Abstract
The systematic investigations on the value of social wasps as a food resource are deficient, in spite of the long history of the utilization of social wasps as food and pharmaceutical bioresources. Vespa velutina nigrithorax is an invasive alien wasp species that is currently dominating in East Asia and Europe, bringing huge economic damages. As a control over alien species is made when the valuable utilization of the invasive species as a potential resource are discovered, investigations on the potential of V. v. nigrithorax as a useful bioresource are also in demand. Nutritional and heavy metal analyses of the larvae revealed their balanced and rich nutritional value and safety as a food resource. The larval saliva amino acid composition was investigated for further study on amino acid supplementation and exercise enhancement.
Keywords: edible insects, alternative food resource, wasp larva, Vespa velutina nigrithorax
1. Introduction
Social wasps are recognized as harmful insects. They are a threat to humans due to their stinging behaviors that might lead to death [1,2] and their aggressive nature to hunt for honeybees brings huge economic losses in the beekeeping industry [1,3,4]. However, especially in East Asia, social wasps are bioresources that have been utilized for medicinal and food purpose for a long time. In Korea and China, nests of social wasps (Nidus vespae) have been used as an ingredient of crude drugs [5,6], or an ingredient of health-promoting traditional liquors [5]. The entomophagic records about wasps are mostly related to consumption of larvae, which are available in Papua New Guinea [7], China [8] and Laos [9]. In Japan, larvae of wasps, ‘hachinoko’ in Japanese, have long been consumed, and even canned products of the larvae are available at markets [10]. However, wasp larvae have been rather delicacies—not part of daily diets—due to their limited supply from nature. Particularly in China and Japan, where wasp materials provided by wasp hunters are traded at high prices and where wasps were traditionally used as food or pharmacological resources, there have been attempts to develop wasp breeding techniques to ensure sufficient supply of materials [8,11]. Fortunately, in some cases, embryo nest breeding was often successful, but none of the reliable systematic breeding technology has been established yet [8]. To establish wasp larvae as a regular food resource, techniques for mass rearing to ensure sufficient quantities of materials is required [12]. However, prior to the supply issue, it is necessary to evaluate if wasp larvae are worth development for a regular food resource in regards to their nutritional value and food safety. The investigation on Vespa mandarinia larvae by Kim and Jung [13] in 2013 is the only attempt to evaluate the nutritional value of wasp larvae to investigate potential as a food material including taxonomic identification.
Invasion of V. v. nigrithorax is a momentous ecological issue to Europe and East Asia now. Since the first invasion of V. v. nigrithorax into South Korea in 2003, it has spread throughout the country [1,5,14,15,16], and invaded Europe [17] and Japan [18]. V. v. nigrithorax have a serious economic impact as they hunt honeybees in apiaries [2,19] and a public health impact as they sting people in urban areas [1]. This wasp is also causing ecosystem disturbances through competition and interference among Vespa species within the ecosystem [19,20,21]. The southern region of Korea is densely populated by V. v. nigrithorax, and this wasp is the most prevalent in urban and forest areas [22]. In this context of momentous significance, V. v. nigrithorax was selected for investigation.
In this study, we collected larvae from V. v. nigrithorax nests and examined the amino acid composition of their saliva, and nutritional analyses of larvae were performed. The nutritional value of V. v. nigrithorax larvae have not been revealed, though V. v. nigrithorax is now one of the frequently encountered species in East Asia.
2. Materials and Methods
2.1. Wasp Larvae Collection and Preparation
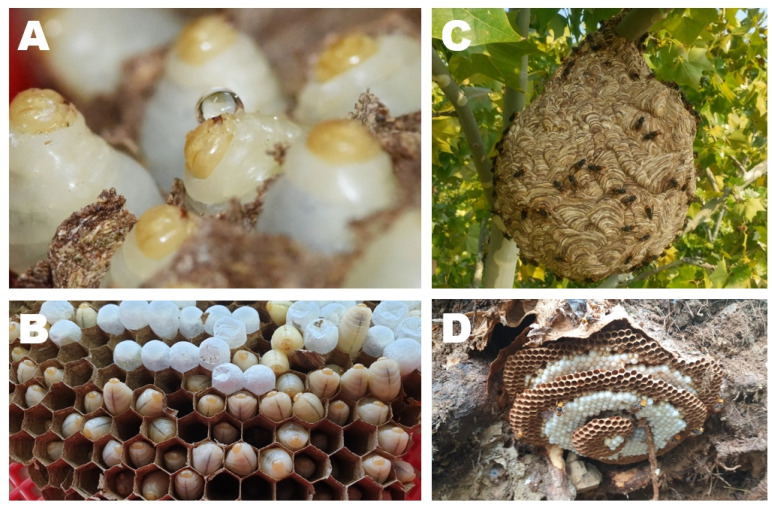
Two V. v. nigrithorax nests were collected from tree branches at heights of approximately 10–15 m in October 2018 in Daegu, South Korea (Figure 1). One of the authors (M.B.C.) identified the builders of these nests as V. v. nigrithorax. The nests contained mature colonies with approximately 1200–1400 adults. The adults were removed from the nests and the combs were separated. Healthy larvae of the fourth or fifth stage were selected for saliva collection. The rest of the larvae were preserved at −80 °C for nutritional analysis.
Figure 1.
The nest of V. v. nigrithorax that hovers larvae. (A) Larvae of V. v. nigrithorax secreting saliva; (B) larvae of V. v. nigrithorax in cells; (C) the nest of V. v. nigrithorax hung on a tree; (D) the nest of V. mandarinia.
2.2. Larval Saliva Preparation
To induce salivation, capillary pipette tips were used to gently tap the mouth parts of the larvae and collect saliva. A 50–200 μL volume of clear saliva was collected from every larva. The tubes containing saliva were immediately frozen and kept at −80 °C until analysis.
