Abstract
Background
Community‐acquired respiratory viruses (CARV) cause upper and lower respiratory tract infections (URTI/LRTI) and may be life‐threatening for recipients of an allogeneic stem cell transplantation (allo‐SCT).
Methods
In a prospective study encompassing 4 winter‐seasons, we collected throat gargles (TG) at random time points from allo‐SCT recipients (patients) and controls and followed them up for at least 3 weeks including repetitive sampling and documentation of symptoms. A Multiplex‐PCR system to identify 20 CARV and Mycoplasma pneumoniae was used to detect CARV.
Results
One hundred ninety‐four patients with 426 TG and 273 controls with 549 TG were included. There were more patients with a positive test result (25% vs 11% in the controls), and the patients had a higher number of positive TG (70 = 16%) compared to controls (32 = 6%) (P < .001). Altogether, 115 viruses were detected. Multiple viruses in one TG (11/48, 34%) and prolonged shedding were only observed in patients (13/48, 27%). Patients had more RSV (18/83, 26%) and adenovirus (15/83, 21%) than controls (both viruses 2/32, 6%). Independent risk factors for the detection of CARV included age >40 years (OR 3.38, 95% CI 1.8‐6.4, P < .001) and presence of URTI‐symptoms (OR 3.22, 95% CI 1.9‐5.5, P < .001). No controls developed a LRTI or died whereas 4/48 (8%) patients developed a LRTI (coronavirus in 2, RSV in 1 and influenza A H1N1 in 1 patient). One patient died of CARV (influenza A H1N1).
Conclusion
Allo‐SCT‐recipients have more CARV‐infections, exhibit a different epidemiology, have more cases of co‐infection or prolonged shedding and have a higher rate of LRTI and mortality.
Keywords: CARV, epidemiology, immunosuppression
1. INTRODUCTION
Community‐acquired respiratory viruses (CARV) are increasingly recognized as clinically relevant pathogens. They commonly cause symptoms of upper respiratory tract infection (URTI) such as cough and running nose. URTI is often harmless, but in some cases pave the way for bacterial superinfection and cause lower respiratory tract infection (LRTI) which can be fatal. 1 Usually, the term CARV comprises orthomyxoviridae (influenza A, B, and C), paromyxoviridae (including parainfluenza 1‐4 (PIV), respiratory syncytial virus A and B (RSV), and human metapneumovirus (hMPV)), coronaviridae, picornaviridae (including >100 different serotypes of rhinovirus and enterovirus), adenoviridae, polyomavirus type 1, and bocavirus. Some of them like influenza or RSV follow seasonal patterns whereas others such as rhinovirus and PIV can be detected throughout the whole year. 2 , 3 , 4 The epidemiology of CARV has attracted growing attention with the broad use of nuclear‐acid amplification techniques. The increased sensitivity of PCR‐based methods created more positive findings than culture‐based and serological methods. Commercially available multiplex‐test systems are increasingly used in routine clinical practice. However, the clinical relevance of the identification of CARV in samples from the respiratory tract is not entirely clear. In a patient with symptoms of URTI, the CARV are assumed to be the cause—if detected. Little is known about the true pathogenicity of CARV and about the natural course of the infection. In cancer patients, asymptomatic carriers and long‐term viral shedding have been described, 5 , 6 whereas this seems to be rare in healthy persons. 7 Clinical symptoms caused by different CARV may vary—for example, influenza is usually assumed to be the cause of febrile illnesses during the winter months, whereas rhinoviruses are more associated with common colds. However, symptoms are not specific for a defined CARV. All CARV have been reported to cause fatalities in severely immunosuppressed patients. 8
For patients after allogeneic stem‐cell transplantation (allo‐SCT), contact with friends and family carries the risk of attracting CARV infections. One of the main goals of patient management after successful allo‐SCT is to return to a normal life situation, where the avoidance of CARV exposure may be difficult to achieve, 9 particularly as there are only few data on healthy asymptomatic individuals and the risk they may pose.
This prospective study addresses the question of epidemiology of CARV in allo‐SCT recipients compared with otherwise non‐immunocompromised controls. In addition, risk factors for CARV infection, symptoms of CARV infection and the course of the disease were analyzed. We hypothesize that allo‐SCT recipients and non‐immunocompromised controls have different incidences and a different clinical course of CARV infections.
