At the time of writing this editorial (beginning of May 2020), the world has already been facing for several months a major health threat with unprecedented societal changes. The changes were and will continue to be so unpredictable that it is difficult to anticipate what constraints we will be facing at the time you will be reading this editorial. Hopefully, the pandemic will be under control. The new coronavirus, the SARS‐CoV‐2, has granted us a new, multifaceted disease that started as an infectious disease, but has now become a much higher burden due to acute or post‐acute inflammatory events. Pediatric Allergy and Immunology is lucky to have committed contributors who were able to very quickly write articles either for clinical guidance or to update our knowledge, as well as to provide a hypothesis for the mechanisms of infection. The result is this thematic issue focusing on COVID‐19. The issue starts with the recommendations from the EAACI pediatric section on the management of allergies and immunodeficiencies during the pandemic. 1 The next article by Lu and colleagues explains what immune mechanisms are behind COVID‐19 according to the literature to date. 2 This first section ends with Matricardi, Negro, and Nisini developing an interesting hypothesis for the spreading of COVID‐19 based on the viral load and barrier factors. 3 Two further contributions related to COVID‐19 come later in this issue. The first reports on the outbreak of COVID‐19 among health providers in the perinatal center of a large German hospital, 4 and the second on two patients with X‐linked agammaglobulinemia, suggesting that B cell response might be important, but not strictly required to overcome the disease. 5
Mitselou N, Hallberg J, Stephansson O, et al. Adverse pregnancy outcomes and risk of later allergic rhinitis—Nationwide Swedish cohort study. Pediatr Allergy Immunol. 2020.
Niki Mitselou

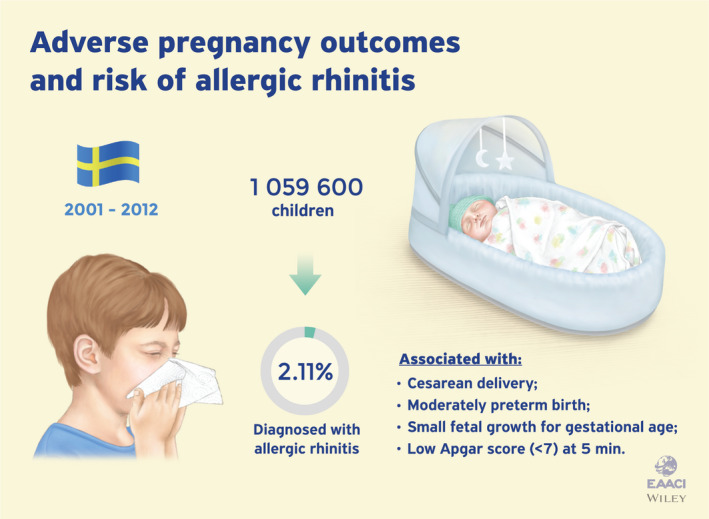
This issue further includes also studies on the various topics usually featured in PAI, and the first study I would like to highlight is a report by Niki Mitselou and colleagues from Stockholm. 6 These investigators analyzed data from a Swedish nationwide longitudinal birth cohort for pregnancy outcome (cesarean delivery, preterm birth, low birth weight) and offspring allergic rhinitis (AR) occurrence. The study period ranged from 2001 to 2013 during which 22,386 children (2.11% of the total) were diagnosed with AR. Children born by cesarean delivery (elective and emergency) had a small increased risk of AR, as well as the children born moderately preterm, large for gestational age, or with a low 5‐minute Apgar score. This large epidemiologic study confirms earlier findings suggesting that gestational circumstances influence the allergic outcome of the child. Among other maternal factors independent of pregnancy outcomes recently reported in PAI, nutritional factors such as the maternal consumption of fermented foods 7 or the early consumption of a diet containing fish by the child 8 have a protective effect on the development of allergies in childhood. Maternal mental health has also been associated with allergy outcomes in the child, 9 besides metabolic factors during pregnancy which may also influence childhood allergies. 10
Padilha LL, Vianna EO, Vale ATM, et al. Pathways in the association between sugar sweetened beverages and child asthma traits in the 2nd year of life: Findings from the BRISA cohort. Pediatr Allergy Immunol. 2020.

