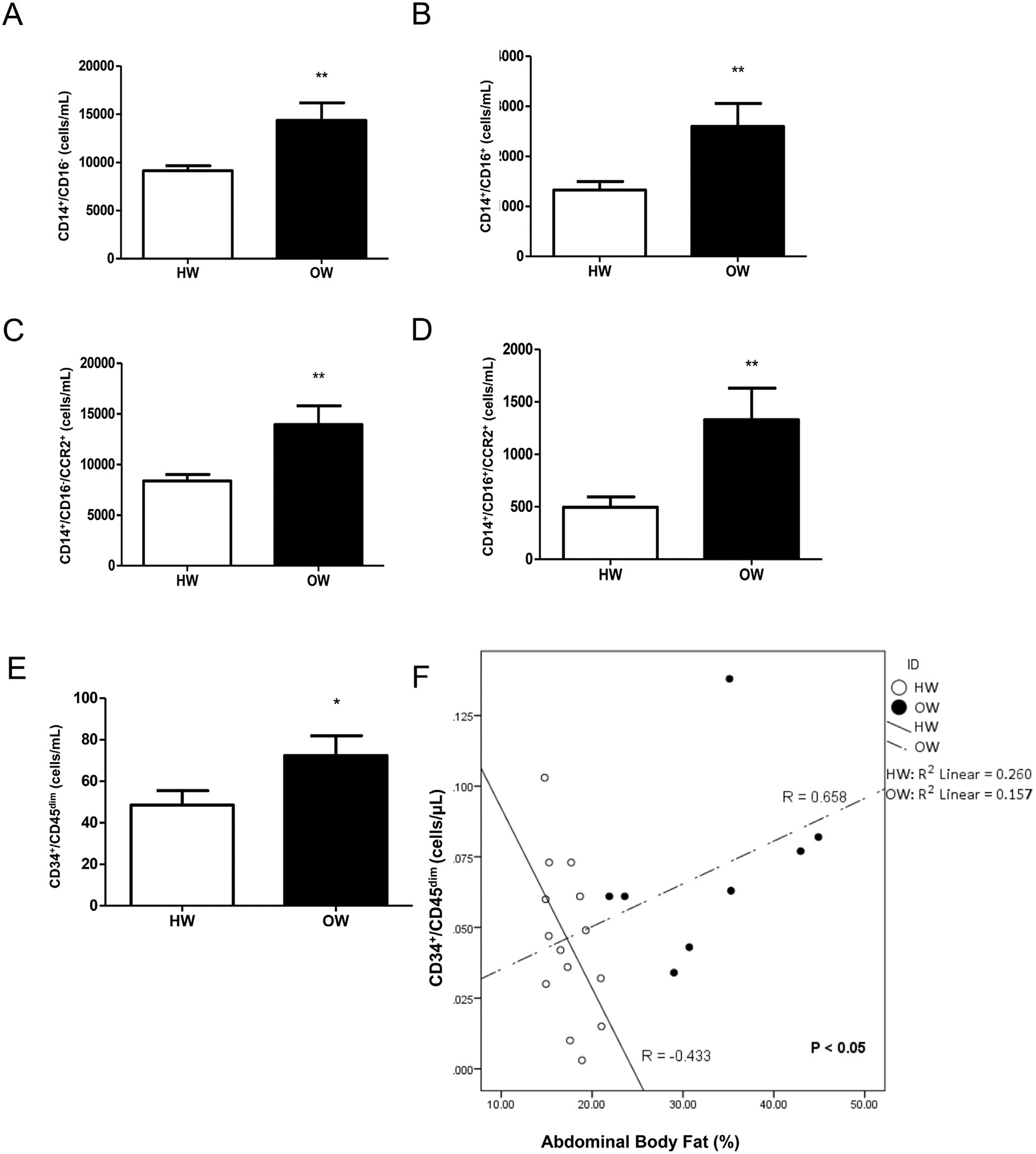
Inflammatory monocyte content and phenotype in HW and OW. Concentrations of classical (A) and non-classical monocytes (B) with and without expression of CCR2 (D and D), and CPCs (E) in the healthy weight (HW) and overweight/obese children (OW); n = 16 HW and n = 11 OW for figures (A–E). Spearman’s Rho correlations (F) between CPCs and abdominal body fat percentage in HW and OW; n = 14 HW and n = 8 OW. Data are presented as mean ± SEM, from, with ** indicating p < 0.01
The quantity of CPCs, a heterogeneous population of circulating stem/progenitor cells that include hematopoietic and endothelial progenitors (Bellows et al., 2011) in peripheral blood, was significantly elevated in OW compared to HW (HW: 49 ± 7 cells/mL vs. OW: 72 ± 9 cells/mL; p < 0.05, Fig. 1E) and showed differential relationships with abdominal body fat% between HW and OW. CPC quantity in HW was negatively correlated with abdominal body fat% (Spearman’s Rho = 0.658), while CPC quantity in OW was positively correlated with abdominal body fat% (Spearman’s Rho = −0.433), with a significant difference in correlation coefficients between groups (p < 0.05; Fig. 1F).
3.2. Differential relationship between CPCs and executive processes between HW and OW children with differential paracrine effects on neuroblast viability
In the present investigation, scores on the WJ III Executive Processing task did not differ between OW and HW participants (OW: 115 ± 9.74; HW: 108.14 ± 14.56; data not shown). Since our primary interest was to compare the relationship between CPCs and executive processing in OW and HW, we performed Spearman’s Rho correlation