Abstract
Caused by a novel type of virus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), coronavirus disease 2019 (COVID‐19) constitutes a global public health emergency. Pregnant women are considered to have a higher risk of severe morbidity and even mortality due to their susceptibility to respiratory pathogens and their particular immunologic state. Several studies assessing SARS‐CoV‐2 infection during pregnancy reported adverse pregnancy outcomes in patients with severe conditions, including spontaneous abortion, preterm labor, fetal distress, cesarean section, preterm birth, neonatal asphyxia, neonatal pneumonia, stillbirth, and neonatal death. However, whether these complications are causally related to SARS‐CoV‐2 infection is not clear. Here, we reviewed the scientific evidence supporting the contributing role of Treg/Th17 cell imbalance in the uncontrolled systemic inflammation characterizing severe cases of COVID‐19. Based on the recognized harmful effects of these CD4+ T‐cell subset imbalances in pregnancy, we speculated that SARS‐CoV‐2 infection might lead to adverse pregnancy outcomes through the deregulation of otherwise tightly regulated Treg/Th17 ratios, and to subsequent uncontrolled systemic inflammation. Moreover, we discuss the possibility of vertical transmission of COVID‐19 from infected mothers to their infants, which could also explain adverse perinatal outcomes. Rigorous monitoring of pregnancies and appropriate measures should be taken to prevent and treat early eventual maternal and perinatal complications.
Keywords: COVID‐19, pregnancy outcomes, SARS‐CoV‐2, systemic inflammation, Th17 cells, Treg cells
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a pneumonia pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 First identified in December 2019 in an outbreak of pneumonia in Wuhan City (Hubei Province, China), COVID‐19 currently affects over 210 countries and territories worldwide. 2 The World Health Organization (WHO) declared COVID‐19 to be a Public Health Emergency of International Concern, 3 then a pandemic, on March 11, 2020. 4 By June 30, 2020, there had been over 10 million confirmed cases, including over 500.000 patient deaths. 2
The majority of COVID‐19 patients manifest mild to moderate symptoms; approximately 15% progress to the severe form of the disease (pneumonia); and approximately 5% eventually develop acute respiratory distress syndrome (ARDS), or multiple organ failure. 5 Elderly patients and those with comorbidities (hypertension, diabetes, cardiovascular disease, and cerebrovascular disease) are at risk of developing the severe form of COVID‐19 and have a high mortality rate. 6 , 7 Due to their singular immune characteristics and susceptibility to respiratory pathogens, pregnant women infected with SARS‐CoV‐2 should be considered to present a higher risk for severe morbidity and even mortality. 8
Numerous studies have reviewed COVID‐19 in pregnant women and reported adverse pregnancy outcomes among these patients. 9 , 10 , 11 , 12 Using four databases (Medline, Web of Science, Scopus, and CINAHL) to search for relevant studies published as at March 25, 2020, Khan et al 9 selected nine studies (involving 101 infected pregnant women) that were summarized employing a narrative synthesis approach. Yan et al 10 evaluated the clinical characteristics and outcomes of pregnancy in 116 pregnant women with COVID‐19 from 25 hospitals in China within and outside of Hubei province between January 20, 2020, and March 24, 2020 In Wuhan, as at March 20, 2020, Chen et al 11 identified 118 infected pregnant women (92% with mild and 8% with severe disease) in 50 COVID‐19‐designated hospitals. Gajbhiye et al 12 reviewed 23 studies (involving 172 pregnant women and 162 neonates), including 20 from China and one each from the USA, Republic of Korea, and Honduras.
The maternal adverse pregnancy outcomes reported in these studies included spontaneous abortion, 10 , 11 premature rupture of membranes, 10 , 12 preterm labor, 9 , 10 , 11 , 12 fetal distress, 12 and cesarean section. 9 , 10 , 11 , 12 Among the neonates of COVID‐19 mothers, preterm birth, 9 , 10 , 11 , 12 neonatal asphyxia, 10 , 12 pneumonia, 9 , 12 low birth weight, 9 , 12 stillbirth, and neonatal death 12 have been reported. These adverse pregnancy outcomes can be attributed to the progression of the disease toward the severe stage (ARDS, septic shock, and multiple organ failure) or are linked to the challenge of treating pregnant women considering the effects of certain therapeutic protocols on the fetus. However, whether these outcomes are causally related to the effects of SARS‐CoV‐2 infection during pregnancy requires further clarification.
In this review, we show that the uncontrolled systemic inflammatory state characterizing COVID‐19 involves an increased number of Th17 cells, and a decrease in Treg cell levels, which could contribute to the occurrence of adverse pregnancy outcomes observed in infected pregnant women.
