Aberrant blood cell counts, most prominently lymphopenia, have been identified as markers of the degree of severity of coronavirus disease 2019 (COVID‐19). 1 , 2 , 3 , 4 , 5 However, most of these studies lack comparison to a COVID‐19‐negative contemporary control group. Additionally, the longitudinal composition and dynamics of lymphocyte subsets in patients with COVID‐19 are insufficiently characterised. We performed a prospective cohort study, in which we compared blood cell and lymphocyte subset counts between (i) hospital‐admitted patients with COVID‐19 and a large contemporary COVID‐19‐negative hospitalised control group, and (ii) non‐critically and critically ill patients with COVID‐19. In addition, we evaluated the impact of these parameters on the in‐hospital mortality rate of COVID‐19. Finally, we assessed the dynamics of lymphocyte reconstitution in patients with COVID‐19 over time.
Results
From 1 March until 1 May 2020, all adult patients who were admitted to our specialised COVID‐19 ward on suspicion of COVID‐19 and tested by polymerase chain reaction (PCR) for severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2) were included. Follow‐up lasted until discharge, death or data lock. A total of 551 patients were enrolled. COVID‐19 was diagnosed in 197 patients, either by PCR (158/197, 80%) or by high‐resolution computed tomography imaging (39/197, 20%). Based on intensive care unit admission or death during hospital admission, 82/197 (42%) of these patients with COVID‐19 were retrospectively labelled as ‘critically ill’. COVID‐19 was ruled out in the remaining 354 patients (controls). The definitive diagnoses in the control group were heterogeneous, ranging from bacterial infections to congestive heart failure and cancer. Baseline characteristics and outcomes of the included patients are presented in Table S1.
Complete routine blood analysis was performed on the day of admission for 349/354 controls, 114/115 non‐critically and 78/82 critically ill patients with COVID‐19 (Table 1, Table S2). Compared to the controls, patients with COVID‐19 had a lower prevalence of anaemia and leucocytosis (>11 × 109/l) and a higher prevalence of thrombocytopenia (<150 × 109/l) (Fig 1A–C). Furthermore, anaemia was significantly more prevalent in critically ill patients with COVID‐19, compared to non‐critically ill patients with COVID‐19. Leucocyte differentiation was performed within the first few days of admission for 152/354 controls, 76/115 non‐critically and 46/82 critically ill patients with COVID‐19 (Table 1, Table S2). The median lymphocyte counts were not significantly different between the controls and the patients with COVID‐19, or between non‐critically and critically ill patients with COVID‐19 (Fig 1D). Correspondingly, the prevalence of lymphopenia (<1·0 × 109/l) was similar between the controls and patients with COVID‐19, and between non‐critically and critically ill patients with COVID‐19. Lymphopenia, defined by a less stringent threshold (<1·5 × 109/l), was present in most patients, but more prevalent in patients with COVID‐19. The controls, compared to the patients with COVID‐19, had a higher mean neutrophil count (Figure S1A). Additionally, compared to non‐critically ill patients with COVID‐19, the mean neutrophil count was higher in critically ill patients with COVID‐19.
Table 1.
Blood cell and lymphocyte subset counts of the patients with COVID‐19 and the controls.
