Abstract
The pandemic condition coronavirus disease (COVID‐19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), can take asymptomatic, mild, moderate, and severe courses. COVID‐19 affects primarily the respiratory airways leading to dry cough, fever, myalgia, headache, fatigue, and diarrhea and can end up in interstitial pneumonia and severe respiratory failure. Reports about the manifestation of various skin lesions and lesions of the vascular system in some subgroups of SARS‐CoV‐2‐positive patients as such features outside the respiratory sphere, are rapidly emerging. Vesicular, urticarial, and maculopapular eruptions and livedo, necrosis, and other vasculitis forms have been reported most frequently in association with SARS‐CoV‐2 infection. In order to update information gained, we provide a systematic overview of the skin lesions described in COVID‐19 patients, discuss potential causative factors, and describe differential diagnostic evaluations. Moreover, we summarize current knowledge about immunologic, clinical, and histologic features of virus‐ and drug‐induced lesions of the skin and changes to the vascular system in order to transfer this knowledge to potential mechanisms induced by SARS‐CoV‐2.
Keywords: allergy, drug hypersensitivity, skin, virus
1. INTRODUCTION
1.1. The story behind the virus with the crown
Coronaviruses belong to a large group of related viruses, named coronavirus family, which can infect humans and animals and lead to diseases of the airways, the gut, liver, and the nervous system. Some members of coronavirus family may infect the upper airways with rather mild courses, and others—as severe acute respiratory syndrome coronavirus SARS‐CoV‐2—may affect the lower respiratory airways with pneumonia and fatal courses (Table 1). 1 , 2 , 3
TABLE 1.
Human coronaviruses
| Upper respiratory airways | Lower respiratory airways |
|---|---|
| HCoV‐229E | SARS‐CoV |
| HCoV‐NL63 | SARS‐CoV‐2 |
| HCoV‐OC43 | MERS‐CoV |
| HCoV‐HKU1 |
Abbreviations: SARS, severe respiratory syndrome coronavirus; MERS, Middle East respiratory syndrome coronavirus.
Belonging to the β‐coronavirus genus, SARS‐CoV‐2 is the pathogen that causes the new infectious respiratory disease, termed as coronavirus disease 19 (COVID‐19), which was reported to have emerged in December 2019, in Wuhan (Hubei province, China), first (or supposedly even earlier) and turned rapidly to be a global pandemic. 2 SARS‐CoV‐2 displays 79% nucleotide identity with SARS‐CoV and 51.8% nucleotide identity with MERS‐CoV, but most importantly shares 96% identity across the entire genome with a bat coronavirus, which is supposed to be the natural origin of SARS‐CoV‐2. 1 , 4 , 5
As other coronaviruses, SARS‐CoV‐2 is an enveloped, positive‐sense, single‐stranded RNA virus with spikes that protrude from the virus surface resembling a crown or “corona” (Figure 1). Most importantly, the spike (S) protein of coronaviruses is essential for viral infection of host cells. During the virus entry procedure, the S protein engages its cellular receptor, angiotensin‐converting enzyme 2 (ACE2), which facilitates viral attachment to the cell surface of target cells. As a next step, the engaged S protein is further primed by the cellular serine protease (transmembrane protease serine 2) TMPRSS2, which mediates membrane fusion and viral entry into the cells (Figure 1). 6 , 7 , 8 , 9 , 10 ACE2 is expressed on the apical surface of the epithelial cells that line conducting airways, as well as alveolar epithelial cells. ACE2 is cleaved from the surface of epithelia by shedding. Soluble ACE2 is catalytically active, interferes with epithelial cell signaling, and has protective defense function. Importantly, as the efficiency of ACE2‐S interaction largely determines SARS‐CoV transmissibility, 11 , 12 , 13 the expression of ACE2 receptors represents a major risk factor for the vulnerability to SARS‐CoV‐2 infection. In humans, the ACE2 receptors are expressed by CD8+ T cells, 14 resting and activated natural killer (NK) cells, 14 alveolar epithelial cells of type II, 15 vascular endothelial cells, macrophages, monocytes, and adipocytes. 16 The nose and the nasal epithelium play an important role for infection and viral spreading. 17 , 18 High ACE2 expression has also been demonstrated on epithelial cells of the oral mucosa, in particular the tongue, so that this receptor might provide an entry route for the virus and designates the oral cavity as a potential organ at high risk for viral spreading from one individual to the other. 19 Tissue distribution of the ACE2 gene includes the small intestine, testis, kidneys, heart, thyroid, and adipose tissue with relatively high expression. 16 It has been recently demonstrated that the inflammatory cytokines interferon (IFN)‐α2 and IFN‐γ increase the expression of ACE2 and is supposed that tissue inflammation may modulate the receptor expression and thereby change the risk of immune cells to be infected by SARS‐CoV‐2. 18 So far, a positive correlation of ACE2 gene expression to CD8+ cells in the skin has been shown 16).
FIGURE 1.

