Abstract
Novel coronavirus disease 2019 (COVID‐19), a 2020 pandemic, has resulted in an unexpected loss in lives, quality of life, and the economy. The COVID‐19 clinical spectrum varies from asymptomatic to death, and its complications may involve various organs. Notwithstanding, the impact of COVID‐19 on endocrine systems is understudied. Previous coronavirus outbreaks such as severe acute respiratory syndrome coronavirus can cause new‐onset diabetes mellitus (DM). However, there were only two previous case reports on newly diagnosed DM in COVID‐19 patients. Here, we described three patients who had newly diagnosed DM associated with COVID‐19. COVID‐19 likely unmasked existing DM by aggravating its metabolic complications rather than causing the new‐onset DM in these patients. However, more research is needed to evaluate if there is a casual relationship between the development of DM, DKA, and COVID‐19.
Keywords: COVID‐19, DKA, DM, SARS‐CoV‐2
1. INTRODUCTION
In 2020, novel coronavirus disease 2019 (COVID‐19), has become a catastrophic pandemic affecting people almost over the entire world and severely affecting public health security. Its pathogen, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), is the third identified human beta‐coronavirus, which is reported to target pulmonary systems. 1 Clinical spectrums of COVID‐19 vary from asymptomatic to lethal pneumonia. Besides this, COVID‐19 has unpredictable effects on many organs. However, data regarding the endocrine impact of COVID‐19 are limited. Herein, we present the three cases of newly diagnosed diabetes mellitus (DM) associated with COVID‐19, raising the question of whether COVID‐19 can cause endocrine abnormalities.
2. CASE PRESENTATION
2.1. Case 1
A 18‐year‐old obese man came to our hospital due to abnormally high blood sugar. He reported fatigue, polydipsia, and polyuria for 10 days. His mother measured his blood sugar, which ranged between 400 and 500 mg/dL. He had no fever nor respiratory symptoms. Family history was remarkable for DM type 2 in many family members, including his mother and father. He denied previous medical problems. The last HbA1C measured 1 year prior was normal (4.8%). Vital signs were normal. He had a body mass index (BMI) of 32. Systemic examination was unremarkable except for mild generalized tenderness around the periumbilical area.
Blood test results are demonstrated in Table 1. Briefly, he had hyperglycemia, high anion gap metabolic acidosis, and ketonemia. Urine analysis showed a large amount of sugar and ketone. Screening SARS‐CoV‐2 reverse‐transcription polymerase chain reaction (RT‐PCR) returned positive. Insulin antibodies, islet cells antibodies, anti‐cyclic citrullinated peptide (anti‐CCP) antibody glutamic acid decarboxylase were all negative. He was diagnosed with diabetic ketoacidosis (DKA) in newly diagnosed DM and asymptomatic COVID‐19 and received treatment with insulin drip, intravenous hydration, replacement of electrolytes, and supportive measures. No specific COVID‐19 treatment was given. His DKA resolved the following day, and he was discharged home with a subcutaneous insulin regimen and metformin on hospital day 3. He did not have worsening pulmonary symptoms nor hypoxemia throughout hospitalization.
Table 1.
Blood test results of each patient
| Parameter | Patient 1 | Patient 2 | Patient 3 | Normal values | Unit |
|---|---|---|---|---|---|
| Random blood sugar | 496 | 795 | 353 | 74‐109 | mg/dL |
| pH | 7.12 | 7.30 | N/A | 7.32‐7.42 | |
| Bicarbonate | 15 | 17 | 24 | 22‐29 | mmol/L |
| White blood cells | 7110 | 8720 | 9430 | 4080‐10 800 | cells/µL |
| Neutrophils | 64 | 71 | 78 | 44‐77 | % |
| Lymphocytes | 27 | 23 | 15 | 20‐45 | % |
| Beta‐hydroxybutarate | >4.4 | >4.4 | 2.06 | <0.27 | mmol/L |
| Anion gap | 25 | 25 | 19 | 8‐16 | mEq/L |
| HbA1C | 10.4 | 12.4 | N/A | <5.7 | % |
2.2. Case 2
A 51‐year‐old man came to our hospital due to worsening fatigue. He reported having tested positive for COVID‐19 2 months prior, in which he had fever and cough, but the symptoms were mild and he did not require hospital treatment. However, since then, he had been feeling fatigued, with lack of energy, and anorexia. Two weeks prior, he started having polyuria and polydipsia. Later, he developed nausea and vomiting for the past 2 days. He also reported some weight loss during this period but denied any other symptoms. None of the family members had DM nor cardiovascular diseases. He denied previous medical problems, did not drink alcohol nor used any drugs. Vital signs were normal. BMI was 34. Systemic physical examination was unremarkable. Chest X‐ray was normal. Repeat SARS‐CoV‐2 RT‐PCR was negative.