| Variable | Controls (no COVID‐19), n = 354 | Patients with COVID‐19, n = 197 | P |
|---|---|---|---|
| Haemoglobin, mmol/l, mean (95% CI) | 7·8 (7·6–7·9) | 8·2 (8·0–8·4) | <0·001 |
| Anaemia, n (%) | 153 (43) | 58 (30) | 0·003 |
| N/A, n | 2 | 4 | |
| Thrombocyte count, × 109/l, mean (95% CI) | 262 (251–274) | 224 (210–237) | <0·001 |
| Thrombocytopenia, n (%) | 46 (13) | 39 (20) | 0·03 |
| N/A, n | 5 | 5 | |
| Leucocyte count, × 109/l, mean (95% CI) | 12·0 (11·4–12·6) | 8·2 (7·1–9·2) | <0·001 |
| Leucocytosis, n (%) | 167 (48) | 34 (18) | <0·001 |
| N/A, n | 3 | 4 | |
| Neutrophil count, × 109/l, mean (95% CI) | 8·9 (8·0–9·8) | 6·2 (5·6–6·8) | <0·001 |
| Neutrophilia, n (%) | 82 (54) | 36 (30) | <0·001 |
| N/A, n | 202 | 75 | |
| Lymphocyte count, × 109/l, median (95% CI) | 1·1 (1·0–1·3) | 1·0 (0·9–1·1) × 109/l | 0·26 |
| Lymphopenia <1·0 × 109/l, n (%) | 77 (51) | 73 (60) | 0·13 |
| Lymphopenia <1·5 × 109/l, n (%) | 107 (70) | 101 (83) | 0·02 |
| N/A, n | 202 | 75 | |
| CD4+‐T‐lymphocyte count, /mm2, median (95% CI) | 589 (444–861) | 581 (486–696) | 0·86 |
| Below reference range, n (%) | 5 (20) | 16 (20) | 0·98 |
| N/A, n | 329 | 118 | |
| CD8+‐T‐lymphocyte count, /mm2, median (95% CI) | 133 (72–278) | 81 (64–101) | 0·25 |
| Below reference range, n (%) | 17 (68) | 70 (89) | 0·02 |
| N/A, n | 329 | 118 | |
| CD4/CD8 ratio, mean (95% CI) | 14·9 (5·8–23·9) | 12·7 (9·0–16·3) | 0·55 |
| B‐lymphocyte count, /mm2, median (95% CI) | 143 (86–204) | 143 (119–174) | 0·85 |
| Below reference range, n (%) | 10 (40) | 33 (42) | 0·88 |
| N/A, n | 329 | 118 | |
| Igκ/Igλ light chain ratio, median (95% CI) | 1·6 (1·4–2·1) | 1·5 (1·4–1·6) | 0·55 |
| NK cell count, /mm2, median (95% CI) | 196 (138–259) | 164 (132–198) | 0·33 |
| Below reference range, n (%) | 7 (28) | 23 (29) | 0·86 |
| N/A, n | 329 | 118 |
Routine blood counting, leucocyte differentiation and immunophenotyping results of patients with COVID‐19 and COVID‐19‐negative controls. P values were calculated using Welch’s t‐test and a Mann–Whitney–Wilcoxon test for normally distributed and non‐normally distributed continuous variables, respectively, and using a chi‐square test for categorical variables. 95% CI, 95% confidence interval; COVID‐19, coronavirus disease 2019; Ig, immunoglobulin; N/A, not available; NK cell, natural killer cell.
Fig 1.
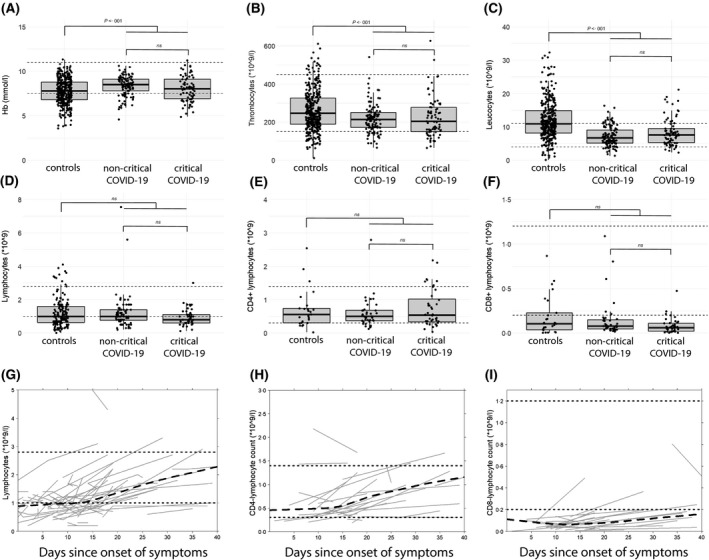
Routine blood analysis, leucocyte differentiation and immunophenotyping. (A–F) Dot plots with routine blood analysis, leucocyte differentiation and immunophenotyping results for controls, non‐critically and critically ill patients with COVID‐19. Each dot represents a single measurement. Upper and lower hinges represent the IQR, whereas the whiskers extend 1·5‐times the IQR. Dotted lines represent the normal reference range. (A–C) routine blood analysis results at day of admission. Significance tested with Student’s t‐test. (D) total lymphocyte counts, quantified by leucocyte differentiation within the first few days of admission. Significance tested with Mann–Whitney–Wilcoxon test. (E,F) CD3+CD4+ and CD3+CD8+‐T‐lymphocyte counts, quantified by immunophenotyping. Significance tested with Mann–Whitney–Wilcoxon test. (G,H) Spaghetti plots illustrating lymphocyte subset counts over time in COVID‐19‐positive patients, with t 0 defined as start of symptoms. Grey lines represent repeated measurements in individual patients, the black dashed line represent the summarised trend of all individual patients and the black dotted lines denote the upper and lower limit of the normal reference range, uncorrected for age. (G) Total lymphocyte counts in patients withCOVID‐19 over time in patients with repeated measurements, including both leucocyte differentiation and immunophenotyping results. (H,I) lymphocyte subset counts over time in COVID‐19‐positive patients. Only patients with repeated immunophenotyping measurements were included. COVID‐19; coronavirus disease 2019; Hb, haemoglobin; IQR, interquartile range; ns, not significant.