analyses between circulating inflammatory cell populations and the WJ III Executive Processing task then compared the correlations between the two populations. No differences were found in the correlations between circulating classical and non-classical monocytes, or monocytes expressing CCR2 and executive processing tasks between HW and OW (data not shown). Conversely, for the WJ III Executive Processing Cluster, which is an amalgamation of the 3 tasks in the cluster, we observed a negative correlation (Spearman’s Rho = −0.462) in HW, while OW had a positive correlation (Spearman’s Rho = 0.571) with a significant difference between the two correlations (P < 0.05 for correlations between HW and OW; Fig. 2A). When we examined the 3 separate tasks that combine to make up the Executive Processing Cluster, we observed the same trend in relationships between HW and OW (data not shown). Since we observed significant differences in the correlations between CPC quantity and executive function as well as CPC quantity and abdominal adiposity between OW and HW, we were interested in evaluating the extent to which abdominal adiposity influenced the relationship between CPCs and executive function scores. Thus, we performed partial correlations on these three outcomes accounting for abdominal adiposity in both weight groups. The significantly different correlations between CPCs and executive processing between HW and OW children was no longer present when abdominal adiposity was accounted for (data not shown). Therefore, these data suggest that abdominal adiposity may have a role in the relationship between CPCs and executive function in OW children.
Fig. 2.