Structure of SARS‐CoV‐2 virus with its typical capsid and the spikes, “corona.” ssRNA, single‐stranded RNA; ACE2, angiotensin receptor 2; TMPRSS2, transmembrane protease serine subtype 2
Binding of SARS‐CoV‐2 to ACE2 downregulates its expression and impacts thereby on its main function, the regulation of the renin‐angiotensin system. This downregulation leads to a dysregulation of the balance of soluble factors, electrolytes, and blood pressure combined with an increase of vascular permeability and lung inflammation. 1 Usually, virus‐specific T cells recruited to the site of inflammation eliminate the virus with neutralizing antibodies generated from B cells and macrophages and prevent thereby virus spreading in an immunocompetent individual. 1 , 20 However, inflammation‐induced cell death of infected cells and damage‐released molecular patterns might induce pro‐inflammatory cytokines and chemokines and recruitment of inflammatory cells to the lung (Figure 2). 21 In patients with severe COVID‐19, HLA class II expression is downregulated and type I interferon‐driven signatures are reduced in peripheral blood cells; that is, monocytes and changes in the function of natural killer cells occur, a mechanism which mirrors evasion of SARS‐CoV‐2 from innate immune sensing and defense. 22 In parallel, inflammatory programs in macrophages resident in the lung are induced. Thus, the lung tissue damage caused by SARS‐CoV‐2 infection and replication, may thereby destroy stepwise the lung structure with the development of pulmonary fibrosis by transformation of adipocytes into myofibroblasts. 23 The knowledge accumulated from SARS and MERS, together with current clinical observations from COVID‐19 patients, suggest that type I IFN‐mediated antiviral responses and activation of both CD4+ Th1 and CD8+ cytotoxic T lymphocytes (CTLs) result in viral clearance in SARS‐CoV‐2‐infected subjects with mild symptoms. However, insufficient initiation of antiviral immune responses, increased production of inflammatory cytokines, and lung infiltration of monocytes and neutrophils contribute to a cytokine storm in severe patients. 24 , 25 Moreover, the cytokine storm elicited from the overproduction of pro‐inflammatory mediators such as interleukin (IL)‐1, IL‐6, IL‐12, and tumor necrosis factor (TNF)‐α, not only leads to increased vascular permeability and inflammation in the lung 24 , 25 but may reach other organs through the vascular system. In the worst case, this might induce injury of multiple other organs including the cardiac, renal, or hepatic system (Figure 2). 1 This cascade of events might lead despite intensive care and a lot of other measures initiated, to fatal courses and death, in particular in elderly patients and individuals with preexisting diseases.
FIGURE 2.
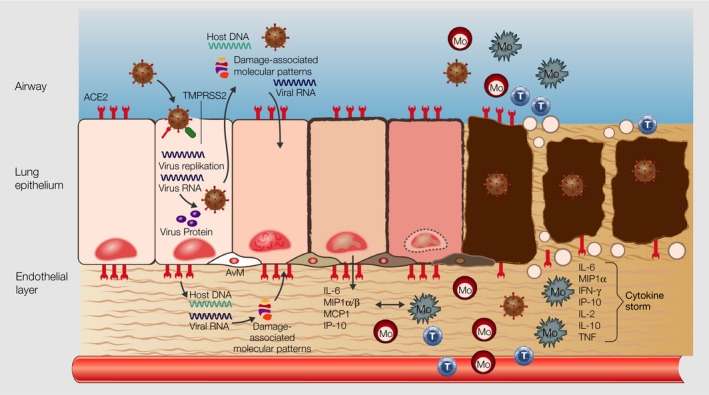
Cascade of events in lung epithelium during SARS‐CoV‐2 infection. SARS‐CoV‐2 infects lung epithelial cells via ACE2 and TMPRSS2 receptor, the host cells undergo apoptosis in consequence of virus replication and release, and the undergoing cell releases damage‐associated molecular patterns, which induce the production of pro‐inflammatory mediators by epithelial and endothelial cells in the neighborhood. Inflammatory cells such as monocytes, macrophages, and T cells are recruited from the blood to the lung epithel, increase the production of inflammatory mediates and further infiltration of the lung by inflammatory cells, leading to damage of the lung structure and a “cytokine storm,” which reaches the vasculature and other organs. ssRNA, single‐stranded RNA; ACE2, angiotensin receptor 2; TMPRSS2, transmembrane protease serine subtype 2; Mo, monocyte, T, T lymphocyte, M, macrophage
2. SARS‐CoV‐2 infection and COVID‐19
2.1. Transmission and symptoms
Transmission of SARS‐CoV‐2 is mediated mainly via respiratory droplets from an infected person. 26 , 27 Of note, SARS‐CoV‐2‐positive patients emit respiratory droplets in high numbers during speaking, which remain in the air up to 8‐14 minutes. 28 The average incubation time until the onset of COVID‐19 is 4‐11 days. 29 The immune responses resulting from SARS‐CoV‐2 infection vary broadly and range from asymptomatic courses, over mild, moderate to severe courses with a need for hospitalization and intensive care. Typical symptoms of COVID‐19 include dry cough, fever, myalgia, headache, anosmia, and ageusia. 30 Depending on the severity of the disease, even other organ systems such as the gastrointestinal tract, the liver, the renal system, and the cardiac system might be involved and become symptomatic as well (Figure 3).
FIGURE 3.