2.3. Amino Acid Analysis of the Larvae and Larval Saliva
Qualitative and quantitative analysis for the composition of amino acids in larval saliva was performed through the amino acid automatic analyzer (L-8900, Hitachi, Tokyo, Japan) equipped with an ion exchange column (No. 2622 SCPF, 4.6 mm × 60 mm). Buffer solutions PF-1, 2, 3, 4, 6, PF-RG, R-3, and C-1 (Wako, Osaka, Japan) were used as the mobile phase with a flow rate of 0.35 mL/min, and the column temperature was maintained at 50 °C. The reaction liquid flow rate was 0.3 mL/min, monitoring the wavelengths of 440 nm from 570 nm, and the injection volume was 20 μL. The concentration of amino acids in larval saliva samples was determined by the peak area of standard samples. For hydrolyzed amino acid analysis, 1 g of freeze-dried larvae and 40 mL of 6 N HCl were placed in a round flask and mixed, followed by hydrolysis by injecting nitrogen gas at 110 °C for 24 h. The hydrochloric acid was concentrated under reduced pressure at 50 °C, and the concentrated sample was diluted with 50 mL of 0.2 N sodium citrate buffer (pH 2.2) and filtered with filter paper (0.45 μm). The filtered sample (30 μL) was analyzed using an amino acid analyzer (L-8900, Hitachi, Tokyo, Japan).
2.4. Nutirtional Analysis of the Larvae
The nutritional composition (ash, moisture, lipid, protein and carbohydrate) of lyophilized larvae was determined by following the standard method of the Korean Food Standard Codex [23]. Briefly, the crude protein content was determined by the Kjeldahl method using a crude protein analyzer (Kjeltec™ 8400, Foss, Hilleroed, Denmark), and was calculated by multiplying the nitrogen content using a coefficient of 6.25. Crude fat was extracted from larvae in a Soxhlet apparatus with ethyl ether as the solvent. Moisture content was determined by drying the sample in an oven at 105 °C until a constant weight was obtained. Ash content was determined by dry-ashing in a furnace (J-FM3, Jisico, Seoul, Korea) at 550 °C for 12 h. Carbohydrate content had been determined by subtracting the sum of the weights including ash, moisture, protein and lipid.
2.5. Hazardous Heavy Metals Ananlyses of the Larvae
The contents of hazardous metals, arsenic (As), cadmium (Cd), mercury (Hg) and lead (Pb) were determined using an inductively coupled plasma atomic emission spectroscopy instrument (ICP/OES, Avio 500, Perkin-Elmer, Waltham, MA, USA). For analysis, 140 mg of the lyophilized larvae underwent a nitric acid-pretreatment and were digested with a microwave digestion system (UltraWAVE, Milestone, Sorisole, Italy). The digested samples were diluted with 2% nitric acid (w/w) and injected for instrumental analysis. The analytical condition used for ICP/OES is described in Table 1.
Table 1.
Analytical condition used for inductively coupled plasma atomic emission spectroscopy instrument (ICP/OES).
| Condition | |
|---|---|
| Parameter | Specification |
| Power | 1500 W |
| Plasma gas flow | 10 L/min |
| Auxiliary gas flow | 0.2 L/min |
| Nebulizer gas flow | 0.6 L/min |
| Nebulizer | Concentric nebulizer, glass |
| Torch | Quartz |
| Spray chamber | Cylconic spary chamber |
| Injector | Alumina, inner diameter 2 mm |
| Pump tubing | Polyvinyl Chloride (PVC) |
| Sample pump flow | 1.5 mL/min |
| Rinse/read delay | 60 s |
| Integration time | 3 (3 replicates) |
| Plasma view | Axial |
3. Results and Discussion
3.1. Amino Acid Composition of Larval Saliva
One of a few examples for the application of wasp materials to food is the development of a supplementary beverage for physical exercise inspired from the amino acid composition of V. mandarinia larval saliva [24]. The beverage products do not contain the actual larval saliva of V. mandarinia, but an amino acid mixture composed of the same amino acid composition of V. mandarinia larval saliva. The products were developed on the basis of a series of investigations on exercise endurance enhancement effects by supplementation of the amino acid mixture of which the composition is the same as the larval saliva of V. mandarinia [25,26,27]. This idea has come from trophallaxis, a unique way of survival observed in social wasps. Trophallaxis is common among wasps; carnivorous larvae which feed on meat pellets prepared by adult workers, while larvae produce and pass an oral exudate to adult workers in response [27,28]. For social wasps, larval saliva is considered a source of energy and motivation for adult workers that fly a distance of about 100 km daily only to prepare meat pellets for larvae [29]. Takashi and colleagues reported the composition of amino acids in the larval saliva from five species of hornets including Vespa mandarinia, Vespa crabo, Vespa tropica, Vespa analis and Vespa xanthoptera, collected in central Japan, which has a temperate climate [27]. Based on this research, other related researches were performed subsequently, which were focused on observations of the metabolism of animals administered an amino acid mixture of an identical composition to the larval saliva of V. mandarinia [25,26,29,30,31,32]. The vespa amino acid mixture (VAAM) was coined, based on the free amino acid composition of the larval saliva of V. mandarina, which may be related to aggressiveness and the longest distance flown for hunting [25,27,29]. Studies on the metabolism using of exercising animals revealed that amino acids in larval saliva may act as energy sources and enhance exercise performance through controlling the metabolism [25,26,29,30,31]. Vespa mandarinia, the most dominant hornet species in central Japan, has been used as a reference, as it boasts the longest flight distance and the most extensive hunting domains [25,27,29].