2. METHODS
This study was a prospective cohort trial including allo‐SCT recipients (patients) and controls. It was approved by the local institutional review board (Nr 3891‐09/13) and is in part registered in the German Clinical Trials Registry (DRKS00005367, cohort healthy persons only). The study was initially designed to analyze controls for 2 consecutive winter seasons and was amended after 1 year to include patients as well. After 3 years, the recruitment of controls was extended for a third winter season (Figure 1).
Figure 1.
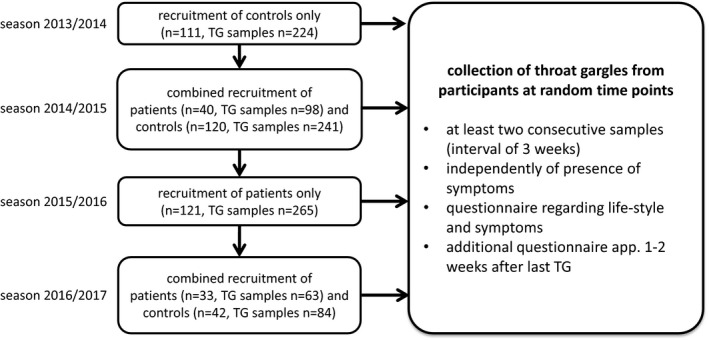
Study flowchart: Patients were recruited in the outpatient clinic of the stem cell transplantation program during routine visits; controls were recruited amongst students, members of a choir and among patients without any immunosuppression undergoing minor surgical procedures such as elective cholecystectomy, glaucoma surgery or orthopedic interventions, recruitment was independent of whether or not the patient presented with symptoms of a CARV‐infection
During the winter months from 2013/2014 till 2016/2017, we collected throat gargles (TG) from patients and controls at random time points after they had given their written informed consent. Patients were recruited in the outpatient clinic of the stem cell transplantation program during routine visits. Controls were recruited among students, among members of a choir and among patients without any immunosuppression attending preoperative routine visits prior to elective minor surgical procedures such as cholecystectomy, glaucoma surgery, or orthopedic interventions. TG were collected at least twice at a 3‐week interval independently of symptoms. In addition, patients and controls were questioned regarding their lifestyle and possible symptoms at the time of collection of TG. An additional questionnaire was completed approximately 1‐2 weeks after the last TG to account for any symptoms that may have developed in the meantime.
LRTI was defined as (a) clinical presentation with typical symptoms of a lower respiratory tract infection (like shortness of breath, weakness, fever, or cough), (b) infiltrates on CT‐scans, and (c) a positive result for virus testing in corresponding TG samples.
For collection of TG, trial participants were advised to perform a TG by using 10 mL of sterile physiological saline solution. Specimens were collected and subjected to RNA extraction using QiAmp Viral RNA Mini Kit according to the manufacturer's instruction (Quiagen). Samples were then stored at −20°C until blockwise blinded testing.
For detection of viral RNA, the multiplex PCR kit FTD respiratory pathogens 21‐Kit was used according to the manufacturer's instructions (Fast Track Diagnostics Ltd.). The analysis was performed on a LightCycler Roche 480 (Roche). The FTD 21 kit allows the simultaneous detection of the following 20 viral respiratory pathogens: influenza A and B viruses, influenza A (H1N1) swl virus, human rhinovirus, human coronaviruses 229E, OC43, HKU1 and NL63, human parainfluenza viruses 1‐4, human metapneumoviruses A/B, human respiratory syncytial virus A/B, human adenovirus, human bocavirus, enterovirus, and human parechovirus. In addition, Mycoplasma pneumoniae, the only bacterial pathogen, can also be detected. Of note, the FTD 21 kit is licensed for the detection of these pathogens in respiratory samples including swabs, bronchoalveolar lavage, and sputum.
Statistics: Analyses were performed by using IBM® SPSS® Statistics version 21 or version 22. Descriptive statistics were used for the description of findings (frequencies, mean/median, range as appropriate). Differences between groups were assessed by the chi‐square test or Fisher's exact test. To identify risk factors, generalized estimating equations were used. A P‐value < .05 was considered statistically significant.
3. RESULTS
During the winter months from 2013 till 2017 (four seasons), we collected a minimum of two to a maximum of seven TG from 194 patients (426 TG) and 273 controls (549 TG) independently of the presence or absence of symptoms.
Participants in the patient cohort were older and had more often children in their household (Table 1 and Figure 1 Supplementary Material). We collected two TG from most patients (81.4%) and 3 or more TG from 14.9% of patients. Seven patients (3.6%) had only one TG and dropped out of the study. Among controls, we collected two TG from 93.4%, three TG from 2.9%, and 4 TG from 0.4% (1 patient). Nine controls (3.3%) dropped out after the first TG.