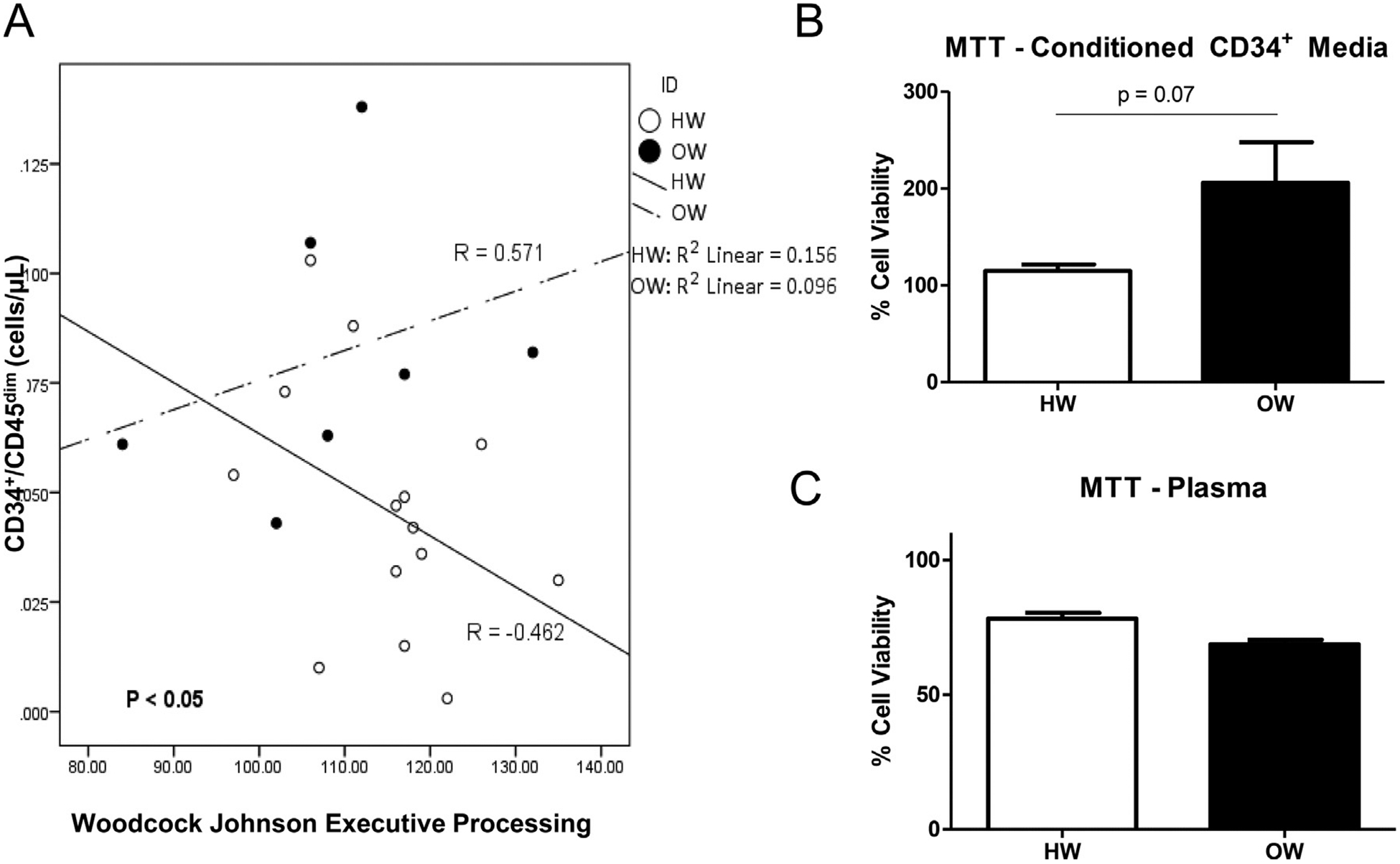
Relationships between CPCs and Executive Processes in HW and OW and CPC conditioned media on neuroblast viability. Spearman’s Rho correlations between CPC concentrations and Woodcock Johnson Tests of Executive Processes, specifically executive processing (A), n = 14 for HW and n = 7 for OW. Percent cell viability of SH-SY5Y neuroblastomas treated with conditioned media from CPCs (B) or plasma (C) collected from healthy weight (HW) and overweight/obese (OW) children. For both experiments, cell viability is expressed at percent difference from neuroblasts grown in neuroblast growth media. Serum free growth media was used as a negative control. N = 6 for HW and n = 6 for OW, with ** indicating p < 0.05.