2. IMMUNE PATHOGENESIS OF SARS‐COV‐2 INFECTION
SARS‐CoV‐2 is a positive‐sense single‐stranded RNA virus (betacoronavirus genus) in the same subgenus as the severe acute respiratory syndrome (SARS) virus, but in a different clade. 13 This novel virus likely originated in chrysanthemum bats; the pangolin is reported to be an intermediate host between bats and humans; and person‐to‐person viral transmission is thought to occur mainly via respiratory droplets. 14 The incubation time varies from 2 to 14 days after infection. Clinically, SARS‐CoV‐2 infection can be asymptomatic or can result in mild to severe symptomatic disease. 15 Both innate and adaptive immune cells synergistically participate in anti‐viral responses during SARS‐CoV‐2 infection. 16
The virus uses a spike glycoprotein (S) protein to bind its receptor (angiotensin‐converting enzyme 2 (ACE2) receptor) on target cells. 17 The virion then enters the host cells (endocytosis) and releases its RNA genome, which constitutes a pathogen‐associated molecular pattern (PAMP). Inside host cells (immune or non‐immune cells), the viral RNA (PAMP) is recognized by pathogen recognition receptors (PRRs), which collect the specific signal adapter protein and activate IRF3 and IRF7 before being translocated to the nucleus to promote the synthesis of type I interferons (IFNs). 17 In infected innate immune cells (macrophages and dendritic cells (DCs)), type I IFNs lead to an increase in antigen presentation activity and production of immune response mediators, such as cytokines and chemokines (Figure 1A). 18 Antigen presentation to T cells induces T‐cell activation and differentiation, including the production of cytokines associated with different T‐cell subsets (such as Th17), followed by a massive release of cytokines for immune response amplification (Figure 1B). 19 In adaptive immunity, type I IFNs may also act by increasing antibody production by B cells (IgM and IgG) and amplify the effector function of T cells. 18 Type I IFNs produced by infected non‐immune cells (fibroblasts and epithelial cells) induce an intracellular antimicrobial program that limits the spread of infectious agents in infected and neighboring cells. 18
FIGURE 1.
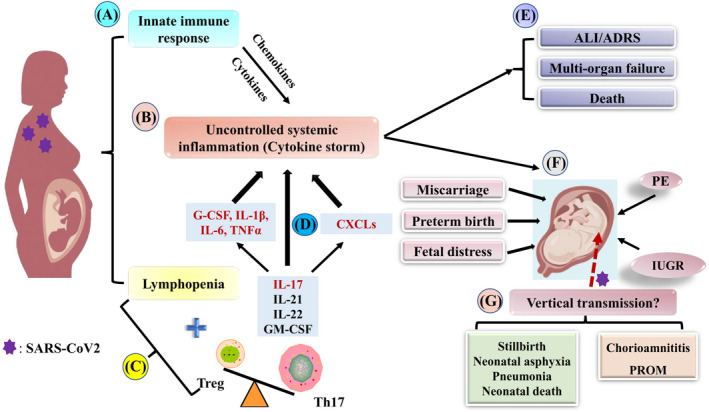
Treg/Th17 cell imbalance and related outcomes in severe COVID‐19 pregnant women A, Infected innate immune cells (macrophages and dendritic cells) produce type I interferons (IFNs). Type I IFNs lead to an increase in antigen presentation activity and the production of cytokines and chemokines to ensure pathogen clearance and help to mobilize adaptive immune responses. B, In the case of prolonged inflammation, continual activation and recruitment of effector cells might establish a feedback loop that perpetuates inflammation and results in the release of large amounts of pro‐inflammatory cytokines and chemokines by immune effector cells (cytokine storm). C, In this severe condition, adaptive immune cells, particularly T cells, are impaired. B cells, T cells, and NK cell numbers are significantly decreased (lymphopenia). Although the lymphopenia also involves CD4+ T cells, the proportion of Treg cells and Th17 cells is affected by SARS‐CoV‐2 in a different way. Indeed, the increased circulating IL‐6 levels induce the differentiation of naïve CD4+ T cells toward Th17 cells, while inhibiting Treg cells, leading to Treg/Th17 ratio imbalance. D, IL‐17 released by Th17 cells activates other downstream cascades involving cytokines (G‐CSF, IL‐1β, IL‐6, TNF‐α) and chemokines (CXCLs), followed by the recruitment of more effector cells and massive release of inflammatory cytokines that amplify the uncontrolled systemic inflammation (cytokine storm). E, Uncontrolled systemic inflammation might provoke acute respiratory distress syndrome (ARDS), multiorgan failure, and death. F, Uncontrolled systemic inflammation might also cause adverse pregnancy outcomes (miscarriage, preterm birth, fetal distress, preeclampsia, and intrauterine growth restriction). G, In the case of vertical transmission of SARS‐CoV‐2, anticipated complications might involve infection of the placenta (chorioamnititis, premature membrane rupture), and adverse neonatal outcomes, such as stillbirth, neonatal asphyxia, pneumonia, and neonatal death