Table 1.
Characteristics of patients and controls
| Patients N = 194 | Controls N = 273 | P‐value | |
|---|---|---|---|
| Age (y; median, range) | 56 (21‐72) | 25 (18‐86) | <.001 |
| Male (n, %) | 115 (59%) | 137 (50%) | .05 |
| Smoker (n,%) | 26 (13%) | 37 (14%) | n.s. |
| Lives alone | 26 (13%) | 51 (19%) | n.s. |
| Children in household | 50 (26%) | 26 (10%) | <.001 |
| Vaccinated against influenza | 47 (24%) | 68 (25%) | n.s. |
| Duration since SCT (d; median, range) | 679 (23‐9936) | ||
| GvHD at time of sampling (n, %) | 72 (37%) | ||
| Immunosuppression at time of sampling (n, %) | 119 (61%) |
Abbreviations: GvHD, graft‐vs‐host disease; SCT, allogeneic stem‐cell transplantation.
Of the collected 975 TG, 102 (10%) were positive. Patients had more often positive results (48/194; 25%) than controls (31/273; 11%; P < .001). Furthermore, the number of positive TG was higher in patients (70/426; 16%) than in controls (32/549; 6%) (P < .001).
The different types of viruses detected according to health status and different seasons are depicted in Table 2. Overall, more positive samples were found in the patient group. Virus detection frequencies between groups were significantly different for corona viruses, RSV, and adenoviruses. We did not detect a positive result for bocavirus, parechovirus, or Mycoplasma pneumoniae. TG from controls contained 1 CARV at the most, whereas we could detect 2 or 3 CARV in 11 TG from patients. There was a notable influence of age with negative findings in young participants (median age 32 years, range 18‐86) compared to a positive TG at some point during the study period in the older participants (median age 53 years, range 18‐72; P < .001).
Table 2.
Results of TG according to season and health status
| 2013/2014 | 2014/2015 | 2015/2016 | 2016/2017 | All seasons | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Controls | Patients | Controls | P‐value | Patients | Patients | Controls | P‐value | Patients | Controls | P‐value | |
| Number of tests | n = 215 | n = 98 | n = 228 | n = 265 | n = 63 | n = 84 | n = 426 | n = 549 | |||
| Positive samples | 9 (4%) | 27 (28%) a | 13 (5%) | <.001 | 31 (12%) b | 12 (19%) d | 10 (12%) | .24 | 70 (16%) c | 32 (6%) | <.001 |
| Number of viruses | 9 | 34 | 13 | <.001 | 34 | 15 | 10 | .06 | 83 | 32 | <.001 |
| Viruses | |||||||||||
| Influenza | 0 | 1 (3%) | 4 (31%) | .03 | 5 (15%) | 0 | 0 | ‐ | 6 (9%) | 4 (13%) | .42 |
| Corona | 4 (44%) | 6 (18%) | 4 (31%) | .36 | 8 (24%) | 8 (53%) | 8 (80%) | .93 | 22 (31%) | 16 (50%) | .02 |
| PIV | 0 | 4 (12%) | 1 (8%) | .65 | 5 (15%) | 0 | 0 | ‐ | 9 (13%) | 1 (3%) | .09 |
| hMPV | 1 (11%) | 0 | 0 | ‐ | 1 (3%) | 0 | 0 | ‐ | 1 (1%) | 1 (3%) | .56 |
| RV | 3 (33%) | 3 (9%) | 2 (15%) | .55 | 7 (21%) | 2 (13%) | 1 (10%) | .80 | 12 (16%) | 6 (18%) | .59 |
| RSV | 0 | 13 (38%) | 1 (8%) | .06 | 3 (9%) | 2 (13%) | 1 (10%) | .80 | 18 (26%) | 2 (6%) | .013 |
| Adeno | 1 (11%) | 7 (21%) | 7 (21%) | .03 | 5 (15%) | 3 (20%) | 0 | ‐ | 15 (21%) | 2 (6%) | .05 |
Influenza: A n = 7 and B n = 3; Corona: 229E n = 18, OC43 n = 11, HKU1 n = 4 and NL63 n = 5; PIV: type 1 n = 2, type 2 n = 2, type 3 n = 6; hMPV: types A/B n = 2; RV: Rhinovirus n = 17, Enterovirus n = 1; RSV: types A/B n = 20; Adeno: n = 17.