Reports on V. v. nigrithorax invasions to temperate regions sparked an increasing interest in them as their distribution is expanding [1,16,33,34]. However, studies on trophallaxis and the larval saliva of V. v. nigrithorax has not been performed despite the significance of these topics. According to previous research by Takashi et al. [27], each wasp species has a peculiar larval saliva amino acid composition and larval saliva is the principal component of trophallaxis in hornets. There may be a number of factors that influenced the expansion of the V. v. nigrithorax territory beyond the tropical region. In addition, V. v. nigrithorax are found in numbers of 1500–2500 individuals per nest, and their nests are 2–3 times larger than those of other Vespa spp [2,35,36]. The amino acid contents in the larval saliva of V. v. nigrithorax might be correlated with the energy required for exercise and metabolism and could have influenced the growth of their population, which might inspire us to coin a supplementary diet formula. Thus, the amino acid composition of the larval saliva of V. v. nigrithorax was investigated.
As V. mandarinia has been the standard for the physiological studies involved in vespa amino acid mixture (VAAM) supplementation, our results on the amino acid composition of the larval saliva of V. v. nigrithorax were also presented alongside the amino acid composition of the larval saliva of V. mandarinia reported by Takashi et al. [27] (Table 2). A notable feature of the amino acid composition of the larval saliva of V. v. nigrithorax was the high proportion of the physiological amino acids compared to the total amount of amino acids measured from the hydrolyzed product of larval saliva. The total amount of physiological amino acids in the larval saliva of V. v. nigrithorax was 38.29 μmol/mL and the total amount of amino acids deduced from the hydrolyzed product was 67.24 μmol/mL; these results suggest that the larval saliva of V. v. nigrithorax has a higher ratio of free amino acids than that of V. mandarinia. Physiological amino acids are valuable nutrients for adult wasp workers; adult wasps are able to consume only liquid foods and hardly digest polymer-type nutrients, owing to their short and simplified gut structure and narrow waists [37]. Worker wasps in particular, spend most of their time flying for hunting purposes; therefore, free amino acids in larval saliva could help them prolong their flight time, as has been reported in previous studies on animal exercise physiology and VAAM supplementation [25,29]. The amino acids that composed the physiological amino acid mixture of the larval saliva of V. v. nigrithorax were similar to those of saliva from other wasps, as revealed by Takashi et al. [27]. A notable feature of the physiological amino acid composition of the larval saliva of V. v. nigrithorax was the high proportion of proline. The content of proline is believed to be correlated with the daily flight distance and hunting domain size of Vespa spp. [27], as proline is metabolized by insects to produce energy for hunting [38]. Branched chain amino acids (BCAAs) such as Ile, Val, and Leu were detected in substantial amounts (3.28, 5.45, and 3.75 mol%, respectively). In animals, BCAAs are directly metabolized in muscles as energy sources and the intake of BCAAs helps prevent muscular loss caused by endurance exercise [39]. The other essential amino acids (threonine, phenylalanine, lysine, and histidine) were detected in fair amounts (5.48, 5.34, 9.88, and 4.28 mol%, respectively), except for tryptophan and methionine.
Table 2.
Physiological amino acid compositions of larval saliva of V. v. nigrithorax compared to V. mandarinia.
| Amino Acids | V. mandarinia | V. v. nigrithorax | V. mandarinia | V. velutina |
|---|---|---|---|---|
| (μmol/mL) a | (μmol/mL) | (mol%) a | (mol%) | |
| phosphoserine | - | 0.08 | - | 0.22 |
| Tau | 0.39 | 0.82 | 0.51 | 2.15 |
| NH3 | 0.87 | 2.70 | 1.13 | 7.04 |
| Asp | 0.12 | 0.06 | 0.16 | 0.16 |
| Thr | 5.40 | 2.10 | 7.03 | 5.48 |
| Ser | 1.86 | 0.96 | 2.42 | 2.50 |
| Glu | 2.40 | 1.71 | 3.13 | 4.46 |
| sarcosine | - | 0.98 | - | 2.57 |
| Gly | 14.37 | 3.30 | 18.72 | 8.63 |
| Ala | 4.54 | 0.74 | 5.91 | 1.93 |
| Val | 4.40 | 2.09 | 5.73 | 5.45 |
| Cys | - | 0.36 | - | 0.94 |
| Ile | 3.40 | 1.26 | 4.43 | 3.28 |
| Leu | 4.62 | 1.44 | 6.02 | 3.75 |
| Tyr | 4.48 | 1.06 | 5.84 | 2.76 |
| Phe | 2.89 | 2.04 | 3.76 | 5.34 |
| β-Ala | 0.15 | 0.04 | 0.20 | 0.10 |
| β-amino isobutyric acid | - | 0.20 | - | 0.52 |
| GABA | 0.26 | 0.07 | 0.34 | 0.17 |
| EtAm | 0.82 | 0.64 | 1.07 | 1.67 |
| Orn | 0.82 | 0.57 | 1.07 | 1.50 |
| Lys | 6.48 | 3.78 | 8.44 | 9.88 |
| His | 1.94 | 1.64 | 2.53 | 4.28 |
| 3-MeHis | 0.36 | 0.12 | 0.47 | 0.31 |
| Arg | 2.64 | 0.74 | 3.44 | 1.92 |
| hydroxy proline | - | 0.16 | - | 0.41 |
| Pro | 13.55 | 8.65 | 17.65 | 22.58 |
| Total | 76.76 | 38.29 | 100.00 | 100.00 |
a Amino acid contents in larval saliva of V. mandarinia is quoted from the previous research by Takashi et al. [27].
The amino acid content of the hydrolyzed product of the larval saliva of V. v. nigrithorax revealed that a few amino acids were present as peptide constituents (Table 3). Acidic amino acids aspartate and glutamate were detected in significantly different amounts in the physiological and hydrolyzed amino acid mixtures. Aspartate and glutamate were detected in amounts of 0.06 and 1.71 μmol/mL, respectively, in the physiological amino acid mixture, while they were detected in amounts of 3.21 and 10.71 μmol/mL, respectively, in the hydrolyzed amino acid mixture. Other amino acids such as glycine, alanine, isoleucine, and lysine were detected in the hydrolyzed amino acid mixture in quantities that were about 2–3 times higher than those in the physiological amino acid mixture. The larval saliva of V. v. nigrithorax did not contain higher amino acid and protein amounts than that of V. mandarinia. Takashi et al. [27] suggested that high amino acid and protein contents in larval saliva may be correlated with the body weight and the ecological dominance of wasps in nature. However, the larval saliva of the invasive alien species V. v. nigrithorax has not been subjected to amino acid analysis up to this point.