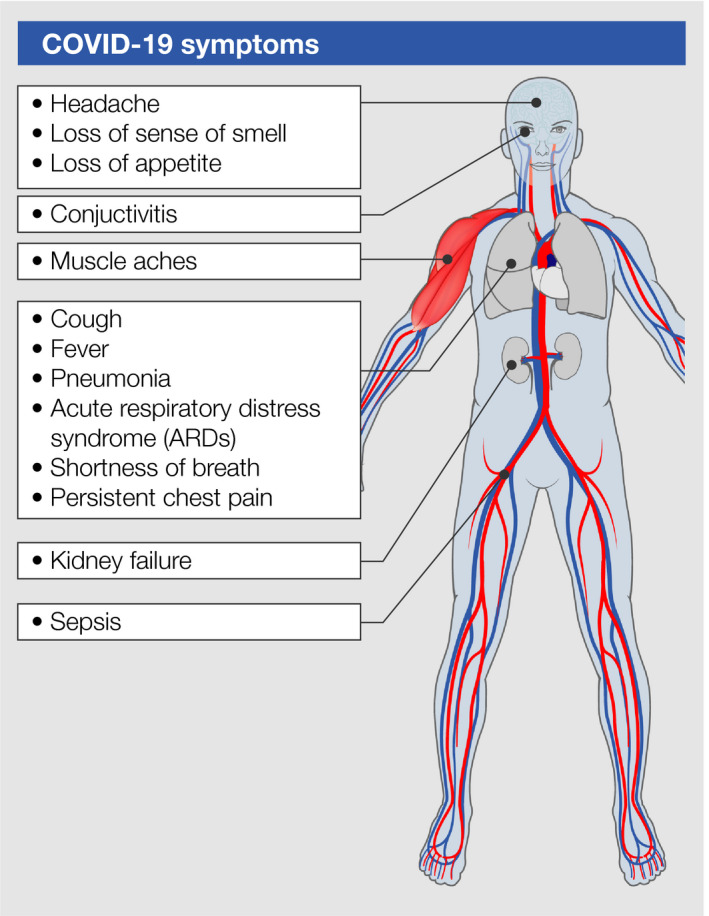
Symptoms of COVID‐19
2.2. Factors associated with poor prognosis
2.2.1. Comorbidities, gender, and senescence of the immune system
Comorbidity rate increases the rate of mortality of COVID‐19 with cardiovascular diseases, hypertension, poorly controlled diabetes mellitus, and obesity being major risk factors. 31 , 32 , 33 Since adipocytes express ACE2 and might serve as a reservoir for SARS‐CoV‐2, this fact might explain in part the higher risk of obese patients and obesity being a predictor for mortality among COVID‐19 patients. 23 , 34
Altered immune function and impaired defense against infectious pathogens and modulations of the innate as well as the adaptive immune system underlie the higher risk for severe courses of patients with diabetes mellitus. 32 On the other hand side, COVID‐19 impacts also on preexisting diabetes mellitus with alterations of the glucose metabolisms, which lead to severe metabolic complications. 35 Endothelial dysfunction, which goes along with aging, decline of sex hormones, increased number of endothelial microparticles, an inflammatory immune state and reactive oxygen species, might among other factors represent a risk factor for patients with cardiovascular diseases. 36 SARS‐CoV‐2 might also enter cardiomyocytes, which express ACE2 and induce myocardial damage. 37
Senescence of the immune system with lower antigen response of T and B cells from elder individuals as well as weaker effector functions and limited number of CD8+ memory cells capable to react do different pathogens might among other factors play a role for attenuated virus clearance and lower number of anti‐inflammatory cytokines and mediators, innate immune regulatory proteins, or regulatory cell mechanisms in higher age groups. 38 , 39
2.2.2. ACE2 distribution and expression
One reason besides a higher number of comorbidities and drugs take in the group of elder patients might be that ACE2 expression increases with age. 40
In a study on patients with asthma, male gender, African Americans race, and history of diabetes mellitus were associated with higher expression of ACE2 and TMPRSS2 in sputum cells of patients with asthma. 41
Higher expression of ACE2 and CD147‐related genes in bronchial biopsies, bronchoalveolar lavage, or peripheral blood cells was observed in patients with asthma, COPD, hypertension and obesity, as well as in smokers and males. 42
2.2.3. Gender and sex hormones
Circulating plasma levels of ACE2 have been demonstrated to be higher in men with heart failure than in women and might indicate higher tissue expression of the SARS‐CoV‐2 entry receptor in man as a risk factor for more fatal courses of COVID‐19. 43 The expression of ACE2 in the myocard is regulated by androgen in mouse models. 44 , 45 Male mice with hypertension had higher ACE2 expression, which was reduced after orchiectomy. These data indicate that testosterone might negatively impact on the risk for COVID‐19. 44 , 45
Whether gender differences in terms of nature and frequency of the skin lesions exist, needs to be elucidated in further studies.
However, several gender differences in relation to virus responses and responses to viral vaccines have been described in the past. These include higher susceptibility of men to infectious diseases or stronger antibody titers inducible by several different types of vaccines in women as opposed to men. 46 , 47 Moreover, plasmacytoid dendritic cell‐mediated type I IFN responses have been demonstrated to be stronger in women than in men, another factor of putative importance for viral clearance even in the context of SARS‐CoV‐2 infections. 46 , 47 Higher susceptibility, need for hospitalization, longer duration of the disease, and higher rate of mortality in men versus women have been also described for other virus infections such as with influenza virus. 48 Together, these observations point to SARS‐CoV‐2 specific as well as general differences in viral immunity in man versus women.
2.2.4. Ethnic and genetic risk factors
Patients with blood group A type are more susceptible to severe courses of COVID‐19 and association with genetic variations in a gene cluster on chromosome 3p21.31 detected in a genome‐wide association study of severe COVID‐19 cases with respiratory failure imply a genetic risk of this group. In the same study, a protective effect in blood group 0 type was observed. 49
Another reason might be the presence of allelic variants interfering with the binding of the S protein of SARS‐CoV‐2 to ACE2 and putative protection of the carriers from SARS‐CoV‐2 infection. 50 In contrast, higher allele frequencies of variants in the ACE2 coding gene region, which go along with higher ACE2 expression in the tissue, have been observed in populations from East Asia 51 and might indicate a specific risk, related to ethnic groups.
2.3. Skin lesions observed in relation to SARS‐CoV‐2
Besides the typical symptoms described above, more and more reports about skin lesion and lesions of the vascular system observed in patients tested positive for SARS‐CoV‐2 and patients suspected to be infected by SARS‐CoV‐2 are coming up in countries all over the world. 52 , 53 , 54 , 55 The percentage of patients with skin lesions varies from lower than 1% to up to 20% of all SARS‐CoV‐2 patients and has to be estimated exactly in systematic studies on this issue.