Including 3 double‐positive and 2 triple‐positive samples accounting for 34 detected viruses,
including 3 double‐positive samples accounting for 34 viruses,
due to 6 double‐positive and 2 triple‐positive samples,
including 3 double positive samples.
Most participants with a positive TG complained of symptoms (Table 3). Very few positive TG (6/102, 6%) were not associated with any symptoms. Except for fever, which was significantly more common in people suffering from influenza, no other symptom was significantly different for any of the viruses. In general, the symptoms were consistent with an URTI. LRTI was observed in 4/48 (8%) patients with a positive test for CARV but not in controls. Coronavirus was found in 2, RSV in 1 and influenza A H1N1 in 1 patient, who died.
Table 3.
Symptoms associated with CARV
|
n/N a (%) |
Influenza N = 10 | Corona N = 38 | PIV N = 10 | hMPV N = 2 | RV N = 18 | RSV N = 20 | Adeno N = 18 | P |
|---|---|---|---|---|---|---|---|---|
| Cough | 8/10 (80%) | 18/35 (51%) | 6/9 (7%) | 2/2 (100%) | 9/18 (50%) | 14/19 (74%) | 11/14 (79%) | .19 |
| Running nose | 7/10 (70%) | 23/35 (66%) | 5/8 (63%) | 2/2 (100%) | 13/18 (72%) | 12/18 (67%) | 9/13 (69%) | .98 |
| Sore throat | 6/10 (60%) | 10/34 (29%) | 2/7 (29%) | 1/2 (50%) | 11/17 (65%) | 5/17 (29%) | 7/12 (58%) | .16 |
| Fever | 6/10 (60%) | 4/35 (11%) | 1/7 (14%) | 1/2 (50%) | 1/18 (6%) | 4/18 (22%) | 1/13 (8%) | .008 |
| Malaise | 8/10 (80%) | 17/35 (49%) | 3/7 (43%) | 0/2 (0%) | 5/18 (28%) | 9/17 (53%) | 5/13 (38%) | .19 |
| Asymptomatic | 0 | 3/35 (9%) | 1/10 (10%) | 0 (0%) | 1/18 (6%) | 1/17 (6%) | 0 (0%) | .85 |
Not all patients provided answers to all questions, most patients had more than one symptom.
To assess risk factors for the detection of CARV, the following variables were analyzed: health status (patients versus controls), age >40 years, presence of symptoms typical of URTI, smoking, living alone versus in a family/community, children in the household, contact to potentially infectious persons and vaccination‐status. The presence of GvHD and the duration after allo‐SCT (100, 365 and 1200 days) was also assessed in the patient cohort.
Univariate analysis revealed the following risk factors for the detection of CARV: health status in patients, age >40, typical symptoms and children in the household. In the multi‐variate analysis, only age >40 and typical symptoms could be confirmed, whereas all other risk factors were not significant (Table 4). When analyzing the control‐cohort, children in the household could not be confirmed as a risk factor whereas age >40 and typical symptoms remained independent risk factors for the detection of CARV. In contrast, in the patient cohort only typical symptoms could be confirmed as risk factor whereas GvHD or time after allo‐SCT were not associated with a higher risk (Table 4 and data not shown).
Table 4.
Risk factors for detection of CARV
| Risk Factors |
All OR (95% CI) |
Uni‐variate P |
All OR (95% CI) |
Multi‐variate P |
controls OR (95% CI) |
Uni‐variate P |
OR (95% CI) |
Multi‐variate P |
Patients OR (95% CI) |
Uni‐variate P |
|---|---|---|---|---|---|---|---|---|---|---|
| Patient vs controls | 3.18 (2.0‐5.1) | <.001 | 1.57 (0.9‐2.8) | .13 | na | na | na | na | na | na |
| Age >40 | 3.87 (2.4‐6.5) | <.001 | 3.38 (1.8‐6.4) | <.001 | 3.2 (1.6‐6.6) | .002 | 3.74 (1.8‐7.8) | <.001 | 2.21 (0.8‐5.8) | .11 |
| Children in Household | 2.1 (1.2‐3.4) | .005 | 1.36 (0.8‐2.4) | .29 | 2.3 (0.9‐6.3) | .096 | na | na | 1.35 (0.7‐2.5) | .34 |
| URTI‐Symptoms a | 2.47 (1.5‐4.2) | .001 | 3.22 (1.9‐5.4) | <.001 | 2.83 (1.1‐7.4) | .034 | 3.41 (1.3‐9.0) | .014 | 2.9 (1.6‐5.4) | .001 |
| GvHD | na | na | na | na | na | na | na | na | 1.0 (0.5‐1.9) | .99 |
URTI‐Symptoms included cough, running nose, sore throat, and fever.