Table 3.
Hydrolyzed amino acid compositions of larval saliva of V. v. nigrithorax compared to that of V. mandarinia.
| Amino Acids | V. mandarinia | V. v. nigrithorax | V. mandarinia | V. v. nigrithorax |
|---|---|---|---|---|
| μmol/mL a | μmol/mL | mol% a | mol% | |
| Asp | 12.29 | 3.21 | 5.62 | 4.77 |
| Thr | 6.71 | 3.18 | 3.07 | 4.74 |
| Ser | 8.47 | 1.59 | 3.87 | 2.36 |
| Glu | 32.17 | 10.71 | 14.71 | 15.93 |
| Gly | 29.85 | 8.24 | 13.65 | 12.25 |
| Ala | 8.90 | 2.14 | 4.07 | 3.18 |
| Cys | 0.67 | 0.57 | 0.31 | 0.85 |
| Val | 11.96 | 4.26 | 5.47 | 6.34 |
| Met | 3.15 | 0.33 | 1.44 | 0.49 |
| Ile | 15.27 | 3.29 | 6.98 | 4.90 |
| Leu | 15.98 | 3.44 | 7.31 | 5.11 |
| Tyr | 7.74 | 0.76 | 3.54 | 1.13 |
| Phe | 6.12 | 3.45 | 2.80 | 5.13 |
| Lys | 22.81 | 7.29 | 10.43 | 10.85 |
| His | 14.13 | 4.00 | 6.46 | 5.96 |
| Arg | 12.75 | 1.60 | 5.83 | 2.38 |
| Pro | 9.72 | 9.17 | 4.44 | 13.64 |
| Total | 218.69 | 67.24 | 100.00 | 100.00 |
a Amino acid contents in larval saliva of V. mandarinia is quoted from the previous research by Takashi et al. [27].
3.2. Nutritional Composition of the Larvae
Table 4 represents the nutrient composition of the lyophilized V. v. nigrithorax larvae compared to other representative edible insect larvae, Protaetia brevitarsis seulensis and Tenebrio molitor. The nutrient compositions of V. mandarinia, P. b. seulensis and T. molitor larvae were quoted from previous reports [35,36,37]. Among carbohydrates in the larvae, the sugar content was 6.08 g/100 g. And the content of saturated fat was 4.31 g/100 g, while the content of trans fat was detected under the limit of quantification. Total cholesterol content was 31.97 mg/100 g. Sodium content was 60.91 mg/100 g.
Table 4.
Nutritional composition of V. v. nigrithorax larvae, compared to those of V. mandarinia, P. b. seulensis and T. molitor larvae (g/100 g).
| Nutrients | Vespa velutina nigrithorax | Vespa mandarinia a | Protaetia brevitarsis seulensis a | Tenebrio molitor a |
|---|---|---|---|---|
| Moisture | 2.78 | - | 6.66 | 5.33 |
| Crude protein | 48.64 | 59.7 | 57.86 | 46.44 |
| Crude fat | 13.23 | 20.6 | 16.57 | 32.70 |
| Crude ash | 3.04 | 4.1 | 8.36 | 2.86 |
| Carbohydrate | 32.31 | 15.6 | 10.56 | 12.67 |
The content of protein in V. v. nigrithorax larvae was similar to that of T. molitor larvae, and less than in P. b. seulensis larvae. The content of fat in V. v. nigrithorax larvae was slightly less than in P. b. seulensis larvae and far less than in T. molitor larvae. Interestingly, carbohydrate content in V. v. nigrithorax larvae was nearly three times of the content in other larvae. Overall nutrient contents in V. v. nigrithorax larvae imply the larvae may provide a nutritionally balanced diet, as they contain sufficient and balanced amounts of essential nutrients compared to other representative edible insect larvae, P. b. seulensis and T. molitor. Compared to dry whole milk, the protein content in V. v. nigrithorax larvae (48.64%) was an overwhelmingly great amount, as protein takes 26.3% of dry milk weight [42]. The protein content in V. v. nigrithorax larvae was even more than commercial dried whey protein concentrate products (protein contents 35–39%) [43].
In 2010, the South Korean government enacted the “Act of Fosterage and Support of the Insect Industry” to establish a legal basis for supporting the growth of the insect industry [44]. Along with the approved insect food ingredients (crickets, T. molitor larvae, P. b. seulensis larvae, and silkworm), wasps are often ingested in Korea even if they are not approved as food ingredients yet [13,45]. However, the nutritional analysis may provide firm grounds for approval of wasp larvae as a food ingredient along with the entomophagic records of wasp larvae in Japan [46], Papua New Guinea [7] and Laos [9].
3.3. Amino Acid Composition in Larvae
Composition of free amino acids in the larvae and amino acids in the hydrolyzed products of the larvae (Table 5.) demonstrated that the larvae of V. v. nigrithorax might be a balanced source for essential amino acids. Total content of free amino acids was nearly a quarter of constitutional amino acids of the larvae, which reflected that the larvae might be good resources for amino acid supplement. Twenty-seven amino acids were found in the free amino acid mixture, and sixteen amino acids were detected in hydrolyzed products of the larvae. In particular, essential amino acid contents, except Met and Trp (Arg, His, Ile, Leu, Lys, Phe, Thr and Val), were detected in substantial amounts in hydrolyzed products of the larvae (7.74–24.86 mg/g).
Table 5.