In 25/354 controls, 39/115 non‐critically and 40/82 critically ill patients with COVID‐19, lymphocyte subsets were quantified by immunophenotyping during admission (Table 1, Table S2). A list of diagnoses in the 25 controls from whom immunophenotyping data were obtained is presented in Table S3. The median CD4+‐ and CD8+‐T‐lymphocyte counts were similar between the controls, non‐critically and critically ill patients with COVID‐19 (Fig 1D,F). The majority of both controls and patients with COVID‐19 had diminished CD8+‐T‐cell counts (<200/mm2), but this proportion was larger in the COVID‐19 group. Compared to non‐critically ill patients with COVID‐19, critically ill patients with COVID‐19 had a higher mean CD4/CD8 ratio (Figure S1C) and a lower median natural killer (NK) cell count (Figure S1D).
To assess the impact of blood cell and lymphocyte subset counts on the in‐hospital mortality risk in patients with COVID‐19, we performed univariable survival analyses (Figure S2). Patients with thrombocytopenia or leucocytosis at admission had an increased risk of in‐hospital mortality (Figure S2A,B). Although lymphopenia (stringently or leniently defined) was not predictive of in‐hospital mortality for COVID‐19 (not shown), the presence of CD4+‐T‐lymphopenia (<300/mm2) was associated with an increased risk of in‐hospital mortality (Figure S2C).
Immunophenotyping was not performed at a standardised time point for each patient. Additionally, immunophenotyping was performed two‐ or three‐times in 29 and four patients with COVID‐19 respectively. This allowed us to perform a longitudinal analysis in which we included all patients with repeated measurements and defined the date of first symptom as t 0. Although patients with COVID‐19 generally had lymphopenia at admission (Fig 1D), the lymphocyte counts gradually increased to reference values around disease day 14 (Fig 1G). CD4+‐T lymphocytes experienced a similar rise after 2 weeks (Fig 1H), but CD8+‐T‐lymphocyte reconstitution was much slower, and average CD8+‐T‐cell counts did not reach normal limits even after 40 days (Fig 1I).
Discussion and conclusions
The core strength of our present study is the inclusion of a large, contemporary, COVID‐19‐negative control population, which allowed us to critically reappraise the haematological features of patients with COVID‐19. At admission, anaemia, leucocytosis and neutrophilia were more prevalent in the controls than in the patients with COVID‐19. In agreement with recent reports, thrombocyte counts were lower in patients with COVID‐19, and thrombocytopenia was associated with an increased risk of in‐hospital mortality. 6 , 7
Lymphopenia, previously presented as a hallmark of COVID‐19, 1 , 8 , 9 is a common feature of critical illness in general. 10 Indeed, in our present cohort, neither the median lymphocyte counts nor the prevalence of lymphopenia (<1·0 × 109/l) were different between the patients with COVID‐19 and the controls. This suggests that lymphopenia per se, at least stringently defined, is not a discriminatory feature of COVID‐19.