Blood test results revealed hyperglycemia, high anion gap metabolic acidosis, and ketonemia (Table 1). He was admitted to the COVID‐19 floor diagnosed with newly diagnosed DM and starvation ketoacidosis, and received treatment with subcutaneous insulin, aggressive intravenous hydration, electrolyte replacement, and supportive measures. The patient was discharged with a subcutaneous insulin regimen on day 3.
2.3. Case 3
A 64‐year‐old woman came to our hospital due to polyuria and polydipsia for 10 days. She tested positive for COVID‐19 10 weeks prior, wherein she had a fever and cough. Her symptoms were completely resolved in a few days without hospital admission or specific treatment. She had a positive family history of DM in her siblings. Her past medical history included breast cancer in remission, and she is currently taking tamoxifen. Vital signs were normal. Systemic physical examination was unremarkable.
Blood test results showed hyperglycemia (Table 1). Urine analysis showed a large amount of sugar and a small amount of ketone. She was newly diagnosed with DM, received intravenous fluid, and discharged home on oral metformin.
3. DISCUSSION
DM negatively impacts COVID‐19 outcomes and worsens mortality. 2 However, the effect of COVID‐19 on DM has not yet been elucidated. In our institutional experience, we encountered many cases of DKA precipitated by COVID‐19 in patients with type 2 diabetes, which is uncommon. Approximately 15% of our hospitalized COVID‐19 patients with DM had developed DKA. Besides this, the mortality of COVID‐19 patients with DKA in our institution is almost 50%.
There were two previously reported cases of newly diagnosed DM in COVID‐19 patients. Chee et al 3 described a 37‐year‐old previously healthy man who presented with DKA from newly diagnosed DM and COVID‐19. 3 The patient had an HbA1C of 14.2%, which indicated that he might have pre‐existing DM aggravated by COVID‐19, causing metabolic complications and DKA. Later, Heaney et al 4 published a 54‐year‐old male case with multiple comorbidities presenting with dyspnea and found to have DKA, newly diagnosed DM, and COVID‐19. The HbA1C result was not available, but assuming his multiple comorbidities including morbid obesity and hypertension, he might have had undiagnosed DM. Both patients had favorable COVID‐19 courses, did not require oxygen supplement, and were discharged home in a few days without complications.
We report three cases of newly diagnosed DM associated with COVID‐19. The first case developed concurrent COVID‐19 and DKA, while the second and third cases had the diagnosis of DM following COVID‐19 illnesses. Although the causal relationship between COVID‐19 and DM cannot be confirmed, it raises the concern of whether COVID‐19 can precipitate the development of new‐onset DM.
Rubino et al 5 proposed that SARS‐CoV‐2 leads to ketosis‐prone diabetes via binding to its cellular entry—ACE‐2 receptors, which are abundant in pancreatic beta cells and adipose tissue, leading to glucose metabolism abnormalities, and pancreatic beta cell destruction (Figure 1). This mechanism underlies the development of DM in SARS‐CoV patients. 6 Besides this, the aberrant immunity caused by SARS‐CoV‐2 may induce an autoimmune attack to the pancreatic islet cells mimicking the pathogenesis of insulin‐dependent DM.
Figure 1.