The spectrum of skin lesions in SARS‐CoV‐2 patients described and published so far contain skin lesions occurring in other infectious and viral diseases such as maculopapular exanthema, vesicular exanthema, or urticarial eruptions. 52 , 56 , 57 , 58 , 59 , 60 , 61 Furthermore, the skin lesions might also be related to virus‐induced or indirectly induced vascular dysfunctions, such as livedo reticularis, petechiae, or cutaneous acro‐ischemia. 52 , 53 , 54 , 55 , 56 , 57 , 62 Chilblain lesions have been described as well, 63 , 64 , 65 but a direct relation to SARS‐CoV‐2 infection is questionable. Considering frequency, most reports exist on maculopapular exanthema/vesicular exanthema and urticarial lesions. 56 Fewer reports exist on acro‐ischemia or livedo reticularis and other skin lesions.
Based on the largest collection of skin lesions from patients from Spain, lesions have been recently categorized into five main groups 56 summarized in Figure 4A‐E.
Vesicular eruptions, predate in about 15% COVID‐19 symptoms or occur with symptoms, last 10‐12 days and go along with COVID‐19 with intermediate severity, patients affected are middle aged, and lesions are accompanied by moderate itch (Figure 4A).
Maculopapular exanthema manifests together with COVID‐19 symptoms, often in more severe cases with a mortality rate up to 2%, lasts 7‐9 days, and is in over 50% of the cases accompanied by itch (Figure 4B).
Urticarial eruptions occur at the same time as other symptoms in more severe COVID‐19 cases are accompanied by itching and last 6‐8 days (Figure 4C).
Livedo or necrosis and other vasculitis forms tend to occur in older and more severe cases, with relatively high mortality (up to 10%), and the onset was together with COVID‐19 symptoms (Figure 4D).
Chilblain was observed in younger patients with mild or even asymptomatic courses or negative SARS‐CoV‐2 testing, the onset is late, duration 12‐14 days and in one third are the lesions accompanied by pain and itch 28 , 29 (Figure 4E).
FIGURE 4.
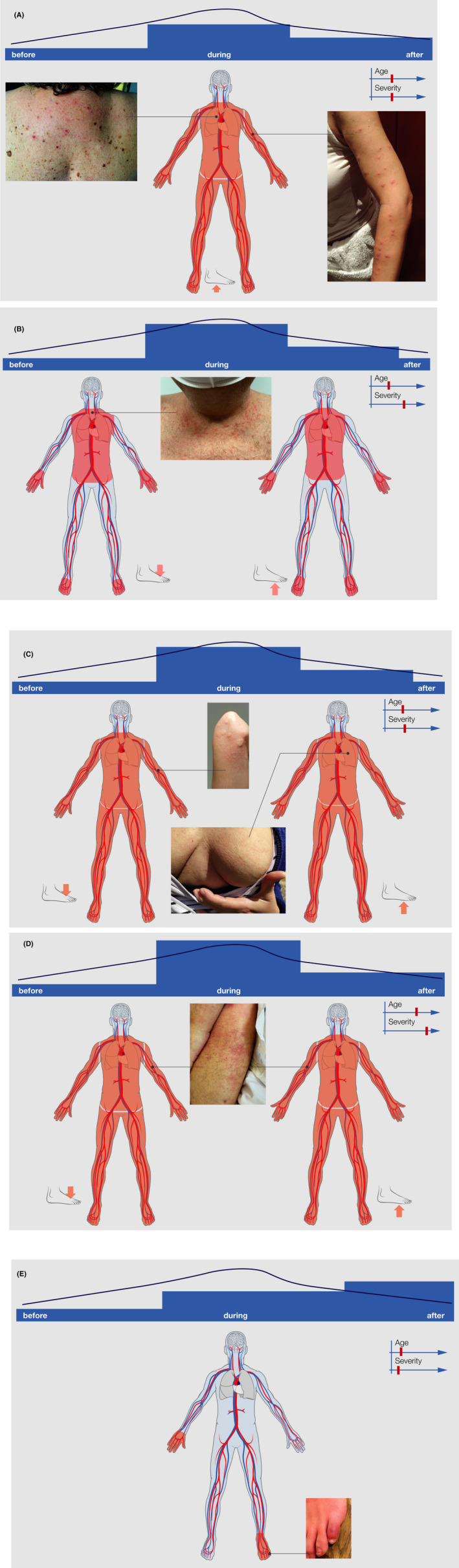
A: Typical skin lesions (vesicular eruptions occurring early during COVID‐19 (A), with symptoms of COVID‐19, that is, maculopapular exanthema (B), urticarial rash (C), vasculitis (D), and later during COVID‐19 disease Chilblain eruptions (E). The bar on top indicates the onset of skin symptoms in relation to COVID‐19 symptoms, as well as average age and severity of COVID‐19 patients, and typical predilection sites of the lesions are also depicted
Since it is quite difficult to verify, in which context the skin lesions occur in terms of SARS‐CoV‐2 infections, we would like to summarize in the next few sections current knowledge about the pathophysiologic background of virus‐induced and drug‐induced lesions of the skin and the vascular system.
2.4. Possible pathogenic mechanisms involved for skin manifestations
2.4.1. Viral rashes
Viral exanthema is defined as a skin rash, which is sometimes associated with an enanthem and goes along with fever and other systemic symptoms. 66 , 67 In this context, it is important to notice that also the ACE2 receptor has been described to be expressed in the oral cavity, 15 and lesions of the mucous membranes have not been reported so far to occur very frequently together with skin lesions in SARS‐CoV‐2‐positive cases.