To examine if paracrine factors secreted from CPCs influenced the growth of neuroblasts in vitro, we performed conditioned media (CM) experiments where neuroblasts were treated with CM from CPCs isolated from HW or OW children. We observed a strong trend for an increase in neuroblast viability when they were treated with CM from CPCs isolated from OW versus HW (1.79 fold increase; p = 0.07, Fig. 2B). To determine if factors released from CPCs specifically, or if the general systemic milieu was promoting the increase in neuroblast viability, we repeated the CM experiments described above, but substituted plasma from OW and HW children for CPC-CM. No difference in viability was seen between neuroblasts treated with plasma from HW or OW (Fig. 2C).
4. Discussion
Developing a better understanding of the potential relationship between immune system and cognition in OW children is necessary given the increasing prevalence of overweight/obesity and children, and because childhood is a time of rapid brain and cognitive development. The main findings from our study are that: (1) the content of CPCs, monocytes, and monocytes expressing CCR2 were all significantly elevated in OW children, (2) abdominal adiposity is differentially related to CPC content in HW and OW preadolescents, (3) the relationship between CPC quantity and WJ III executive was significantly different in HW and OW, and (4) paracrine factors released from CPCs isolated from OW children trended to increase neuroblast viability to a greater extent than paracrine factors released from CPCs isolated from HW children. Contrary to our initial hypothesis, these data suggest that increased CPC quantity in OW children may have a role in combating the negative cognitive effects associated with overweight/obesity.
Consistent with previous investigations in obese adults (Krinninger et al., 2014; Rogacev et al., 2010), and obese children (Breslin et al., 2012; Schipper et al., 2012), we observed a higher amount of circulating monocyte content in our OW population compared to HW. A novel aspect of the present study is the additional evaluation of co-expression of CCR2 on monocyte populations. CCR2 is involved in cell migration to sites of inflammation via interaction with its ligand, chemokine ligand 2 (CCL2; also known as monocyte chemoattractant protein [MCP]-1) (Shantsila et al., 2011; Krinninger et al., 2014). We observed a significant elevation in the quantity of both classical and non-classical monocytes expressing CCR2 in OW. Thus, not only did we observe a higher content of total monocytes in OW, but a higher amount of the proportion of monocytes that express markers associated with homing to inflamed tissues compared to HW. Previous studies have examined the relationships between cognition and CD16+ monocytes in individuals with HIV-associated cognitive decline (Williams et al., 2012; Vehmas et al., 2004; Shikuma et al., 2012), older adults with Alzheimer’s disease and/or dementia (Saresella et al., 2014), and natural cognitive decline associated with aging (Freeman et al., 2014; McAfoose and Baune, 2009). The present study is the first to compare the relationship between inflammatory monocytes and executive function between OW and HW children. The within group correlations in monocytes and executive function were not different between OW and HW in our study. These findings suggest that the relationship between monocytes and cognition is present independent of BMI.
The present investigation is the first to demonstrate that CPC content is greater in overweight/obese children. Furthermore, our data show that CPC content is differentially related to abdominal adiposity in HW and OW. These data in children are consistent with previous findings showing a positive correlation between CPC quantity and BMI in adults (Bellows et al., 2011). The physiological role of CPCs has not been fully elucidated, but they have been suggested to be involved in tissue repair (Palermo et al., 2005; Mocco et al., 2014). To examine if CPCs had a differential relationship to cognitive function in OW children who have previously been shown to have decreased performance on executive control tasks and academic achievement tests in OW compared to HW children (Kamijo et al., 2012a,b), we compared the relationship between CPC content and performance on the WJ III test of Executive Processing in OW and HW. We observed a significant difference in the relationship between CPC content and executive function between OW and HW children with CPC content being positively correlated to performance in OW, and negatively correlated to performance in HW. This differential relationship was no longer present when abdominal adiposity was accounted for. This observation suggests that specific aspects associated with abdominal adipose tissue may underlie this relationship. We hypothesized that differences in the CPC secretome may underlie the differential relationship between CPCs and cognition in OW and HW preadolescents. Previous in vitro experiments have shown that certain cytokines, such as IL-6 and INF-γ, inhibit neuroblast proliferation but enhance neurogenesis, while others, such as TNF-α and IL-10, increase neuroblast proliferation and inhibit neurogenesis (Borsini et al., 2015). Furthermore, animal studies have shown that in response to inflammatory stimuli, cytokine secretion by CPCs exceeds that of mature immune cells (Zhao et al., 2014). In particular, LPS-stimulated CPCs have been shown to secrete high levels of TNF-α, IL-6, and IL-10 (Zhao et al., 2014), which are also active regulators of neuronal progenitors in vitro (Borsini et al., 2015). Although it was not possible to evaluate cytokine production or secretion from isolated CPCs in the present investigation, our in vitro data suggest that the secretome of CPCs from OW was more favorable for neuroblast viability compared to the secretome of HW. Furthermore, although these in vitro studies are informative for determining mechanisms, their direct relevance to in vivo conditions with a complex cellular and soluble factor milieu within the neurogenic niche is debatable.