Unfortunately, we were only able to perform immunophenotyping in a proportion of patients. Furthermore, immunophenotyping could not be performed at a standardised time point, which bears the risk of comparing patients at different stages of their disease. Nevertheless, the range in timing of the immunophenotyping, in combination with repeated sampling in some patients, allowed us to demonstrate that in patients with COVID‐19, reconstitution of CD4+‐T lymphocytes starts around disease day 14, whereas CD8+‐T‐lymphocyte reconstitution was much slower. This possibly reflects the functional exhaustion reported in these cells in the context of COVID‐19. 8
In conclusion, we demonstrate that red cell, white cell, and platelet counts are significantly different between COVID‐19‐positive and ‐negative patients, whereas lymphopenia is not a distinguishing feature of COVID‐19. Our present data help to identify patients at higher risk of a critical disease course, who could potentially most benefit from pre‐emptive interventions.
Methods
For the methods section, please see the supporting material.
Conflict of Interest
The authors do not have any conflict of interest to report.
Supporting information
Fig S1. Routine blood analysis, leucocyte differentiation and immunophenotyping.
Fig S2. Survival analysis in COVID‐19‐positive patients.
Table S1. Baseline clinical characteristics and outcomes.
Table S2. Blood cell‐ and lymphocyte subset counts of non‐critically and critically ill patients with COVID‐19.
Table S3. Definitive diagnoses in controls who underwent immunophenotyping.
Data S1. Supplementary methods.
Acknowledgements
Mark‐David Levin was principal investigator and conceived the study. Aaram O. Khader, Inge G. P. Geelen, Eske A. van Baalen, Eva Jansen, Ömer Balak, Peter E. Westerweel and Mark‐David Levin were involved in patient inclusion and data acquisition. Linda H. A. de Bruin and Jurgen A. Riedl performed the flow cytometry. Paul J. Hengeveld, Aaram O. Khader, Inge G. P. Geelen, Eske A. van Baalen, Eva Jansen and Mark‐David Levin extracted the data from the electronic patient files. Paul J. Hengeveld, Nathalie I. Bouwer, Anton W. Langerak and Mark‐David Levin advised and performed the data analysis. Paul J. Hengeveld wrote the manuscript. All authors critically reviewed and approved the final manuscript.
References
- 1. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, et al. Hematological findings and complications of COVID‐19. Am J Hematol. 2020;95:834–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID‐19) infected patients. Br J Haematol. 2020;189:428–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang F, Nie J, Wang H, Zhao Q, Xiong Y, Deng L, et al. Characteristics of peripheral lymphocyte subset alteration in COVID‐19 pneumonia. J Infect Dis. 2020;221:1762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao Q, Meng M, Kumar R, Wu Y, Huang J, Deng Y, et al. Lymphopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a systemic review and meta‐analysis. Int J Infect Dis. 2020;96: 131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: a meta‐analysis. Clin Chim Acta. 2020;506:145–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maquet J, Lafaurie M, Sommet A, Moulis G, Alvarez M, Amar J, et al. Thrombocytopenia is independently associated with poor outcome in patients hospitalized for COVID‐19. Br J Haematol. 2020. [Epub ahead of print]. DOI: 10.1111/bjh.16950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. Functional exhaustion of antiviral lymphocytes in COVID‐19 patients. Cell Mol Immunol. 2020;17:533–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bermejo‐Martin JF, Almansa R, Menéndez R, Mendez R, Kelvin DJ, Torres A. Lymphopenic community acquired pneumonia as signature of severe COVID‐19 infection. J Infect. 2020;80:e23–e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hohlstein P, Gussen H, Bartneck M, Warzecha KT, Roderburg C, Buendgens L, et al. Prognostic relevance of altered lymphocyte subpopulations in critical illness and sepsis. J Clin Med. 2019;8:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Routine blood analysis, leucocyte differentiation and immunophenotyping.
Fig S2. Survival analysis in COVID‐19‐positive patients.
Table S1. Baseline clinical characteristics and outcomes.
Table S2. Blood cell‐ and lymphocyte subset counts of non‐critically and critically ill patients with COVID‐19.
Table S3. Definitive diagnoses in controls who underwent immunophenotyping.
Data S1. Supplementary methods.