Amino acids composition in the larvae.
| Amino Acids | Free Amino Acids in The Larvae (mg/g) | Free Amino Acids in the Larvae (mol%) | Hydrolyzed Amino Acids in the Larvae (mg/g) | Hydrolyzed Amino Acids in the Larvae (mol%) |
|---|---|---|---|---|
| phosphoserine | 0.63 | 0.53 | ||
| Tau | 8.14 | 10.17 | ||
| NH3 | 0.30 | 2.76 | ||
| Asp | 0.45 | 0.53 | 24.86 | 7.38 |
| Thr | 3.17 | 4.16 | 13.35 | 4.43 |
| Ser | 1.59 | 2.36 | 11.37 | 4.28 |
| Glu | 2.87 | 3.05 | 44.71 | 12.02 |
| Gly | 10.26 | 21.37 | 17.51 | 9.22 |
| Ala | 2.85 | 5.00 | 12.12 | 5.38 |
| Val | 2.79 | 3.72 | 18.08 | 6.10 |
| Cys | - | - | 3.31 | 1.08 |
| Ile | 2.68 | 3.19 | 13.61 | 4.10 |
| Leu | 3.31 | 3.95 | 21.81 | 6.57 |
| Tyr | 3.62 | 3.12 | 12.07 | 2.63 |
| Phe | 2.68 | 2.54 | 12.90 | 3.09 |
| β-Ala | 0.56 | 0.99 | ||
| β-amino isobutyric acid | 0.30 | 0.46 | ||
| EtAm | 0.15 | 0.51 | ||
| Orn | 0.62 | 0.73 | ||
| Lys | 5.17 | 5.53 | 21.79 | 5.89 |
| His | 1.29 | 1.30 | 7.74 | 3.23 |
| 3-MeHis | 0.10 | 0.10 | ||
| Arg | 6.43 | 5.77 | 14.21 | 1.97 |
| hydroxy proline | 0.37 | 0.44 | ||
| Pro | 12.24 | 16.62 | 24.58 | 13.21 |
| Total | 74.41 | 100 | 277.68 | 100 |
The amount of each hydrolyzed amino acid reflects that V. v. nigrithorax larvae could be a good supplementation for essential amino acids. Compared to the guideline of the World Health Organization (WHO) for daily adult essential amino acid requirement [47], the essential amino acids that might be provided by V. v. nigrithorax larvae are balanced and sufficient. Table 6 represents daily adult amino acid requirement suggested by the WHO, the amount of each essential amino acid required for an adult of 70 kg weight, and the amount of each essential amino acid equivalent to amino acid contained in 100 g of the larvae. On the basis of the guideline from the WHO, the essential amino acid requirements of a 70 kg adult was deduced, and could be mostly fulfilled by supplementation of amino acids provided from V. v. nigrithorax larvae. The amounts of Thr, Cys, Phe, Tyr, Lys, and His contained in 100 g of the larvae were more than the daily amino acid requirements for a 70 kg adult (104–143% of the requirement). Val and Ile provided by 100 g of the larvae were estimated to account for 99% and 97% of the requirements, respectively. The Leu content in 100 g of the larvae was 80% of the requirement. Except Trp and Met which were not detected in the larvae, the majority of the essential amino acids in the larvae are sufficient to fulfil the daily requirements of amino acids in adults.
Table 6.
Comparison of the essential amino acids amounts required for an adult per day and amino acids contained in 100 g of V. v. nigrithorax larvae.
| Amino Acids | Daily Adult Amino Acid Requirements (mg/kg) a | Daily Amino Acid Requirements for a 70-kg Adult (mg) | Amino Acid Provided by 100 g of V. v. nigrithorax Larvae (mg) b | Ratio of Amino Acid Provided to Requirements (%) |
|---|---|---|---|---|
| Thr | 15.0 | 1050 | 1335 | 127 |
| Val | 39.0 | 1820 | 1808 | 99 |
| Cys | 4.1 | 287 | 331 | 115 |
| Ile | 20.0 | 1400 | 1361 | 97 |
| Leu | 39.0 | 2730 | 2181 | 80 |
| Phe + Tyr | 25.0 | 1750 | 2497 | 143 |
| Lys | 30.0 | 2100 | 2179 | 104 |
| His | 10.0 | 700 | 774 | 111 |
| Trp | 4.0 | 280 | - | |
| Met | 10.4 | 728 | - |
3.4. Hazardous Heavy Metal Analysis
By the analysis using the ICP/OES instrument, contents of As, Pb and Cd were not detected, while 23.56 ppb of Hg was detected in the lyophilized larvae. Compared to other edible insects, wasp larvae have an extraordinary carnivorous nature; they are fed on smaller insects hunted by adult wasps and adults are fed on saliva of the larvae. In nature, it is common to contain more heavy metals in the body if an animal species is a higher predator. However, hazardous heavy metal contents in the larvae were not as considerable as a carnivorous animal which might accumulate heavy metals from prey. There is no regulation legislated by the Ministry of Food and Drug Safety (formerly Korea Food & Drug Administration, KFDA)for edible insect products concerning heavy metal contents yet. For fishery products, up to 500 ppb of Hg is allowed to be detected, therefore, the amount of Hg in the larvae would not harm consumers.
3.5. General Discussion
The habitat of V. v. nigrithorax is quickly expanding northward, particularly in Korea [48], since its first reported appearance in 2003 [16]. V. v. nigrithorax has serious negative public health, economic, and ecological impacts [1]. In recent years, invasion of alien species has gradually increased in Korea. However, in most of the cases, the controls over the invasive species were not successful, and various damages are rapidly increasing through the nationwide spread [49,50,51,52]. However, in some cases, the control of alien species is made when the valuable utilization of the invasive species as a potential resource are discovered [53]. V. v. nigrithorax was designated an ecological disturbance species in 2019, causing comprehensive impact in Korea, but the attempts to discover the utilization as a useful bioresource from this species are rare [54].