In conclusion, the data presented herein suggest that increased CPC content in OW children may play a role in maintaining cognitive performance. Future studies will be necessary to determine if CPCs can enter the central nervous system in OW children, and the precise milieu of paracrine factors secreted by CPCs. CPC content (King and Goodell, 2011; Liu et al., 2015), migration (Palermo et al., 2005; Mocco et al., 2014), and cytokine secretion (Zhao et al., 2014) has been shown to increase in response to acute inflammatory stimuli. Furthermore, the blood brain barrier is more permeable in older obese mice (Tucsek et al., 2014), and immune cells increase migration into the CNS in acute inflammatory conditions (Borlongan et al., 2012). These data combined with data from the present study suggest that CPCs may be an important cell population with a role in cognitive function and brain development in OW children.
Acknowledgments
We thank Dr. Barbara Pilas and Angela Kouris at the Roy J. Carver biotechnology center at the University of Illinois at Urbana-Champaign, and Dr. Tor Jensen at the Carle Foundation Hospital for technical assistance with flow sorting, as well as Dr. Lara Pilutti and Dr. Sa Shen for consultation on statistical analyses. Funding was provided by Centers for Health, Aging, and Disability, UIUC and from the National Institutes of Health (HD 069381). LaurenB. Raine was supported by the National Institute for Agriculture under the Illinois Transdisciplinary Obesity Prevention Program grant (2011-67001-30101) to the Division of Nutritional Sciences at the University of Illinois.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- André C, Dinel A-L, Ferreira G, Layé S, Castanon N, 2014. Diet-induced obesity progressively alters cognition, anxiety-like behavior and lipopolysaccharide-induced depressive-like behavior: focus on brain indoleamine 2,3-dioxygenase activation. Brain. Behav. Immun 41, 10–21. [DOI] [PubMed] [Google Scholar]
- Armstrong L, 2006. ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins. [Google Scholar]
- Bauer CCC et al. , 2015. Child overweight and obesity are associated with reduced executive cognitive performance and brain alterations: a magnetic resonance imaging study in Mexican children. Pediatr. Obes 10, 196–204. [DOI] [PubMed] [Google Scholar]
- Bellows CF, Zhang Y, Simmons PJ, Khalsa AS, Kolonin MG, 2011. Influence of BMI on level of circulating progenitor cells. Obesity 19, 1722–1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C et al. , 2012. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 22, 2095–2100. [DOI] [PubMed] [Google Scholar]
- Borlongan CV, Glover LE, Sanberg PR, Hess DC, 2012. Permeating the blood brain barrier and abrogating the inflammation in stroke: implications for stroke therapy. Curr. Pharm. Des 18, 3670–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsini A, Zunszain PA, Thuret S, Pariante CM, 2015. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 38, 145–157. [DOI] [PubMed] [Google Scholar]
- Breslin WL et al. , 2012. Obese Mexican American children have elevated MCP-1, TNF-α, monocyte concentration, and dyslipidemia. Pediatrics 129, e1180–e1186. [DOI] [PubMed] [Google Scholar]
- Buckman LB et al. , 2014. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain. Behav. Immun 35, 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H et al. , 2006. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. J. Neurobiol 66, 552–563. [DOI] [PubMed] [Google Scholar]