In principle, skin manifestation of viral infections can derive by:
direct inoculation of the virus,
dissemination or reactivation of the virus from another site, or
interaction of the virus with the immune system in general and related cellular and humoral immune responses including virus‐specific lymphocytes and antibodies. 66
The onset of the skin lesions in relation to viremia and general symptoms can help to understand the pathophysiologic mechanisms behind the lesions in COVID‐19 patients. In the case of skin lesions precede general symptoms or might even be the only sign for a putative infection, they might serve as important early indicators of the disease or indicator for asymptomatic virus carriers. On the other hand, skin symptoms, which occur quite late during infection or even after resolution of main symptoms, might indicate a lack of viral clearance and cascades of immune responses induced by the virus.
Viremia often predates the typical COVID‐19 symptoms, and virus‐specific virology might be both false negative and false positive due to cross‐reactivity to other members of the coronavirus family, but could provide important information about acute or previous infection by the analysis of virus‐specific IgM and IgG. 68 Nucleotide testing of tissue specimens is highly sensitive and specific, but stability of samples and RNA, contamination, and other issues may occur and limit information gained, so that even part of smear taken from the mouth remains false negative. 67
Virus‐induced maculopapular exanthema
Although skin biopsies are rarely taken from viral skin lesions, because the features observed are mostly not very specific, biopsies might be an option to differentiate viral exanthema from drug‐induced exanthema in COVID‐19 patients; in these cases, it is essential to do the differential diagnosis and rule out the pharmacological cause.
To distinguish infectious exanthema from exanthema induced by drug hypersensitivity reactions, histologic features and immunologic changes in the skin might help to diagnostically differentiate both entities.
Immunologic features of maculopapular virus exanthema known so far are summarized in Figure 5A. Typical histologic features of infectious exanthema induced by viremia or dissemination of infectious agents through the blood are shown in Table 2 and Figure 6A.
FIGURE 5.
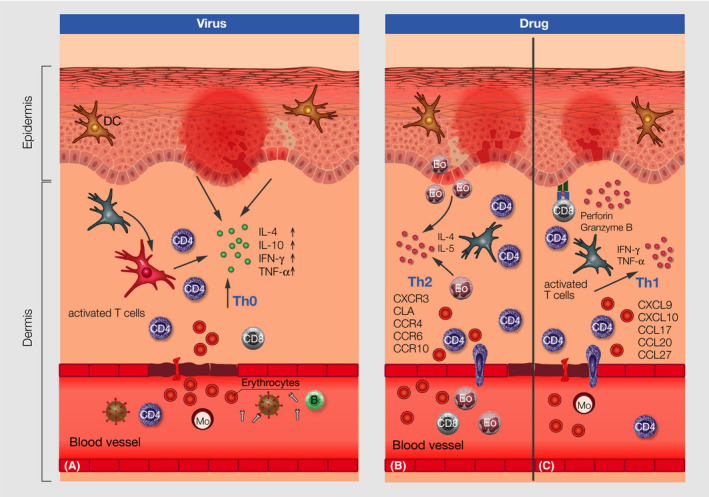
Immunologic features in the skin of virus‐induced maculopapular exanthema (A) with virus antibody activation and interaction with immune cells in the blood, recruitment of activated cells to the skin, extravasation of erythrocytes through the blood vessels, cytokine production and keratinocyte apoptosis and related changes to the dermis and epidermis and drug‐induced maculopapular rush type IVb (B) and IVc (C) derived by activated T cells, extravasation of erythrocytes, perivascular infiltrates and perforin and granzyme B and Th1 cytokines as well as different chemokines and recruitment of T cells and eosinophils to the dermis and respective changes to the dermis and epidermis; DC, dendritic cell; B, B cell; Mo, monocyte, Eo, eosinophil
TABLE 2.
Histologic characteristics of infectious exanthemas (adapted from 66 )
| Location | Histology | Skin lesion |
|---|---|---|
| Capillary endothelium dermis | Damage of vessels, endothelial swelling, perivascular edema, hemorrhage | Macules, papules, petechiae |
| Dermis | Edema, cellular infiltrate, hemorrhage, visualization of organism by electron microscopy | Papules, urticaria, purpura, vesicles |
| Epidermis | Cytopathic effects of the virus, that is, inclusion, ballooning, vacuolar degeneration, necrosis | Papules, vesicles, ulcer |
FIGURE 6.
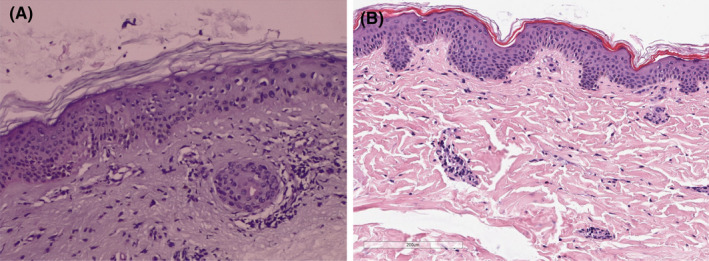
Histologic features of skin biopsies taken from two SARS‐CoV‐2‐positive patients with maculopapular eruptions. (A Skin sections showing epidermis with mild hyperkeratosis, keratinocytes with frosted glass nuclei, with intranuclear and occasionally multinucleate inclusions, reminiscent of cytopathic damage. Dermis without edema, perivascular inflammatory infiltrate extending focally to the basal layer, causing slight vacuolate damage and pigmentary incontinence. No eosinophils are observed. (B) Histology (H&E stain) showing an inconspicuous epidermis and a very subtle perivascular lymphohistiocytic infiltrate in the upper dermis with admixture of few eosinophilic granulocytes
Virus‐induced vesicular eruptions
Virus‐induced vesicular eruptions are quite common for herpes simplex virus or varicella zoster virus‐induced skin lesions in which the vesicles are caused by intraepidermal blister and epithelial necrosis. Either reticular degeneration of the epidermis or ballooning of keratinocytes might cause intraepidermal vesicle formation 69 , 70 (Table 2). Another example for virus‐induced vesicular eruptions is caused by Coxsackie A16 virus, an enterovirus, which is responsible for Hand‐foot‐and‐mouth diseases. Intraepidermal vesicles with reticular degeneration and ballooned cells are characteristic features. Papillary edema and mild perivascular infiltrates might be detectable as well. In all of these virus‐induced skin eruptions, viral antigens are detectable in various cutaneous structures using different methods such as antibody staining, electron microscopy, or others. 69 , 70
Whether vesicular eruptions observed in part of the COVID‐19 patients are caused by SARS‐CoV‐2, needs to be evaluated in further studies.