Owing to environmental pressures, food and feed insecurity, and a growing demand for protein, the consumption of insects to ease the problem of global food shortages has been advocated since 1975 [55]. Along with the approved insect food ingredients (crickets, mealworm, Protaetia brevitarsis larvae, and silkworm), wasps are also often ingested in Korea even if they are not yet approved as food ingredients by KFDA. The history of utilizing the social wasps as a food and pharmaceutical resources suggests that V. v. nigrithorax could be a valuable bioresource.
4. Conclusions
From this study, the potential of V. v. nigrithorax larvae as a food resource has been exhibited. They contain decent amount of nutrients, while hazardous metal contents are scarce. However, additional investigations in sanitary issues and sustainable production technology are necessary to accept the wasp larvae as an alternative food source. The larval saliva of V. v. nigrithorax will not provide original food sources, but the amino acid contents of it can be mimicked and might inspire the development of amino acid supplement formulas for exercise enhancement. This study will initiate future systematic investigations on the potential of V. v. nigrithorax as a valuable bioresource.
Author Contributions
Conceptualization, M.B.C. and D.H.; methodology, D.H., H.J. and B.K.; validation, J.M.K. and M.B.C.; formal analysis, M.B.C. and J.-O.N.; investigation, H.J. and J.M.K.; resources, M.B.C.; data curation, J.M.K. and J.-O.N.; writing—original draft preparation, H.J., D.H. and M.B.C.; writing—review and editing, D.H. and M.B.C.; visualization, H.J. and B.K.; supervision, D.H. and M.B.C.; project administration, M.B.C.; funding acquisition, M.B.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT, No. 2018R1D1A1B07050065).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Choi M.B., Kim J.K., Lee J.W. Increase trend of social hymenoptera (wasps and honeybees) in urban areas, inferred from moving-out case by 119 rescue services in Seoul of South Korea. Entomol. Res. 2012;42:308–319. doi: 10.1111/j.1748-5967.2012.00472.x. [DOI] [Google Scholar]
- 2.Choi M.B., Kwon O. Occurrence of Hymenoptera (wasps and bees) and their foraging in the southwestern part of Jirisan National Park, South Korea. J. Ecol. Environ. 2015;38:367–374. doi: 10.5141/ecoenv.2015.038. [DOI] [Google Scholar]
- 3.Abrol D. Ecology, behaviour and management of social wasp Vespa velutina Smith (Hymenoptera: Vespidae), attacking honeybee colonies. Korean J. Apic. 1994;9:5–10. [Google Scholar]
- 4.Shah F., Shah T. Vespa velutina, a serious pest of honey bees in Kashmir. Bee World. 1991;72:161–164. doi: 10.1080/0005772X.1991.11099099. [DOI] [Google Scholar]
- 5.Kwon H.-O., Kim C.-S., Lee Y.-S., Choi M.B. Abundance of diet-derived polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans in the bodies and nests of the yellow-legged hornet Vespa velutina nigrithorax and risks to human health in South Korea. Sci. Total Environ. 2019;654:1033–1039. doi: 10.1016/j.scitotenv.2018.11.060. [DOI] [PubMed] [Google Scholar]
- 6.Wang B., Zhang C., Gao P., Wu X., Zhao Y. Research progress on Nidus vespae, a traditional Chinese medicine derived from insents. J. Pharm. Sci. Innov. 2013;2:1–9. doi: 10.7897/2277-4572.02680. [DOI] [Google Scholar]
- 7.Mercer C. Sago grub production in Labu swamp near Lae, Papua New Guinea. Klinkii. 1994;5:30–34. [Google Scholar]
- 8.Feng Y., Zhao M., Ding W., Chen X. Overview of edible insect resources and common species utilisation in China. J. Insects Food Feed. 2020;6:13–25. doi: 10.3920/JIFF2019.0022. [DOI] [Google Scholar]
- 9.Boulidam S. Edible insects in a Lao market economy. Edible For. Insects. 2010:131–141. [Google Scholar]
- 10.Mitsuhashi J. Insects as traditional foods in Japan. Ecol. Food Nutr. 1997;36:187–199. doi: 10.1080/03670244.1997.9991514. [DOI] [Google Scholar]
- 11.Nonaka K., Yanagihara H. Reviving the consumption of insects in Japan: A promising case of hebo (Vespula spp., wasps) by high school club activities. J. Insects Food Feed. 2020;6:45–50. doi: 10.3920/JIFF2019.0005. [DOI] [Google Scholar]
- 12.Feng Y., Zhao M., He Z., Chen Z., Sun L. Research and utilization of medicinal insects in China. Entomol. Res. 2009;39:313–316. doi: 10.1111/j.1748-5967.2009.00236.x. [DOI] [Google Scholar]
- 13.Kim H., Jung C. Nutritional characteristics of edible insects as potential food materials. Korean J. Apic. 2013;28:1–8. [Google Scholar]
- 14.Choi M.B., Kim T.G., Kwon O. Recent trends in wasp nest removal and Hymenoptera stings in South Korea. J. Med Entomol. 2019;56:254–260. doi: 10.1093/jme/tjy144. [DOI] [PubMed] [Google Scholar]
- 15.Do Y., Kim J.B., Shim J., Kim C.J., Kwon O., Choi M.B. Quantitative analysis of research topics and public concern on V. velutina as invasive species in Asian and European countries. Entomol. Res. 2019;49:456–461. doi: 10.1111/1748-5967.12390. [DOI] [Google Scholar]