- De Lisio M, Jensen T, Sukiennik RA, Huntsman HD, Boppart MD, 2014. Substrate and strain alter the muscle-derived mesenchymal stem cell secretome to promote myogenesis. Stem Cell Res. Ther 5, 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, 2013. Executive functions. Annu. Rev. Psychol 64, 135–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinel A-L et al. , 2011. Cognitive and emotional alterations are related to hippocampal inflammation in a mouse model of metabolic syndrome. PLoS One 6, e24325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djukic M et al. , 2006. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain 129, 2394–2403. [DOI] [PubMed] [Google Scholar]
- Donzis EJ, Tronson NC, 2014. Modulation of learning and memory by cytokines: signaling mechanisms and long term consequences. Neurobiol. Learn. Mem, 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer E, O’Hare ED, Bunge SA, 2009. Fluid reasoning and the developing brain. Front. Neurosci 3, 46–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LR, Haley-Zitlin V, Rosenberger DS, Granholm A-C, 2014. Damaging effects of a high-fat diet to the brain and cognition: a review of proposed mechanisms. Nutr. Neurosci 17, 241–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH et al. , 2014. Effects of the FITKids randomized controlled trial on executive control and brain function. Pediatrics 134, e1063–e1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzaugarat ME et al. , 2014. Alterations in innate and adaptive immune leukocytes are involved in paediatric obesity. Pediatr. Obes 9, 381–390. [DOI] [PubMed] [Google Scholar]
- Kamijo K et al. , 2012a. The association of childhood obesity to neuroelectric indices of inhibition. Psychophysiology 49, 1361–1371. [DOI] [PubMed] [Google Scholar]
- Kamijo K et al. , 2012b. The relation of adiposity to cognitive control and scholastic achievement in preadolescent children. Obesity 20, 2406–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo K et al. , 2014. The negative association of childhood obesity to cognitive control of action monitoring. Cereb. Cortex 24, 654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan NA et al. , 2015. Central adiposity is negatively associated with hippocampal-dependent relational memory among overweight and obese children. J. Pediatr 166, 302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KY, Goodell MA, 2011. Inflammatory modulation of HSCs: viewing the HSC as a foundation for the immune response. Nat. Rev. Immunol 11, 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Derecki NC, Yang C, Scrable H, 2008. Immunity and cognition: what do age-related dementia, HIV-dementia and ‘chemo-brain’ have in common? Trends Immunol. 29, 455–463. [DOI] [PubMed] [Google Scholar]
- Krinninger P et al. , 2014. Peripheral monocytes of obese women display increased chemokine receptor expression and migration capacity. J. Clin. Endocrinol. Metab 99, 2500–2509. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ et al. , 2000. CDC growth charts: United States. Adv. Data 1–27. [PubMed] [Google Scholar]
- Liu A et al. , 2015. Cutting edge: hematopoietic stem cell expansion and common lymphoid progenitor depletion require hematopoietic-derived, cell-autonomous TLR4 in a model of chronic endotoxin. J. Immunol. Baltim. Md 1950 (195), 2524–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin I, Kipnis J, 2013. Learning and memory … and the immune system. Learn. Mem 20, 601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfoose J, Baune BT, 2009. Evidence for a cytokine model of cognitive function.Neurosci. Biobehav. Rev 33, 355–366. [DOI] [PubMed] [Google Scholar]
- McGrew KS, Woodcock RW, 2001. Woodcock-Johnson III Technical Manual. (Riverside Pub.). [Google Scholar]