Pulmonary and systemic inflammatory responses associated with COVID‐19 are often triggered by the innate immune system when it recognizes the viruses. 20 Depending on the general health and genetic background (HLA) of patients, 21 this immune response promotes virus clearance, inhibits viral replication, induces tissue repair, and triggers a prolonged adaptive immune response against the infectious agent. 20
2.1. Uncontrolled systemic inflammation (cytokine storm)
When patients are not in good general health, or when they are susceptible to infections (unfavorable genetic background), protective immune responses can be impaired. 21 The virus will propagate, and massive destruction of the affected tissues will occur, especially in organs that have high ACE2 expression, such as the lungs, intestines, and kidneys. Lung inflammation is the leading cause of life‐threatening respiratory disorders when at a severe stage of disease. 22 Indeed, prior reports show that at the critical stage of COVID‐19, ARDS is the main cause of death. 23 ARDS is mostly provoked by cytokine storms (CSs), which occur in viral infections when a large number of cytokines are produced. A deadly uncontrolled systemic inflammatory response results from the release by immune effector cells of large amounts of pro‐inflammatory cytokines (IFN‐α, IFN‐γ, IL‐1β, IL‐6, IL‐12, IL‐17, IL‐18, IL‐33, TNF‐α, TGFβ, etc) and chemokines (CCL2, CCL3, CCL5, CXCL8, CXCL9, CXCL10, etc) (Figure 1B). 23 CSs can worsen the condition of patients and cause multiorgan failure, which is frequently fatal (Figure 1E).
2.2. Impaired adaptive immune responses in COVID‐19 patients
During SARS‐CoV‐2 infection, dysregulation of the immune system and involving T cells has been reported. 24 Differences in blood cell counts are observed between severe and non‐severe groups. Compared with non‐severe patients, most severe cases of COVID‐19 have lower percentages of monocytes, eosinophils, and basophils. 24 , 25 Moreover, they exhibit lower lymphocyte counts (lymphopenia) (Figure 1C), higher leukocyte and neutrophil numbers, and, consequently, higher neutrophil‐to‐lymphocyte ratio (NLR). 24 , 25 Lymphopenia in COVID‐19 patients has been reported in several other studies. 22 , 26 , 27 , 28 , 29 , 30 B cells, T cells, and NK cell numbers are significantly decreased in patients with COVID‐19, and this is more evident in severe cases. 22 , 24 , 31 , 32 However, T‐cell numbers are more affected than other cell types. 24 Analyzing different T‐cell subsets, Qin et al 24 found that both suppressor T cells (CD3+CD8+) and helper T cells (CD3+ CD4+) were below normal levels. In addition, the percentage of naïve helper T cells (CD3+CD4+CD45RA+) increased, while memory helper T cells (CD3+CD4+CD45RO+) decreased in severe cases, indicating the severity of immune system impairment. Although the number of T cells decreased, their functions remained within the normal range 24 or were even hyperactivated, as evidenced by the high proportion of HLA‐DR (CD4) and CD38 (CD8) double‐positive fractions. 22 The impaired adaptive immune response observed in severe COVID‐19 patients might be caused by the pulmonary recruitment of immune cells from peripheral blood and the infiltration of lymphocytes into the airways as suggested by several authors. 33 , 34 , 35 , 36 The release of pro‐inflammatory cytokines (such as IFN‐γ and IL‐6) and chemokines (such as CCL2 and CXCL10) attracts immune cells, notably monocytes and T lymphocytes, but not neutrophils, from the blood to the infected site. 22 , 37 This will result in lymphopenia, eosinopenia, and increased neutrophil‐lymphocyte ratio in peripheral blood, 38 , 39 as well as ARDS in COVID‐19 patients. 35
In summary, these data show that, in its severe stage, the pathogenesis of COVID‐19 involves impaired adaptive immune responses and uncontrolled release of pro‐inflammatory cytokines, which cause systemic inflammation, ADRS, multiple organ failure, and finally lead to death.