Luana Lopes Padilha

The second report presented here addresses the association between sugar‐sweetened beverages (SSB) and childhood asthma at 2 years of age, in the frame of a Brasilian cohort study. 11 Luana Lopes Padilha et al studied this association by modeling pathways mediated by the BMI of the child and allergic inflammation. They first found that a high intake of daily calories from sugars added to SSBs was directly associated with higher values of child asthma traits. In addition, increased levels of eosinophils were also directly associated with child asthma traits, but no mediation pathways were observed via greater BMI z‐scores or eosinophil counts. The study concludes by warning that even in early life, as early as two years of age, exposure to SSB would be a risk factor for childhood asthma traits. Nutrition has definitely been identified as an important factor influencing the arousal of asthma and allergies. 12 And more specifically, ultra‐processed foods are among factors having a potential to unfavorably influence allergy later in life. 13 , 14 Not only foods but more globally the environment may have an impact on childhood asthma, 15 possibly by providing inflammatory signals. 16
Engelkes M, Baan EJ, de Ridder MAJ, et al. Incidence, risk factors and re‐exacerbation rate of severe asthma exacerbations in a multinational, multidatabase pediatric cohort study. Pediatr Allergy Immunol. 2020;31.

Esme Baan

The third study highlighted here reports on severe asthma exacerbation by examining incidence and risk factors influencing the re‐exacerbation rate. 17 Marjolein Engelkeis, Esme Baan and a multinational group of investigators have collected data from 5‐ to 17‐year‐old asthma patients with ≥1 year of follow‐up from six European electronic databases from the Netherlands, Italy, the UK, Denmark, and Spain during the years 2008‐2013. The overall exacerbation rate in children with asthma ranged from 17 to 198/1000 patient‐years and was increased in children with severe asthma. It was the highest in those with severe asthma with a history of exacerbations (283‐1000/1000 patient‐years). Rehospitalization following a previous hospitalization was high in the overall asthma cohort (17%‐29% being rehospitalized in a year). It was higher in children with severe asthma, as up to 79% were rehospitalized within a year. The authors underline the importance of enhanced patient care in children with asthma in order to achieve the ultimate goal of preventing asthma exacerbations. Risk domains in the long‐term management of asthma have recently been developed in PAI, 18 as well as various environmental and infectious factors promoting asthma exacerbations. 19 , 20
At the end of this editorial, I would like to acknowledge the strong commitment of our contributors, the reviewers, and the editorial team for a fast and efficient production of this thematic issue of PAI I hope you will enjoy reading.
REFERENCES
- 1. Brough HA, Kalayci O, Sediva A, et al. COVID‐19 pandemic. Pediatr Allergy Immunol. 2020;31(5):442‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lu X, Xiang Y, Du H, et al. SARS‐CoV‐2 infection in children – Understanding the immune responses and controlling the pandemic. Pediatr Allergy Immunol. 2020;31(5):449‐453. [DOI] [PubMed] [Google Scholar]