- 16.Kim J.-K., Choi M., Moon T.-Y. Occurrence of Vespa velutina Lepeletier from Korea, and a revised key for Korean Vespa species (Hymenoptera: Vespidae) Entomol. Res. 2006;36:112–115. doi: 10.1111/j.1748-5967.2006.00018.x. [DOI] [Google Scholar]
- 17.Villemant C., Barbet-Massin M., Perrard A., Muller F., Gargominy O., Jiguet F., Rome Q. Predicting the invasion risk by the alien bee-hawking Yellow-legged hornet Vespa velutina nigrithorax across Europe and other continents with niche models. Biol. Conserv. 2011;144:2142–2150. doi: 10.1016/j.biocon.2011.04.009. [DOI] [Google Scholar]
- 18.Ueno T. Establishment of the invasive hornet Vespa velutina (Hymenoptera: Vespidae) in Japan. Int. J. Chem. Environ. Biol. Sci. 2014;2:3. [Google Scholar]
- 19.Monceau K., Maher N., Bonnard O., Thiéry D. Evaluation of competition between a native and an invasive hornet species: Do seasonal phenologies overlap? Bull. Entomol. Res. 2015;105:462–469. doi: 10.1017/S0007485315000280. [DOI] [PubMed] [Google Scholar]
- 20.Cini A., Cappa F., Petrocelli I., Pepiciello I., Bortolotti L., Cervo R. Competition between the native and the introduced hornets Vespa crabro and Vespa velutina: A comparison of potentially relevant life-history traits. Ecol. Entomol. 2018;43:351–362. doi: 10.1111/een.12507. [DOI] [Google Scholar]
- 21.Yamasaki K., Takahashi R., Harada R., Matsuo Y., Nakamura M., Takahashi J.-I. Reproductive interference by alien hornet Vespa velutina threatens the native populations of Vespa simillima in Japan. Sci. Nat. 2019;106:1–5. doi: 10.1007/s00114-019-1609-x. [DOI] [PubMed] [Google Scholar]
- 22.Park J., Jung C. Risk prediction of the distribution of invasive hornet, Vespa velutina nigrothorax in Korea using CLIMEX model. Korean J. Apic. 2018;31:293–303. doi: 10.17519/apiculture.2016.11.31.4.293. [DOI] [Google Scholar]
- 23.Codex K.F.S. KFDA, 10. General Test Methods. Korea Food Drug Administration; Seoul, Korea: 2010. [Google Scholar]
- 24.Abe T., Tsuchita H., Iida K. Amino Acid Composition. No. 6,224,861. U.S. Patent. 2001
- 25.Abe T., Takiguchi Y., Tamura M., Shimura J., Yamazaki K.-I. Effects of Vespa Amino Acid Mixture (VAAM) lsolated from Hornet Larval Saliva and Modified VAAM Nutrients on Endurance Exercise in Swimming Mice. Jpn. J. Phys. Fit. Sports Med. 1995;44:225–237. doi: 10.7600/jspfsm1949.44.225. [DOI] [Google Scholar]
- 26.Sasai H., Matsuo T., Fujita M., Saito M., Tanaka K. Effects of regular exercise combined with ingestion of vespa amino acid mixture on aerobic fitness and cardiovascular disease risk factors in sedentary older women: A preliminary study. Geriatr. Gerontol. Int. 2011;11:24–31. doi: 10.1111/j.1447-0594.2010.00630.x. [DOI] [PubMed] [Google Scholar]
- 27.Takashi A., Yoshiya T., Hiromitsu M., Yasuko Y.K. Comparative study of the composition of hornet larval saliva, its effect on behaviour and role of trophallaxis. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1991;99:79–84. doi: 10.1016/0742-8413(91)90079-9. [DOI] [Google Scholar]
- 28.Matsuura M., Yamane S. Biology of the Vespine Wasps. Springer-Verlag Berlin Heidelberg; Berlin, Germany: 1990. [Google Scholar]
- 29.Tsuchita H., ShiraiMorishita Y., Shimizu T., Abe T. Effects of a Vespa amino acid mixture identical to hornet larval saliva on the blood biochemical indices of running rats. Nutr. Res. 1997;17:999–1012. doi: 10.1016/S0271-5317(97)00064-X. [DOI] [Google Scholar]
- 30.Ogasawara J., Abe T. Amino acid mixture identical to vespa larval saliva increases both leptin secretion and basal lipolysis in rat adipocytes. Food Sci. Technol. Res. 2008;14:95–98. doi: 10.3136/fstr.14.95. [DOI] [Google Scholar]
- 31.Shinozaki F., Abe T. Synergistic effect of Vespa amino acid mixture on lipolysis in rat adipocytes. Biosci Biotechnol. Biochem. 2008;72:1860–1868. doi: 10.1271/bbb.80119. [DOI] [PubMed] [Google Scholar]
- 32.Shinozaki F., Abe T., Kamei A., Watanabe Y., Yasuoka A., Shimada K., Kondo K., Arai S., Kumagai K., Kondo T., et al. Coordinated regulation of hepatic and adipose tissue transcriptomes by the oral administration of an amino acid mixture simulating the larval saliva of Vespa species. Genes Nutr. 2016;11:21. doi: 10.1186/s12263-016-0534-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franklin D.N., Datta S., Keeling M.J., Franklin D.N., Keeling M.J., Brown M.A., Datta S., Keeling M.J., Cuthbertson A.G.S., Budge G.E. Invasion dynamics of Asian hornet, Vespa velutina (Hymenoptera: Vespidae): A case study of a commune in south-west France. Appl. Entomol. Zool. 2017;52:221–229. doi: 10.1007/s13355-016-0470-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi J., Okuyama H., Kiyoshi T., Takeuchi T., Martin S.J. Origins of Vespa velutina hornets that recently invaded Iki Island, Japan and Jersey Island, UK. Mitochondrial DNA Part A. 2019;30:434–439. doi: 10.1080/24701394.2018.1538366. [DOI] [PubMed] [Google Scholar]