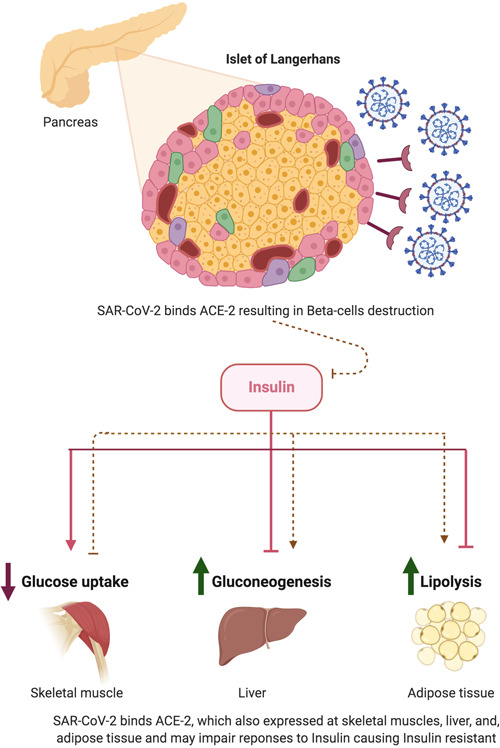
Proposed mechanisms of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) inducing diabetes mellitus
Notwithstanding, the elevated HbA1C and the presence of DM risk factors in several patients may indicate that the newly diagnosed DM is a result of metabolic disturbances from COVID‐19 illnesses unmasking the existing DM rather than causing the new‐onset of disease. However, the unusual high incidences of DKA in type 2 DM raise the issue of whether COVID‐19 can further damage pancreatic islet cells leading to insulin deficiency states. More research is needed to investigate the causal relationship between DM, DKA, and COVID‐19.
Interestingly, all reported patients had a very favorable course of COVID‐19; none had developed hypoxemia, despite the presence of the other negative prognostic factors such as advanced age, obesity, male sex, and multiple comorbidities. This result contradicts the verified theory that DM patients have poor COVID‐19 outcomes. 7 , 8 Several reasons may underlie this unexpected finding. First, the duration of DM may be more important than the presence of DM itself. Second, the worsening COVID‐19 prognoses may be a result of other confounding factors in the patients who had DM, such as obesity, kidney disease, cardiovascular disease, and the use of certain medications. Third, it may be just sampling bias, as there were only five patients reported so far.
In conclusion, we reported three cases of new diagnoses DM associated with COVID‐19 illnesses. More research is needed to evaluate the association between DM, DKA, and COVID‐19.
Suwanwongse K, Shabarek N. Newly diagnosed diabetes mellitus, DKA, and COVID‐19: Causality or coincidence? A report of three cases. J Med Virol. 2021;93:1150–1153. 10.1002/jmv.26339
REFERENCES
- 1. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265‐269. 10.1038/s41586-020-2008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. 10.1016/s0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chee YJ, Ng SJH, Yeoh E. Diabetic ketoacidosis precipitated by Covid‐19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. 10.1016/j.diabres.2020.108166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heaney AI, Griffin GD, Simon EL. Newly diagnosed diabetes and diabetic ketoacidosis precipitated by COVID‐19 infection. Am J Emerg Med. 2020. 10.1016/j.ajem.2020.05.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubino F, Amiel SA, Zimmet P, et al. New‐onset diabetes in Covid‐19 [published online ahead of print June 12, 2020]. N Engl J Med. 2020. 10.1056/NEJMc2018688 [DOI] [Google Scholar]
- 6. Yang J‐K, Lin S‐S, Ji X‐J, Guo L‐M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith SM, Boppana A, Traupman JA, et al. Impaired glucose metabolism in patients with diabetes, prediabetes, and obesity is associated with severe COVID‐19. J Med Virol. 2020. 10.1002/jmv.26227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyazawa D. Why obesity, hypertension, diabetes, and ethnicities are common risk factors for COVID‐19 and H1N1 influenza infections. J Med Virol. 2020. 10.1002/jmv.26220 [DOI] [PMC free article] [PubMed] [Google Scholar]