3.1. Epidemiology of different cohorts
To address the question whether patients and controls have the same epidemiology of CARV‐infections, we analyzed the two seasons with parallel recruitment separately. Also, we included only the first positive result. There was a marked difference in the CARV detected in TG from patients versus those from controls with a predominance of coronavirus and influenza in controls. In contrast, patients had more frequent detection of RSV, PIV, and adenovirus (Figure 2, P = .033). As patients and controls in the entire cohort had a marked age‐difference and age appears to be an independent risk factor, we performed a separate analysis including only TG from trial participants >42 years of age (64 patients with a median age of 57 years and 45 controls with a median age of 55 years). In this smaller cohort, the same pattern could be detected, although it failed to reach statistical significance (P = .23, Figure 2) probably due to small sample size. On a closer look, we found an increase of RSV positive cases in the patient cohort within a close time frame: Three of altogether 18 patient samples tested positive for RSV were detected within 1 week in springtime 2015. Because the samples were frozen after collection and analyzed blockwise later on, treating clinicians were unaware of the results and no measures whatsoever were taken. Clinically, there was no suspicion of an outbreak and no patient developed LRTI or died.
Figure 2.
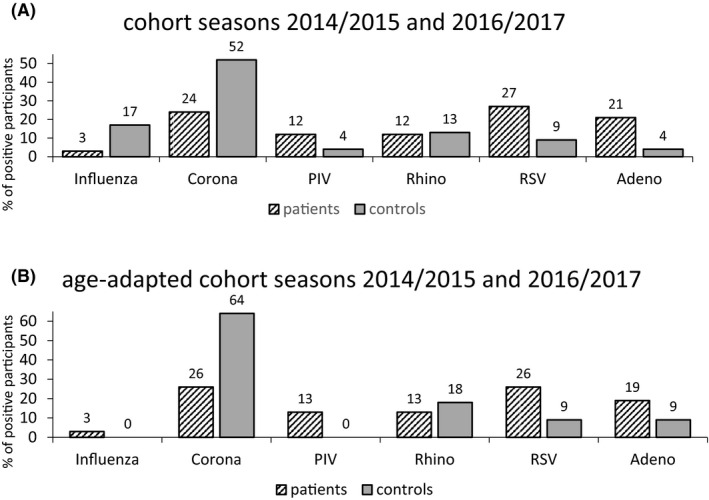
A, Cohort seasons 2014/2015 and 2016/2017. Only first positive results, P = .033. Corona: Coronavirus; PIV: Parainfluenza, hMPV: human Metapneumovirus; RV: Rhino‐ and Enterovirus; RSV: Respiratory Syncytial Virus; Adeno: Adenovirus; B, Age‐adapted cohort seasons 2014/2015 and 2016/2017 (Age >42 y, TG from controls n = 90 with 11 positive, TG from patients n = 136 with 34 positive). Only first positive results, P = .23. Corona: Coronavirus; PIV: Parainfluenza, hMPV: human Metapneumovirus; RV: Rhino‐ and Enterovirus; RSV: Respiratory Syncytial Virus; Adeno: Adenovirus
3.2. Shedding
In 13 of our patients, the same respiratory virus was detected in consecutive samples indicating a prolonged shedding of the virus. In contrast, no shedding could be observed in controls. Median duration of shedding was 28 days (range 13‐58 days). Duration of virus detection for each patient and virus is shown in Figure 3. We found no differences regarding age, gender, time after transplant, smoking status, or number of children in the household in those patients with prolonged shedding versus those with rapid clearance (data not shown). Of note, most patients, in which viruses could be detected in consecutive TG were symptomatic, suggesting a clinically relevant infection with the detected virus.
Figure 3.