- 3. Matricardi PM, Negro RWD, Nisini R. The first, holistic immunological model of COVID‐19: implications for prevention, diagnosis, and public health measures. Pediatr Allergy Immunol. 2020;31(5):454‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kabesch M, Roth S, Brandstetter S, et al. Successful containment of COVID‐19 outbreak in a large maternity and perinatal center while continuing clinical service. Pediatr Allergy Immunol. 2020;31(5):560‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Soresina A, Moratto D, Chiarini M, et al. Two X‐linked agammaglobulinemia patients develop pneumonia as COVID‐19 manifestation but recover. Pediatr Allergy Immunol. 2020;31(5):565‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitselou N, Hallberg J, Stephansson O, et al. Adverse pregnancy outcomes and risk of later allergic rhinitis—Nationwide Swedish cohort study. Pediatr Allergy Immunol. 2020;31(5):471‐479. [DOI] [PubMed] [Google Scholar]
- 7. Celik V, Beken B, Yazicioglu M, et al. Do traditional fermented foods protect against infantile atopic dermatitis. Pediatr Allergy Immunol. 2019;30:540‐546. [DOI] [PubMed] [Google Scholar]
- 8. Vasileiadou S, Wennergren G, Strömberg Celind F, et al. Eating fish and farm life reduce allergic rhinitis at the age of twelve. Pediatr Allergy Immunol. 2018;29:283‐289. [DOI] [PubMed] [Google Scholar]
- 9. Rusconi F, Gagliardi L, Gori E, et al. Perinatal maternal mental health is associated with both infections and wheezing in early childhood. Pediatr Allergy Immunol. 2019;30:732‐738. [DOI] [PubMed] [Google Scholar]
- 10. Berger K, Eskenazi B, Balmes J, et al. Prenatal high molecular weight phthalates and bisphenol A, and childhood respiratory and allergic outcomes. Pediatr Allergy Immunol. 2019;30:36‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Padilha LL, Vianna EO, Vale ATM, et al. Pathways in the association between sugar sweetened beverages and child asthma traits in the 2nd year of life: Findings from the BRISA cohort. Pediatr Allergy Immunol. 2020;31(5):480‐488. [DOI] [PubMed] [Google Scholar]
- 12. Botha M, Basera W, Facey‐Thomas HE, et al. Nutrition and allergic diseases in urban and rural communities from the South African Food Allergy cohort. Pediatr Allergy Immunol. 2019;30:511‐521. [DOI] [PubMed] [Google Scholar]
- 13. Melo B, Rezende L, Machado P, et al. Associations of ultra‐processed food and drink products with asthma and wheezing among Brazilian adolescents. Pediatr Allergy Immunol. 2018;29:504‐511. [DOI] [PubMed] [Google Scholar]
- 14. Azeredo CM, Cortese M, dos Costa C, et al. Pelotas birth cohort study. Pediatr Allergy Immunol. 2004;2020(31):27‐37. [DOI] [PubMed] [Google Scholar]
- 15. Huang C‐C, Chiang T‐L, Chen P‐C, et al. Risk factors for asthma occurrence in children with early‐onset atopic dermatitis: An 8‐year follow‐up study. Pediatr Allergy Immunol. 2018;29:159‐165. [DOI] [PubMed] [Google Scholar]
- 16. de Mendes FC, Paciência I, Rufo JC, et al. The inflammatory potential of diet impacts the association between air pollution and childhood asthma. Pediatr Allergy Immunol. 2020;31:290‐296. [DOI] [PubMed] [Google Scholar]
- 17. Engelkes M, Baan EJ, de Ridder MAJ, et al. Incidence, risk factors and re‐exacerbation rate of severe asthma exacerbations in a multinational, multidatabase pediatric cohort study. Pediatr Allergy Immunol. 2020;31(5):496‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hamelmann E, von Mutius E, Bush A, et al. Addressing the risk domain in the long‐term management of pediatric asthma. Pediatr Allergy Immunol. 2020;31:233‐242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abe N, Yasudo H, Fukano R, et al. Multi‐season analyses of causative pathogens in children hospitalized with asthma exacerbation. Pediatr Allergy Immunol. 2019;30:724‐731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen B‐Y, Chen C‐H, Chuang Y‐C, et al. Changes in the relationship between childhood asthma and ambient air pollution in Taiwan: Results from a nationwide survey repeated 5 years apart. Pediatr Allergy Immunol. 2019;30:188‐194. [DOI] [PubMed] [Google Scholar]