3. ROLE OF TREG/TH17 CELL RATIO IN COVID‐19 PATHOGENESIS
Although helper T‐cell (CD3+ CD4+) levels were below normal, the proportion of Treg cells and Th17 cells was affected by SARS‐CoV‐2 in different ways. Indeed, severe COVID‐19 patients had a significant decrease in Treg cell levels (CD3+CD4+CD25+CD127low+), 24 , 40 increased levels of Th17 cells (CCR6+ Th17), 22 , 41 with a consequent decrease in the Treg/Th17 cell ratio (Figure 1C).
Treg and Th17 cells are part of the complex machinery that constitutes the immune system. The differentiation of Th17 and Treg cells from naïve CD4+T cells is mediated by TGF‐β. However, in the presence of IL‐6 or IL‐21 (together with TGF‐β), naïve CD4+ T cells differentiate into Th17 cells. 42 These two CD4+ T‐cell subsets have different actions. 43 Th17 cells are mainly characterized by their production of inflammatory cytokines such as IL‐17A, 42 which activates target cells and induces chemokine (C–X–C motif) ligands (CXCLs). 44 CXCLs then attract myeloid cells such as neutrophils to infected or injured tissues. 44 In contrast, Treg cells express anti‐inflammatory cytokines (IL‐4, IL‐10, and TGF‐β) and control excessive immune responses. 45 , 46 The Treg/Th17 balance plays an important role in the severity of lung injury 47 and in uncontrolled systemic inflammation characterizing acute lung injury (ALI)/ ARDS (Figure 1C). 48
In patients with severe COVID‐19, the increase in Th17 cells is accountable, at least in part, to severe immune injury in such patients. 22 Indeed, among the pro‐inflammatory cytokines identified during deadly CSs, several cytokines are involved in Th17‐type responses. 41 Th17 cells produce IL‐17, GM‐CSF, IL‐21, and IL‐22 (Figure 1C). 42 IL‐17 recruits monocytes and neutrophils to the site of infection, and activates other downstream cytokine cascades (G‐CSF, IL‐1β, IL‐6, TNF‐α) and chemokines (CXCL1, CXCL‐2, CXCL10, CXCL10, and CCL20) (Figure 1D), and matrix metalloproteinases. 19 , 41 , 49 , 50
Treg cells play a crucial role in weakening or dampening overactive innate immune responses for the maintenance of self‐tolerance and immune homeostasis during viral. 51 , 52 In severe COVID‐19 cases, decreased Treg numbers indicate insufficient regulation of pro‐inflammatory immune responses that may further aggravate hyperinflammation and tissue injury. 53
In addition, several studies have reported a significant increase in IL‐6 levels among other elevated inflammatory cytokines in COVID‐19 patients, with significantly higher levels in severe rather than in mild cases. 16 , 24 , 30 , 54 , 55 IL‐6 is a pleiotropic cytokine with mainly a pro‐inflammatory function affecting several processes including immunity, tissue repair, and metabolism. 56 Indeed, in response to tissue damage and infections, 57 IL‐6 is promptly and transiently produced by a range of cells including fibroblasts, keratinocytes, mesangial cells, vascular endothelial cells, mast cells, macrophages, dendritic cells, and T and B cells. 58 IL‐6 trans‐signaling promotes the recruitment of monocytes to the inflammation site. 59 Moreover, IL‐6 promotes specific differentiation of naïve CD4+ T cells, thus performing an important function in the linking of innate to acquired immune response infections. 57 Indeed, in combination with the transforming growth factor‐beta 1(TGF‐β1), IL‐6 induces the generation of Th17 cells from naïve T cells and inhibits TGF‐β1‐induced Treg (iTreg) differentiation. 60 , 61 Thus, by promoting Th17 cell lineages and inhibiting the induction of Treg cells, 55 elevated circulating IL‐6 levels contribute to deregulation of the Treg/Th17 cell ratio toward an increase in Th17 cells.
These data suggest that the increase in Th17 cells associated with a decrease in Treg cells may contribute to the uncontrolled release of pro‐inflammatory cytokines and chemokines in COVID‐19 patients, which might result in aggravated inflammatory responses, production of CSs, worsening the damage to tissues, and leading to multiorgan failure and death.
4. TREG/TH17 CELL IMBALANCE AND ADVERSE PREGNANCY OUTCOMES
In a healthy pregnancy, the immune system not only recognizes and fights infections (defense), but also regulates undesired immune responses against tissue self‐antigens or harmless non‐self‐organisms (tolerance). Treg and Th17 cells are active players in the establishment of this singular immunologic state. Treg cells and their related cytokines allow the growth and development of the allogeneic fetus, 62 while Th17 cells and their related cytokines are involved in defense against various pathogens.