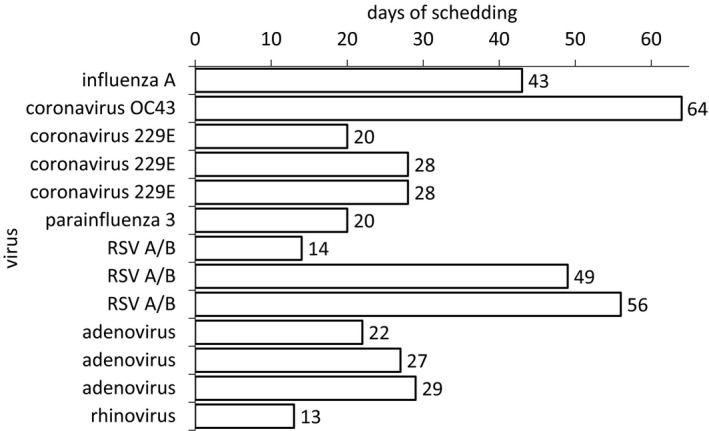
Duration of shedding in patients with repeatedly positive TG in days
4. DISCUSSION
In this prospective study of patients after allo‐SCT and controls, we found a higher rate of positivity for CARV in TG from patients compared with controls. Interestingly, age was associated with a higher rate of positivity in controls but not in patients. Also, despite a generally high rate of symptoms of URTI in individuals with negative TG, positivity for CARV was almost always associated with typical clinical symptoms. Patients had a different epidemiology of CARV with an increased frequency of RSV, PIV, and adenovirus whereas controls had a predominance of coronavirus.
We were surprised to find an increased risk of CARV in subjects of higher age. It is known that children exhibit the highest risk, 10 but the higher risk of elderly people is increasingly recognized. 11 , 12 , 13 Of note, the effect of age appears to be unique to the healthy population, since it could not be confirmed as independent risk factor in our patients, consistent with other studies. 14 Also, the risk may start earlier than previously assumed. 10 Our data suggest that an age of 40 years and above was associated with an increased risk whereas others have only investigated the age over 65. 10
Typical symptoms proved to be the most relevant risk factor for the presence of CARV, consistent with previous findings from other groups. 15 , 16 However, similar to others we could not find a specific pattern of symptoms for a specific virus with the possible exception of fever, which was most common in influenza infection. 15 Thus, in an individual patient potential testing of viruses should not be guided by specific symptoms. Symptoms of URTI justify testing the widest available range of CARV. In a hospital setting, adherence to isolation precautions is critical in preventing transmission of infectious agents. Allo‐SCT patients who are in the hospital are placed on droplet precautions and may benefit from wearing masks or N95 respirators during the pre‐engraftment period when they are outside their hospital rooms. They are advised to minimize the time spent in crowded areas to avoid exposure to persons with community‐acquired respiratory virus (CRV) infections. 17
In our study cohorts, we could not show an association of CARV detection and contact to children or other lifestyle factors. This finding questions the necessity of social distancing with families or friends with children in the household and supports the opportunity to a faster return to normal life after allo‐SCT.
The potentially most relevant finding of our study is the different epidemiology of CARV in patients after allo‐SCT versus healthy individuals. Especially with regard to the latter group, there is a general lack of available data. To the best of our knowledge, there is no study to date comparing these two populations prospectively in the same regional and seasonal setting. In the literature, RSV appears to be more common in allo‐SCT‐recipients than in otherwise healthy people with a reported incidence of 16%‐18% 5 , 14 in allo‐SCT‐recipients versus 2%‐4% 7 , 18 in the general population. This pattern is reflected in our study population with 26% RSV in patients versus 6% RSV in controls. Three patients were tested positive within 1 week which suggests an RSV outbreak in our outpatient clinic of the stem cell transplantation program. Such outbreaks are well described in the literature. 19 , 20 , 21 However, this occurred in the middle of the typical season (springtime 2015) and clinically there was no reason to suspect an outbreak. It is therefore unlikely that an undetected outbreak is the sole reason for the different epidemiology.
In most studies, influenza appears to be the most prominent CARV in the general population; however, they usually include symptomatic people only 4 , 7 , 18 , 22 , 23 , 24 and did not always test for coronaviruses. 24 Yet, coronaviruses were by far the most common CARV detected in our controls. It may be that they are often underdiagnosed because coronaviruses are often not tested for. Our data suggest that coronaviruses account for a large proportion of common colds, similar to rhinoviruses. In contrast, adenoviruses which are also frequently left out in testing panels seem to play a minor role in healthy adults. In patients on the other hand, they appear to be potentially as relevant as RSV since we could detect them in 21% of positive patients.
In contrast to others, we did not detect any bocaviruses, parechoviruses, Mycoplasma, and only very few hMPV in patients or in controls. 25 This may be a sampling error due to the design of the study since these pathogens do not exhibit such a strict seasonality as influenza and RSV and we only sampled during the winter months. 26 Alternatively, they play a minor role in our setting in the healthy population as well as in patients. Since CARV‐infection requires human contact it seems likely that there are significant regional variations and it seems prudent to acquire knowledge regarding the local epidemiology.