Some reports of erythemato‐violaceous papules and patches with a pseudo‐vesicle in the center, resembling erythema multiforme and an enanthem of the oral cavity in some of those patients during or after improvement of COVID‐19 symptoms, exist. 71 , 72 , 73 , 74 If those lesions are related to SARS‐CoV‐2 infection or drugs applied in this context, then both remain to be elucidated in further studies.
Virus‐induced urticarial rashes
Urticaria and acute urticarial rashes go along with up to 50% of all cases with infections of the upper respiratory tract 75 and are most often mediated by complement activation and serum sickness induced by viral antigens or secondary mechanisms, due to the interaction of the immune system with viral antigens. This could be also the case in part of the SARS‐CoV‐2‐positive patients with urticarial eruptions (Figure 7).
FIGURE 7.
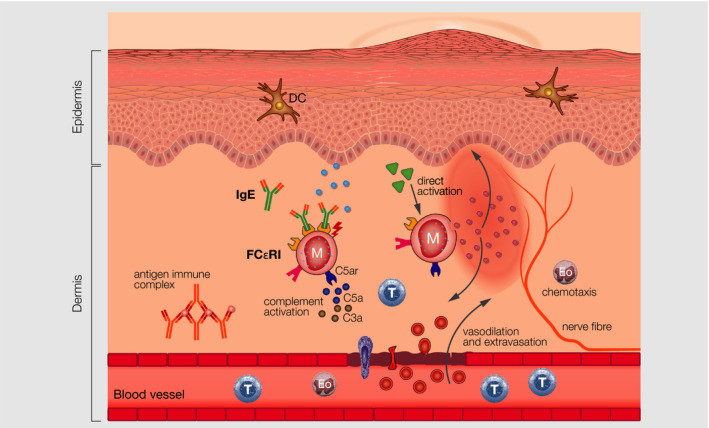
IgE‐, direct‐, and antigen‐immunocomplex–mediated mast cell activation in urticaria
Virus‐induced vasculitis
Infectious diseases are the cause of over 20% of cutaneous vasculitis. 76 Most of them have a self‐limiting course, but the involvement of other organs is possible.
Vasculitis can affect small, medium, and large vessels (Figure 8). Mechanisms might be type III or immune complex‐mediated reactions to viral antigens or part of viral antigens. In this context, large immune complexes might precipitate and accumulate within vessels, a process leading to vascular injury (Figure 8A). 77 Histologic features of vasculitis are an inflammatory infiltrate in wall of dermal or subcutaneous vessels (which can be neutrophilic, lymphocytic, or granulomatous), red blood cell extravasation, variable fibrinoid necrosis of vessel walls, and nuclear debris. Further on, deposits of immunoglobulin, complement, or fibrin in the vessel wall are detectable by direct immunofluorescence staining. 78 Polyarthritis nodosa related to hepatitis B infection is one example for a vasculitis directly induced by a viral antigen. 79 Interestingly, specific virus genotypes of hepatitis B and C virus have been identified, which are associated with particular forms of vasculitis, which explains in part why only a subgroup of virus‐infected patients develops such cutaneous vascular reactions in some viral diseases. 76
FIGURE 8.
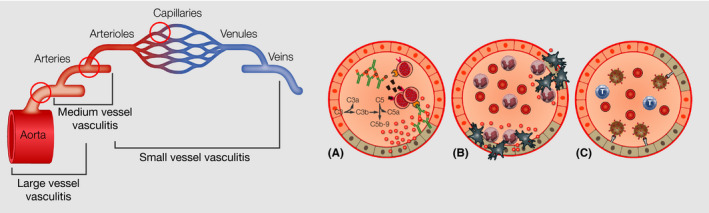
Vasculitis of small, medium, and large vessels, with formation of antigen‐immune complexes, which accumulate within the vessels and damage of the endothel, which leads to extravasation of cells and neutrophil recruitment
Cell‐mediated hypersensitivity, in which exposure to viral antigens induce recruitment of lymphocytes, which release pro‐inflammatory cytokines and further attract macrophages and more lymphocytes leading to tissue damage of the vessels, has also been described as a pathway in some infectious vasculitis types (Figure 8B).
Furthermore, abnormal immune regulation including different expression of adhesion molecules and cytokines in vascular endothelium by different virus‐related processes as well as direct endothelial cell invasion of the virus (Figure 8C) or direct stimulation of the immune system by infectious agents might take place. 78
Whether and which kind of virus‐induced mechanisms of infectious vasculitis play a major role in SARS‐CoV‐2 vascular lesions is unclear, but immune complex‐mediated mechanisms are likely to be of relevance.
Furthermore, since ACE2 expression has been described for endothelial cells, 79 , 80 it is quite speculative but still conceivable at this time point—but even possible—that the virus directly interacts with endothelial cells leading to tissue damage and the resulting skin lesions.
In children, an association of COVID‐19 with Kawasaki‐like disease with mucocutaneous involvement, polymorphic rash, erythema of the palms and soles, firm induration of hands or feet, or both besides other symptoms of Kawasaki disease has been postulated. 81 Kawasaki disease is classified a systemic vasculitis of medium size vessels (Figure 8) and pathogens including different virus types have been suspected as one causative factor in a rather multifactorial pathogenesis. Further studies are needed to demonstrate a correlation of Kawasaki disease with SARS‐CoV‐2 in larger patient groups and time courses.