An appropriate balance between Treg/Th17 cells is critical for healthy fetal implantation and pregnancy development. 63 During healthy pregnancy, the Treg/Th17 ratio shifts in favor of Treg cells. Treg cells also proliferate systemically, as well as at the fetal‐maternal interface, to ensure maternal‐fetal immune tolerance and successful pregnancy. 64 In contrast, uncontrolled Th17 cell proliferation is unfavorable because it is associated with fetal allograft rejection at the feto‐maternal interface. 43 The dysregulation of this tight balance between Treg and Th17 cells has been shown to be involved in the pathogenesis of adverse pregnancy outcomes. 63 Indeed, decreased Treg cell numbers 65 , 66 , 67 , 68 , 69 , 70 , 71 and increased Th17 cell percentages 68 , 72 , 73 , 74 are associated with pregnancy complications such as miscarriage, preeclampsia (PE), and preterm labor (Figure 1F).
The pathogenesis of severe COVID‐19 involves deregulation of the Treg/Th17 cell ratio toward an increase in Th17 cells, resulting in uncontrolled systemic inflammation. Thus, in SARS‐CoV‐2‐infected pregnant women, Treg/Th17 cell imbalance might be potentially associated with adverse pregnancy outcomes such as pregnancy loss, preterm birth, and PE. Nevertheless, further investigations are required to validate their causal relationship.
5. SARS‐COV‐2 INFECTION AND FETAL/NEONATAL RISK
In COVID‐19‐infected pregnant women, abortion or preterm delivery can be induced by the severity of the disease, and by the challenge to identify appropriate treatment. However, in the cases of spontaneous abortion or preterm birth, Treg/Th17 cell imbalance and uncontrolled systemic inflammation may be involved in their occurrence.
Another important question raised by the issue of SARS‐CoV‐2 infection during pregnancy is to determine whether COVID‐19 can be transmitted from the mother to the fetus. Currently, the limited data available do not show evidence of intrauterine vertical transmission of SARS‐CoV‐2 from infected pregnant women to their fetuses. 11 , 75 , 76 , 77 However, the presence of anti‐SARS‐CoV‐19 immunoglobulins in newborns has been reported in recent studies involving IgG and IgM. 78 , 79 The lack of amniotic fluid or placental examinations are the main limitations of these studies. Subsequently, in reviewing 23 studies published as at March 31, 2020, Gajbhiye et al 12 estimated a possible vertical transmission rate of 11% based on laboratory methods (RT‐qPCR or by the presence of IgM antibodies) used to confirm diagnosis within the first 48 hours of life.
One of the factors determining viral tropism in the decidua and placenta is viral entry receptor expression in these tissues. 80 In their study, Li et al 81 demonstrated the expression of SARS‐CoV‐2 receptors at the maternal‐fetal interface and fetal organs. Moreover, Alzamora et al 82 reported a severe case of COVID‐19 in a pregnant woman who delivered a baby which was nasopharyngeal swab‐positive for SARS‐CoV‐2 by RT‐qPCR 16 hours after delivery. These recent findings add to the accumulating evidence regarding the possibility of vertical transmission of COVID‐19, and further studies are needed.
Thus, the possibility of intrauterine transmission of SARS‐CoV‐2 exposes the placenta and fetus to viral infection (and inflammation), which could result in severe birth defects or pregnancy loss (Figure 1G). 80
6. CONCLUSIONS
Several studies have reported adverse pregnancy outcomes among infected pregnant women, including spontaneous abortion and preterm delivery. However, there is no evidence of direct effects of SARS‐CoV‐2 infection on pregnancy fate. Here, we showed that severe COVID‐19 involves impaired adaptive immune responses, mainly affecting T cells (lymphopenia). Moreover, analysis of CD4+ T‐cell subsets showed that, in severe COVID‐19 patients, there was a significant increase in Th17 cell numbers, while the proportion of Treg cells decreased. The deregulated Treg/Th17 cell imbalance contributes to the induction of an uncontrolled release of pro‐inflammatory cytokines (CSs), which can cause systemic inflammation and multiple organ failure, ultimately leading to death. Treg/Th17 cell imbalance and subsequent systemic inflammation are involved in the pathogenesis of pregnancy complications. Thus, we proposed that, through Treg/Th17 cell imbalance and subsequent uncontrolled systemic inflammation, SARS‐CoV‐2 infection may lead to pregnancy complications. We also discussed the possibility of intrauterine transmission of COVID‐19 to the fetus, which might be associated with adverse perinatal outcomes. Although further supporting studies are needed, the possibility of vertical transmission of COVID‐19 should be considered. Based on the above, cautious monitoring of pregnancies and appropriate measures should be taken to prevent and treat early eventual maternal and perinatal complications.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by research grants from “The Fundamental Research Funds for Central Universities” (2020kfyXGYJ116), and "the Cultivation Plan of International Joint Research Platform of Huazhong University of Science and Technology" (5001519009).