Not surprisingly, our study confirmed the potential of long viral shedding in cancer patients as described previously. 6 , 27 Re‐assuringly, we did not detect any shedding in controls, which supports the practice of stopping contact isolation after resolution of symptoms in otherwise healthy people without the need for a negative test. In cancer patients, however, our study confirms the need for re‐testing to be able to declare a patient free of virus. 8
Generally, infections with CARV can be potentially dangerous even in non‐immunosuppressed people. 28 However, the higher rate of complications, especially LRTI, in cancer patients has been well described. 1 , 29 In the literature, the rate of LRTI is assumed to be around 25% with an associated mortality of 30%. 8 In the patient group, the rate of LRTI was 8%, which is lower than expected, but the associated mortality was 25% which is consistent with published data. In our study, we did not observe any LRTI or fatal course in controls. The low rate of LRTI may be due to the fact that patients were assessed at random time‐points and not only triggered by relevant clinical symptoms and we therefore detected more CARV‐infections (less severe) than would have been detected in routine clinical practice.
In conclusion, allo‐SCT recipients have more CARV‐infections and exhibit a different epidemiology than controls with more RSV and adenovirus. Although we did not find an association with obvious immunosuppression or GvHD, this may still be a symptom of a suppressed immune system. In addition, patients have a more complicated course with more cases of co‐infection and prolonged shedding as well as a higher rate of LRTI and mortality. Prolonged viral shedding was not observed in non–immuno‐suppressed controls. Risk factors for the detection of a CARV are age >40 years and symptoms typical for URTI.
CONFLICT OF INTERESTS
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
TR, JH, KW, BH, and MvLT designed the study. JK, SK, TL, PS, NW, AK, and IH recruited patients and controls and set up the database. JK, SK, KW, and PS performed the experiments. TR, TL, SL, and MvLT analyzed the data. TR, SL, and MvLT wrote the manuscript. TR, MMR, IH, SL, AH, and MvLT revised the manuscript for its final version.
ETHICAL APPROVAL
The study was approved by the local institutional review board (Nr 3891‐09/13) in 2013.
Supporting information
Fig S1
ACKNOWLEDGEMENTS
Open access funding enabled and organized by Projekt DEAL.
Rachow T, Lamik T, Kalkreuth J, et al. Detection of community‐acquired respiratory viruses in allogeneic stem‐cell transplant recipients and controls—A prospective cohort study. Transpl Infect Dis. 2020;22:e13415. 10.1111/tid.13415
REFERENCES
- 1. Hermann B, Lehners N, Brodhun M, et al. Influenza virus infections in patients with malignancies – characteristics and outcome of the season 2014/15. A survey conducted by the Infectious Diseases Working Party (AGIHO) of the German Society of Haematology and Medical Oncology (DGHO). Eur J Clin Microbiol Infect Dis. 2017;36(3):565‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl J Med. 2001;344(25):1917‐1928. [DOI] [PubMed] [Google Scholar]
- 3. Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98(3):573‐578. [DOI] [PubMed] [Google Scholar]
- 4. Kim ES, Park KU, Lee SH, et al. Comparison of viral infection in healthcare‐associated pneumonia (HCAP) and community‐acquired pneumonia (CAP). PLoS One. 2018;13(2):e0192893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mikulska M, Del Bono V, Gandolfo N, et al. Epidemiology of viral respiratory tract infections in an outpatient haematology facility. Ann Hematol. 2014;93(4):669‐676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lehners N, Tabatabai J, Prifert C, et al. Long‐term shedding of influenza virus, parainfluenza virus, respiratory syncytial virus and nosocomial epidemiology in patients with hematological disorders. PLoS One. 2016;11(2):e0148258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ieven M, Coenen S, Loens K, et al. Aetiology of lower respiratory tract infection in adults in primary care: a prospective study in 11 European countries. Clin Microbiol Infect. 2018;24(11):1158‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. von Lilienfeld‐Toal M, Berger A, Christopeit M, et al. Community acquired respiratory virus infections in cancer patients‐Guideline on diagnosis and management by the Infectious Diseases Working Party of the German Society for haematology and Medical Oncology. Eur J Cancer. 2016;67:200‐212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ison MG, Hirsch HH. Community‐acquired respiratory viruses in transplant patients: diversity, impact, unmet clinical needs. Clin Microbiol Rev. 2019;32:e00042‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ambrosioni J, Bridevaux PO, Wagner G, Mamin A, Kaiser L. Epidemiology of viral respiratory infections in a tertiary care centre in the era of molecular diagnosis, Geneva, Switzerland, 2011–2012. Clin Microbiol Infect. 