2.4.2. Previous skin diseases
Skin manifestations, such as urticaria, psoriasis, autoimmune diseases, or others, might result from the SARS‐CoV‐2‐related reactivation or aggravation of preexisting skin diseases.
Chilblain
Idiopathic chilblain or also called acro‐ischemic lesions, presenting as violaceous, infiltrated painful and sometimes even pruritic plaques on erythematous skin with predilection on the back of the toes or feet has been described to occur in a special group of SARS‐CoV‐2‐positive suspected patients as well as asymptomatic or noninfected patients (Figure 4F). 65 , 82 Histologic features of these lesions were typical for a lymphocytic vasculitis, with a superficial and deeper lymphocytic infiltration around vessels and close to eccrine glands, a papillary edema, vacuolar degeneration of the basal layer, and lymphocytic exocytosis to the epidermis. Red cell extravasation and focal thrombosis in papillary dermal capillaries as well as vessels of the reticular dermis were described in some reports as well. 64 , 83 , 84 The pathologic pathways behind these lesions are still unknown and remain to be elucidated. If it is related to SARS‐CoV‐2, infection remains to be demonstrated. Two recent studies hypothesize that Chilblain is not directly associated with SARS‐CoV‐2 infection and COVID‐19. 85 , 86
2.4.3. Cutaneous drug reactions
Cutaneous drug reactions are classified into immediate and nonimmediate drug hypersensitivity reactions. The latter have been subclassified into five groups based on the type of immune mechanisms in the foreground (Table 3). Part of the skin and vascular lesions are most likely related to drug hypersensitivity reactions.
TABLE 3.
Immunologic classification of (cutaneous) drug reactions (modified from 96 )
| I | Specific IgE | Urticarial eruption with or without involvement of other organs | |
|---|---|---|---|
| II | IgG or IgM | Hemolytic anemia | |
| III | IgG or IgM | Serum sickness | |
| Type IVa | Th1 (IFN‐γ) | Monocyte activation | Eczema |
| Type IVb | Th2 (IL‐4/IL‐5) | Eosinophilic inflammation | Maculopapular exanthema, drug rush with eosinophilia and systemic symptoms (DRESS) |
| Type IVc | CTL Perforin/Granzyme B | CD4/CD8 mediated killing of cells |
Maculopapular exanthema Toxic epidermal necrolysis |
| Type IVd | T cells (IL‐8) | Neutrophil activation | Acute generalized exanthematous pustulosis (AGEP) |
| Type IVe | Th2 cells | CD4/CD8 cell activation |
Drug‐induced urticarial rashes
Urticaria and urticarial rashes belong at least in part to cutaneous type I (IgE‐mediated) drug hypersensitivity reactions (Table 3) (Figure 7). Other mechanisms involved in drug‐induced urticarial rashes are direct mast cell activation, immune complex formation/precipitation (Figure 7) and activation of complement during serum sickness, or interaction with metabolic pathways of drugs such as arachidonic acid metabolism. 87 Urticaria or urticarial rashes in SARS‐CoV‐2 patients could be drug‐induced, induced by viral RNA or a mixture of both. Infection‐associated reactivation or exacerbation of preexisting urticaria might be possible as well.
Drug‐induced maculopapular exanthema
In general, about 7% of hospitalized patients develop drug hypersensitivity reactions of variable severity, and exanthema occurs in 2%‐3% of these cases. 88 , 89
Both, drugs and metabolites of drugs might elicit exanthematous drug hypersensitivity reactions (Table 4).
TABLE 4.
Common and less common cutaneous (drug) eruptions (modified from 97 ), which have also been described in COVID‐19 patients
| Common | Less common | Severe cutaneous drug reactions |
|---|---|---|
| Maculopapular exanthems | Lichenoid eruptions | DRESS |
| Urticaria | Pityriasis rosea‐like eruptions | AGEP |
| Purpura | ||
| Vasculitis |
Abbreviaitons: Dress, drug rash and eosinophilia and systemic symptoms; AGEP, acute generalized exanthematous pustulosis.
Maculopapular exanthema with eosinophilia has been described in COVID‐19 patients. 90
Some skin lesions observed in COVID‐19 patients might result from cutaneous type IV (T‐cell mediated) drug hypersensitivity reactions. Typical immunologic characteristics of maculopapular eruptions are summarized in Figure 5B (type IVb) and 5C (type IVc), and histologic features are summarized in Table 5 and shown in Figure 6B. The most common histologic feature of drug‐induced maculopapular exanthema is a perivascular dermal lymphohistiocytic infiltrate with or without infiltration of eosinophilic granulocytes. The epidermis can be normal or exhibit interface changes with vacuolar degeneration of the basal layer, apoptotic keratinocytes, and exocytosis of lymphocytes can be present. Additional features can be edema in the upper dermis, extravasation of red blood cells, and dilatation of blood vessels. It is often impossible to histologically distinguish viral and drug‐induced exanthema. Lichenoid—and less frequently—spongiotic or psoriasiform pattern of reactions may be related to maculopapular drug reactions. Sometimes even systemic eosinophilia and elevated CRP levels might occur. 91 Typical immunologic characteristics of maculopapular eruptions are summarized in Figure 5B (type IVb) and 5C (type IVc), and histologic features are summarized in Table 5 and shown on Figure 6B.
TABLE 5.