Muyayalo KP, Huang D‐H, Zhao S‐J, Xie T, Mor G, Liao A‐H. COVID‐19 and Treg/Th17 imbalance: Potential relationship to pregnancy outcomes. Am J Reprod Immunol. 2020;84:e13304. 10.1111/aji.13304
Kahinho P. Muyayalo and Dong‐Hui Huang contributed equally.
REFERENCES
- 1. Gorbalenya AE, Baker SC, Baric RS, et al. Severe acute respiratory syndrome‐related coronavirus: the species and its viruses – a statement of the Coronavirus Study Group. bioRxiv. 2020;2020. https://www.biorxiv.org/content/10.1101/2020.02.07.937862v1. Accessed March 30th, 2020. [Google Scholar]
- 2. Worldometer . COVID‐19 coronavirus pandemic. 2020; https://www.worldometers.info/coronavirus. Accessed June 30, 2020.
- 3. WHO (WHO) . Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019‐nCoV). 2020; https://www.who.int/news‐room/detail/30‐01‐2020‐statement‐on‐the‐second‐meeting‐of‐the‐international‐health‐regulations‐(2005)‐emergency‐committee‐regarding‐the‐outbreak‐of‐novel‐coronavirus‐(2019‐ncov). Accessed January 31, 2020.
- 4. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Biomed. 2020;91(1):157‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao X. COVID‐19: immunopathology and its implications for therapy. Nat Rev Immunol. 2020;20:269‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ji D, Zhang D, Xu J, et al. Prediction for progression risk in patients with COVID‐19 pneumonia: the CALL Score. Clin Infect Dis. 2020. 10.1093/cid/ciaa414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu H, Wang LL, Zhao SJ, Kwak‐Kim J, Mor G, Liao AH. Why are pregnant women susceptible to COVID‐19? An immunological viewpoint. J Reprod Immunol. 2020;139:103122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khan MMA, Khan MN, Mustagir MG, Rana J, Haque MR, Rahman MM. COVID‐19 infection during pregnancy: a systematic review to summarize possible symptoms, treatments, and pregnancy outcomes. medRxiv. 2020. 10.1101/2020.03.31.20049304 [DOI] [Google Scholar]
- 10. Yan J, Guo J, Fan C, et al. Coronavirus disease 2019 (COVID‐19) in pregnant women: a report based on 116 cases. Am J Obstet Gynecol. 2020;223(1):111.e1‐111.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen L, Li Q, Zheng D, et al. Clinical characteristics of pregnant women with Covid‐19 in Wuhan, China. N Engl J Med. 2020;382(25):e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gajbhiye R, Modi D, Mahale S. Pregnancy outcomes, newborn complications and maternal‐fetal transmission of SARS‐CoV‐2 in women with COVID‐19: a systematic review. medRxiv. 2020. 10.1101/2020.04.11.20062356 [DOI] [Google Scholar]
- 13. McIntosh K, Hirsch MS, Bloom A.Coronavirus disease 2019 (COVID‐19). 2020; https://www.uptodate.com/contents/coronavirus‐disease‐2019‐covid‐19?source=history_widget. Accessed March 30.