- 35.Choi M.B., Martin S.J., Lee J.W. Distribution, spread, and impact of the invasive hornet Vespa velutina in South Korea. J. Asia-Pac. Entomol. 2012;15:473–477. doi: 10.1016/j.aspen.2011.11.004. [DOI] [Google Scholar]
- 36.Rome Q., Muller F., Touret-Alby A., Darrouzet E., Perrard A., Villemant C. Caste differentiation and seasonal changes in Vespa velutina (Hym.: Vespidae) colonies in its introduced range. J. Appl. Entomol. 2015;139:771–782. doi: 10.1111/jen.12210. [DOI] [Google Scholar]
- 37.Morimoto R. Experimental study on the trophallactic behavior in Polistes (Hymenoptera, Vespidae) Acta Hym. Fukuoka. 1960;1:99–103. [Google Scholar]
- 38.Beenakkers A., Van Der Horst D.J., Van Marrewijk W.J.A. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Vol. 10 Pergamon Press; Oxford, UK: 1985. Biochemical process directed to flight muscle metabolism. [Google Scholar]
- 39.Assenza A., Bergero D., Tarantola M., Piccione G., Caola G. Blood serum branched chain amino acids and tryptophan modifications in horses competing in long-distance rides of different length. J. Anim. Physiol. Anim. Nutr. 2004;88:172–177. doi: 10.1111/j.1439-0396.2004.00493.x. [DOI] [PubMed] [Google Scholar]
- 40.Chung M.Y., Gwon E.-Y., Hwang J.-S., Goo T.-W., Yun E.-Y. Analysis of general composition and harmful material of Protaetia brevitarsis. J. Life Sci. 2013;23:664–668. doi: 10.5352/JLS.2013.23.5.664. [DOI] [Google Scholar]
- 41.Ravzanaadii N., Kim S.-H., Choi W.-H., Hong S.-J., Kim N.-J. Nutritional value of mealworm, Tenebrio molitor as food source. Int. J. Ind. Entomol. 2012;25:93–98. doi: 10.7852/ijie.2012.25.1.093. [DOI] [Google Scholar]
- 42.Haytowitz D., Ahuja J., Thomas R., Nickle M., Roseland J., Williams J., Showell B., Somanchi M., Khan M., Nguyen Q. USDA National Nutrient Database for Standard Reference, Release 24. US Department of Agriculture; Washington, DC, USA: 2011. [Google Scholar]
- 43.Evans J., Zulewska J., Newbold M., Drake M., Barbano D. Comparison of composition, sensory, and volatile components of thirty-four percent whey protein and milk serum protein concentrates. J. Dairy Sci. 2009;92:4773–4791. doi: 10.3168/jds.2009-2194. [DOI] [PubMed] [Google Scholar]
- 44.Kim Y.-S., Park C.G., Kim T., Choi J.W. A Study on the Legal Status of Insect Industry. Korean J. Appl. Entomol. 2018;57:401–408. [Google Scholar]
- 45.Jung C. Prospects of insect food commercialization: A mini review. Korean J. Soil Zool. 2013;17:5–8. [Google Scholar]
- 46.Payne C.L., Evans J.D. Nested houses: Domestication dynamics of human–wasp relations in contemporary rural Japan. J. Ethnobiol. Ethnomed. 2017;13:13. doi: 10.1186/s13002-017-0138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.World Health Organization. United Nations University . Protein and amino acid requirements in human nutrition. Vol. 935. World Health Organization; Geneva, Switzerland: 2007. [PubMed] [Google Scholar]
- 48.Choi M.B., Lee S.A., Suk H.Y., Lee J.W. Microsatellite variation in colonizing populations of yellow-legged Asian hornet, Vespa velutina nigrithorax, in South Korea. Entomol. Res. 2013;43:208–214. doi: 10.1111/1748-5967.12027. [DOI] [Google Scholar]
- 49.Choi M.B., Lee S.Y., Yoo J.S., Jun J., Kwon O. First record of the western black widow spider Latrodectus hesperus Chamberlin & Ivie, 1935 (Araneae: Theridiidae) in South Korea. Entomol. Res. 2019;49:141–146. [Google Scholar]
- 50.Lee H., Kim M.J., Bae Y.S., Kim D.E. New occurrence of the invasive alien leaf-footed bug Leptoglossus gonagra (Hemiptera: Coreidae) in South Korea. Entomol. Res. 2020;50:14–22. doi: 10.1111/1748-5967.12402. [DOI] [Google Scholar]
- 51.Lee W., Lee Y., Kim S., Lee J.-H., Lee H., Lee S., Hong K.-J. Current status of exotic insect pests in Korea: Comparing border interception and incursion during 1996–2014. J. Asia-Pac. Entomol. 2016;19:1095–1101. doi: 10.1016/j.aspen.2016.09.003. [DOI] [Google Scholar]
- 52.Sung S., Kwon Y.S., Lee D.K., Cho Y. Predicting the potential distribution of an invasive species, Solenopsis invicta Buren (Hymenoptera: Formicidae), under climate change using species distribution models. Entomol. Res. 2018;48:505–513. doi: 10.1111/1748-5967.12325. [DOI] [Google Scholar]
- 53.Kong J.-Y., Yeon S.-C., Lee H.J., Kang C., Park J.-K., Jeong K.-S., Hong I.-H. Protective Effects of Nutria Bile against Thioacetamide-Induced Liver Injury in Mice. Evid.-Based Complement. Altern. Med. 2019;2019:6059317. doi: 10.1155/2019/6059317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim J., Kim M., Lee M., Lee Y.J., Kim H.R., Nam J.O., Choi M.B., Hahn D. Antibacterial potential of Nidus vespae built by invasive alien hornet, Vespa velutina nigrithorax, against food-borne pathogenic bacteria. Entomol. Res. 2020;50:28–33. doi: 10.1111/1748-5967.12405. [DOI] [Google Scholar]
- 55.Meyer-Rochow V.B. Can insects help to ease the problem of world food shortage. Search. 1975;6:261–262. [Google Scholar]