- Mocco J et al. , 2014. SDF1-A facilitates Lin−/Sca1+ cell homing following murine experimental cerebral ischemia. PLoS One 9, e85615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-López L, Soriano-Mas C, Delgado-Rico E, Rio-Valle JS, Verdejo-García A, 2012. Brain structural correlates of reward sensitivity and impulsivity in adolescents with normal and excess weight. PLoS One 7, e49185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemni R et al. , 1992. Effect of chronic treatment with recombinant interleukin-2 on the central nervous system of adult and old mice. Brain Res. 591, 248–252. [DOI] [PubMed] [Google Scholar]
- Palermo AT, LaBarge MA, Doyonnas R, Pomerantz J, Blau HM, 2005. Bone marrow contribution to skeletal muscle: a physiological response to stress. Dev. Biol 279, 336–344. [DOI] [PubMed] [Google Scholar]
- Rachal Pugh C, Fleshner M, Watkins LR, Maier SF, Rudy JW, 2001. The immune system and memory consolidation: a role for the cytokine IL-1b. Neurosci. Biobehav. Rev 25, 29–41. [DOI] [PubMed] [Google Scholar]
- Rogacev KS et al. , 2010. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur. Heart J 31, 369–376. [DOI] [PubMed] [Google Scholar]
- Saresella M et al. , 2014. A complex proinflammatory role for peripheral monocytes in Alzheimer’s disease. J. Alzheimers Dis. JAD 38, 403–413. [DOI] [PubMed] [Google Scholar]
- Schipper HS et al. , 2012. Systemic inflammation in childhood obesity: circulating inflammatory mediators and activated CD14++ monocytes. Diabetologia 55, 2800–2810. [DOI] [PubMed] [Google Scholar]
- Shantsila E et al. , 2011. Immunophenotypic characterization of human monocyte subsets: possible implications for cardiovascular disease pathophysiology: characterization of human monocyte subsets. J. Thromb. Haemost 9, 1056–1066. [DOI] [PubMed] [Google Scholar]
- Shikuma CM et al. , 2012. Antiretroviral monocyte efficacy score linked to cognitive impairment in HIV. Antivir. Ther 17, 1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancredi V, Zona C, Velotti F, Eusebi F, Santoni A, 1990. Interleukin-2 suppresses established long-term potentiation and inhibits its induction in the rat hippocampus. Brain Res. 525, 149–151. [DOI] [PubMed] [Google Scholar]
- Taub GE, McGrew KS, 2004. A confirmatory factor analysis of Cattell-Horn-Carroll theory and cross-age invariance of the woodcock-johnson tests of cognitive abilities III. Sch. Psychol. Q 19, 72–87. [Google Scholar]
- Tucsek Z et al. , 2014. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampus: effects on expression of genes involved in beta-amyloid generation and Alzheimer’s disease. J. Gerontol. A. Biol. Sci. Med. Sci 69, 1212–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine AD, Meyers CA, Kling MA, Richelson E, Hauser P, 1998. Mood and cognitive side effects of interferon-alpha therapy. Semin. Oncol 25, 39–47. [PubMed] [Google Scholar]
- Vehmas A, Lieu J, Pardo CA, McArthur JC, Gartner S, 2004. Amyloid precursor protein expression in circulating monocytes and brain macrophages from patients with HIV-associated cognitive impairment. J. Neuroimmunol 157, 99–110. [DOI] [PubMed] [Google Scholar]
- Williams DW, Eugenin EA, Calderon TM, Berman JW, 2012. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. J. Leukoc. Biol 91, 401–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaretsky AG, Engiles JB, Hunter CA, 2014. Infection-induced changes in hematopoiesis. J. Immunol 192, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JL et al. , 2014. Conversion of danger signals into cytokine signals by hematopoietic stem and progenitor cells for regulation of stress-induced hematopoiesis. Cell Stem Cell 14, 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, 2007. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J. Leukoc. Biol 81, 584–592. [DOI] [PubMed] [Google Scholar]