- 14. Del Rio C, Malani PN. COVID‐19‐new insights on a rapidly changing epidemic. JAMA. 2020. 10.1001/jama.2020.3072 [DOI] [PubMed] [Google Scholar]
- 15. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tufan A, Avanoglu Guler A, Matucci‐Cerinic M. COVID‐19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk. J Med Sci. 2020;50(SI‐1):620‐632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol. 2014;14(1):36‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92(4):424‐432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schett G, Sticherling M, Neurath MF. COVID‐19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol. 2020;20(5):271‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi Y, Wang Y, Shao C, et al. COVID‐19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27(5):1451‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID‐19. J Pharm Anal. 2020;10(2):102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang B, Zhou X, Zhu C, et al. Immune phenotyping based on neutrophil‐to‐lymphocyte ratio and IgG predicts disease severity and outcome for patients with COVID‐19. medRxiv. 2020;7. 10.3389/fmolb.2020.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan L, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng Y, Huang Z, Ying G, et al. Study of the lymphocyte change between COVID‐19 and non‐COVID‐19 pneumonia cases suggesting other factors besides uncontrolled inflammation contributed to multi‐organ injury. medRxiv. 2020. 10.1101/2020.02.19.20024885 [DOI] [Google Scholar]
- 29. Wan S, Yi Q, Fan S, et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. 2020. 10.1101/2020.02.10.20021832 [DOI] [Google Scholar]
- 30. Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS‐CoV‐2 infected patients. EBioMedicine. 2020;55:102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shi Y, Tan M, Chen X, et al. Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. medRxiv. 2020;160(3):261‐268. 10.1111/imm.13223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Urra JM, Cabrera CM, Porras L, Rodenas I. Selective CD8 cell reduction by SARS‐CoV‐2 is associated with a worse prognosis and systemic inflammation in COVID‐19 patients. Clin Immunol. 2020;217:108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221(11):1762‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun DW, Zhang D, Tian RH, et al. The underlying changes and predicting role of peripheral blood inflammatory cells in severe COVID‐19 patients: a sentinel? Clin Chim Acta. 2020;508:122‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tian S, Hu W, Niu L, Liu H, Xu H, Xiao SY. Pulmonary pathology of early‐phase 2019 novel coronavirus (COVID‐19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guan WJ, Zhong NS. Clinical characteristics of COVID‐19 in China. Reply. N Engl J Med. 2020;382(19):1861‐1862. [DOI] [PubMed] [Google Scholar]
- 39. Qin G, Zhang L, Yu CJ. Nonpharmaceutical interventions and epidemic intensity during the 2019 novel coronavirus disease pandemic. Clin Infect Dis. 2020;ciaa432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang F, Hou H, Luo Y, et al. The laboratory tests and host immunity of COVID‐19 patients with different severity of illness. JCI Insight. 2020;5(10):e137799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wu D, Yang XO. TH17 responses in cytokine storm of COVID‐19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53(3):368‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee GR. The balance of Th17 versus Treg cells in autoimmunity. Int J Mol Sci. 2018;19(3):730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Muyayalo KP, Li ZH, Mor G, Liao AH. Modulatory effect of intravenous immunoglobulin on Th17/Treg cell balance in women with unexplained recurrent spontaneous abortion. Am J Reprod Immunol. 2018;80(4):e13018. [DOI] [PubMed] [Google Scholar]
- 44. Capone A, Volpe E. Transcriptional regulators of T helper 17 cell differentiation in health and autoimmune diseases. Front Immunol. 2020;11:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kleinewietfeld M, Hafler DA. Regulatory T cells in autoimmune neuroinflammation. Immunol Rev. 2014;259(1):231‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. LaMarca B, Cornelius DC, Harmon AC, et al. Identifying immune mechanisms mediating the hypertension during preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2016;311(1):R1‐R9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li G, Cao Y, Sun Y, Xu R, Zheng Z, Song H. Ultrafine particles in the airway aggravated experimental lung injury through impairment in Treg function. Biochem Biophys Res Commun. 2016;478(1):494‐500. [DOI] [PubMed] [Google Scholar]
- 48. Lin S, Wu H, Wang C, Xiao Z, Xu F. Regulatory T cells and acute lung injury: cytokines, uncontrolled inflammation, and therapeutic implications. Front Immunol. 2018;9:1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dutzan N, Abusleme L. T Helper 17 cells as pathogenic drivers of periodontitis. Adv Exp Med Biol. 2019;1197:107‐117. [DOI] [PubMed] [Google Scholar]
- 50. Bunte K, Beikler T. Th17 cells and the IL‐23/IL‐17 axis in the pathogenesis of periodontitis and immune‐mediated inflammatory diseases. Int J Mol Sci. 2019;20(14):3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10(7):490‐500. [DOI] [PubMed] [Google Scholar]