2014;20(9):O578‐O584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McClure DL, Kieke BA, Sundaram ME, et al. Seasonal incidence of medically attended respiratory syncytial virus infection in a community cohort of adults >/=50 years old. PLoS One. 2014;9(7):e102586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chasqueira MJ, Paixao P, Rodrigues ML, et al. Respiratory infections in elderly people: viral role in a resident population of elderly care centers in Lisbon, winter 2013–2014. Int J Infect Dis. 2018;69:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Masse S, Capai L, Falchi A. Epidemiology of respiratory pathogens among elderly nursing home residents with acute respiratory infections in Corsica, France, 2013–2017. Biomed Res Int. 2017;2017:1423718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sim SA, Leung VKY, Ritchie D, Slavin MA, Sullivan SG, Teh BW. Viral respiratory tract infections in allogeneic haematopoietic stem cell transplant recipients in the era of molecular testing. Biol Blood Marrow Transplant. 2018;24(7):1490–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bridevaux PO, Aubert JD, Soccal PM, et al. Incidence and outcomes of respiratory viral infections in lung transplant recipients: a prospective study. Thorax. 2014;69(1):32‐38. [DOI] [PubMed] [Google Scholar]
- 16. Soccal PM, Aubert JD, Bridevaux PO, et al. Upper and lower respiratory tract viral infections and acute graft rejection in lung transplant recipients. Clin Infect Dis. 2010;51(2):163‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yokoe D, Casper C, Dubberke E, et al. Infection prevention and control in health‐care facilities in which hematopoietic cell transplant recipients are treated. Bone Marrow Transplant. 2009;44(8):495‐507. [DOI] [PubMed] [Google Scholar]
- 18. Alimi Y, Lim WS, Lansbury L, Leonardi‐Bee J, Nguyen‐Van‐Tam JS. Systematic review of respiratory viral pathogens identified in adults with community‐acquired pneumonia in Europe. J Clin Virol. 2017;95:26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chu HY, Englund JA, Podczervinski S, et al. Nosocomial transmission of respiratory syncytial virus in an outpatient cancer center. Biol Blood Marrow Transplant. 2014;20(6):844‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kassis C, Champlin RE, Hachem RY, et al. Detection and control of a nosocomial respiratory syncytial virus outbreak in a stem cell transplantation unit: the role of palivizumab. Biol Blood Marrow Transplant. 2010;16(9):1265‐1271. [DOI] [PubMed] [Google Scholar]
- 21. Lehners N, Schnitzler P, Geis S, et al. Risk factors and containment of respiratory syncytial virus outbreak in a hematology and transplant unit. Bone Marrow Transplant. 2013;48(12):1548‐1553. [DOI] [PubMed] [Google Scholar]
- 22. Loubet P, Voiriot G, Houhou‐Fidouh N, et al. Impact of respiratory viruses in hospital‐acquired pneumonia in the intensive care unit: a single‐center retrospective study. J Clin Virol. 2017;91:52‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Piralla A, Mariani B, Rovida F, Baldanti F. Frequency of respiratory viruses among patients admitted to 26 Intensive Care Units in seven consecutive winter‐spring seasons (2009–2016) in Northern Italy. J Clin Virol. 2017;92:48‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Landes MB, Neil RB, McCool SS, et al. The frequency and seasonality of influenza and other respiratory viruses in Tennessee: two influenza seasons of surveillance data, 2010–2012. Influenza Other Respir Viruses. 2013;7(6):1122‐1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zheng XY, Xu YJ, Guan WJ, Lin LF. Regional, age and respiratory‐secretion‐specific prevalence of respiratory viruses associated with asthma exacerbation: a literature review. Arch Virol. 2018;163(4):845‐853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Visseaux B, Burdet C, Voiriot G, et al. Prevalence of respiratory viruses among adults, by season, age, respiratory tract region and type of medical unit in Paris, France, from 2011 to 2016. PLoS One. 2017;12(7):e0180888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Richardson L, Brite J, Del Castillo M, et al. Comparison of respiratory virus shedding by conventional and molecular testing methods in patients with haematological malignancy. Clin Microbiol Infect. 2016;22(4):380.e1‐380.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after laboratory‐confirmed influenza infection. N Engl J Med. 2018;378(4):345‐353. [DOI] [PubMed] [Google Scholar]
- 29. Memoli MJ, Athota R, Reed S, et al. The natural history of influenza infection in the severely immunocompromised vs nonimmunocompromised hosts. Clin Infect Dis. 2014;58(2):214‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1