- 52. Shaw AC, Goldstein DR, Montgomery RR. Age‐dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Neurath MF. Covid‐19 and immunomodulation in IBD. Gut. 2020;69(7):1335‐1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wang F, Nie J, Wang H, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221(11):1762‐1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. The cytokine release syndrome (CRS) of severe COVID‐19 and Interleukin‐6 receptor (IL‐6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55(5):105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tanaka T, Narazaki M, Kishimoto T. IL‐6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mauer J, Denson JL, Bruning JC. Versatile functions for IL‐6 in metabolism and cancer. Trends Immunol. 2015;36(2):92‐101. [DOI] [PubMed] [Google Scholar]
- 59. Hurst SM, Wilkinson TS, McLoughlin RM, et al. Il‐6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14(6):705‐714. [DOI] [PubMed] [Google Scholar]
- 60. Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235‐238. [DOI] [PubMed] [Google Scholar]
- 61. Mangan PR, Harrington LE, O'Quinn DB, et al. Transforming growth factor‐beta induces development of the T(H)17 lineage. Nature. 2006;441(7090):231‐234. [DOI] [PubMed] [Google Scholar]
- 62. Balandya E, Wieland‐Alter W, Sanders K, Lahey T. Human seminal plasma fosters CD4(+) regulatory T‐cell phenotype and transforming growth factor‐beta1 expression. Am J Reprod Immunol. 2012;68(4):322‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Figueiredo AS, Schumacher A. The T helper type 17/regulatory T cell paradigm in pregnancy. Immunology. 2016;148(1):13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jørgensen N, Persson G, Hviid TVF. The tolerogenic function of regulatory T cells in pregnancy and cancer. Front Immunol. 2019;10:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zenclussen AC, Gerlof K, Zenclussen ML, et al. Abnormal T‐cell reactivity against paternal antigens in spontaneous abortion: adoptive transfer of pregnancy‐induced CD4+CD25+ T regulatory cells prevents fetal rejection in a murine abortion model. Am J Pathol. 2005;166(3):811‐822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sasaki Y, Darmochwal‐Kolarz D, Suzuki D, et al. Proportion of peripheral blood and decidual CD4(+) CD25(bright) regulatory T cells in pre‐eclampsia. Clin Exp Immunol. 2007;149(1):139‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Darmochwal‐Kolarz D, Saito S, Rolinski J, et al. Activated T lymphocytes in pre‐eclampsia. Am J Reprod Immunol. 2007;58(1):39‐45. [DOI] [PubMed] [Google Scholar]
- 68. Nakashima A, Shima T, Inada K, Ito M, Saito S. The balance of the immune system between T cells and NK cells in miscarriage. Am J Reprod Immunol. 2012;67(4):304‐310. [DOI] [PubMed] [Google Scholar]
- 69. Koucky M, Malickova K, Cindrova‐Davies T, et al. Low levels of circulating T‐regulatory lymphocytes and short cervical length are associated with preterm labor. J Reprod Immunol. 2014;106:110‐117. [DOI] [PubMed] [Google Scholar]
- 70. Xiong H, Zhou C, Qi G. Proportional changes of CD4+CD25+Foxp3+ regulatory T cells in maternal peripheral blood during pregnancy and labor at term and preterm. Clin Invest Med. 2010;33(6):E422. [DOI] [PubMed] [Google Scholar]
- 71. Eghbal‐Fard S, Yousefi M, Heydarlou H, et al. The imbalance of Th17/Treg axis involved in the pathogenesis of preeclampsia. J Cell Physiol. 2019;234(4):5106‐5116. [DOI] [PubMed] [Google Scholar]
- 72. Darmochwal‐Kolarz D, Kludka‐Sternik M, Tabarkiewicz J, et al. The predominance of Th17 lymphocytes and decreased number and function of Treg cells in preeclampsia. J Reprod Immunol. 2012;93(2):75‐81. [DOI] [PubMed] [Google Scholar]
- 73. Ito M, Nakashima A, Hidaka T, et al. A role for IL‐17 in induction of an inflammation at the fetomaternal interface in preterm labour. J Reprod Immunol. 2010;84(1):75‐85. [DOI] [PubMed] [Google Scholar]
- 74. Fu B, Tian Z, Wei H. TH17 cells in human recurrent pregnancy loss and pre‐eclampsia. Cell Mol Immunol. 2014;11(6):564‐570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Karimi‐Zarchi M, Neamatzadeh H, Dastgheib SA, et al. Vertical transmission of coronavirus disease 19 (COVID‐19) from infected pregnant mothers to neonates: a review. Fetal Pediatr Pathol. 2020;39(3):246‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Peng Z, Wang J, Mo Y, et al. Unlikely SARS‐CoV‐2 vertical transmission from mother to child: a case report. J Infect Public Health. 2020;13(5):818‐820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zeng H, Xu C, Fan J, et al. Antibodies in infants born to mothers with COVID‐19 pneumonia. JAMA. 2020;323(18):1848‐1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dong L, Tian J, He S, et al. Possible vertical transmission of SARS‐CoV‐2 from an infected mother to her newborn. JAMA. 2020;323(18):1846‐1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Racicot K, Mor G. Risks associated with viral infections during pregnancy. J Clin Invest. 2017;127(5):1591‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li M, Chen L, Zhang J, Xiong C, Li X. The SARS‐CoV‐2 receptor ACE2 expression of maternal‐fetal interface and fetal organs by single‐cell transcriptome study. PLoS One. 2020;15(4):e0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Alzamora MC, Paredes T, Caceres D, Webb CM, Valdez LM, La Rosa M. Severe COVID‐19 during pregnancy and possible vertical transmission. Am J Perinatol. 2020;37(8):861‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]