Features of drug‐induced exanthema (modified from 92 )
| Lymphocytic exocytosis |
|---|
| Perivascular lymphohistiocytic infiltrate with or without eosinophils |
| Interface changes (vacuolar degeneration of basal layer, apoptotic keratinocytes, exocytosis of lymphocytes), lichenoid, spongiotic, or psoriasiform changes |
| Papillary dermal edema |
| Extravasation of red blood cells |
| Elevated serum CRP and eosinophilia |
Severe cutaneous adverse drug reaction
There are COVID‐19‐positive cases severe cutaneous adverse drug reactions including DRESS (drug reaction with eosinophilia and systemic symptoms) described, which is a severe cutaneous drug reaction with nonspecific histologic findings (Figure 9A,B). The diagnosis is based on a combination of clinical symptoms and laboratory parameters including frequently occurring hepatic abnormalities, eosinophilia and exanthema with facial swelling, and lymphadenopathy. 92 The culprit drug should be stopped immediately upon identification.
FIGURE 9.
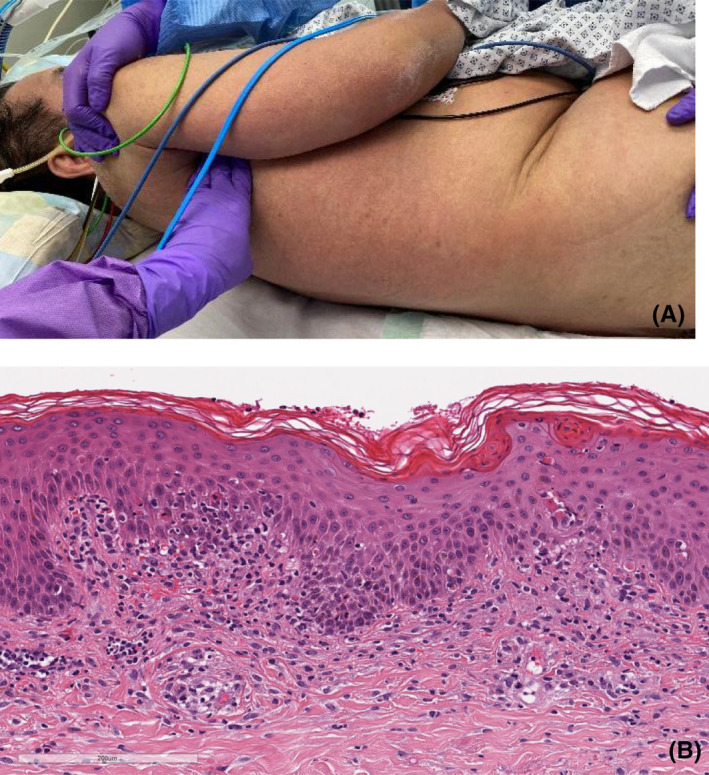
(A) 58‐year‐old male patient diagnosed with COVID‐19 and DRESS syndrome. (B) Histology (H&E stain) showing interface changes (vacuolar degeneration of the basal layer, apoptotic keratinocytes, exocytosis of lymphocytes) and spongiotic changes with hyperparakeratosis. Perivascular lymphohistiocytic infiltrate with admixture of few eosinophilic granulocytes. Mild extravasation of erythrocytes
2.4.4. Drug‐induced vasculitis
Drug hypersensitivity reactions with changes of the vasculature or vascular pathways such as vasculitis, livedo racemosa, or purpura represent a proportion of 10%‐20% of cutaneous reactions to drugs and can occur relatively late 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 during or even after drug exposure, but the time of onset to the related drug varies and depending on the causative drug. 93
Numerous drugs can induce vasculitis, which manifests primarily as cutaneous vasculitis, mediated as III hypersensitivity reactions (Table 3), that is, immune complex deposits with antigen excess in arteries, arterioles, venules, or capillaries (Figure 8). It is of notice that some drugs might also induce antibody production. 78
Drug‐induced vasculitis includes leucocytoclastic or hypersensitivity vasculitis, necrotizing vasculitis, panarteritis nodosa‐like vasculitis and others. 78
Histologic features of vasculitis are an inflammatory infiltrate in the wall of dermal or subcutaneous vessels (which can be neutrophilic, lymphocytic, or granulomatous), red blood cell extravasation, variable fibrinoid necrosis of vessel walls, and nuclear debris. 93
The number of drugs, which have been reported to be applied to COVID‐19 patients all over the world, is high and ranges from specific antiviral drugs, antiphlogistics, antibiotics, anti‐coagulants, and immunosuppressive and immunoregulatory drugs. All of these drugs are approved and tested for other infectious diseases or entities so that not much experience about putative side effects in COVID‐19 patients exists and knowledge gained about skin lesions induced by drugs applied during other diseases can only be used and transferred to speculate on this issue. Leucocytoclastic vasculitis has been described in a patient with severe COVID‐19 recently. 94
Summing up, COVID‐19 skin manifestations could be caused for each one or a mixture of the components mentioned above, that is, reaction to the infection, drug hypersensitivity reaction, preexisting immune state of the skin/immune system, combined with an impaired general health condition, and other co‐factors such as age of the individual. Therefore, it is important to discuss also putative additional trigger factors related primarily to the susceptibility to infection with SARS‐CoV‐2 and the risk to undergo severe courses of COVID‐19 as an indirect factor predisposing subsets of patients to skin and vascular lesions.
3. CONCLUSION
Recent observation of involvement of the skin and the vasculature in subgroups of SARS‐CoV‐2‐infected patients illustrates the need for a precise stratification and differential diagnostic evaluation of those patients in order to gain more insight into mechanisms of this novel virus. So far, pathophysiologic mechanisms behind the skin lesions occurring in the context of COVID‐19 are speculative, could be related to the virus, drugs, and other co‐factors. Knowledge from other viral infections and drug‐induced lesions of the skin and vascular system might be transferred, and observations on SARS‐CoV‐2 patients systematically evaluated to rapidly increase our knowledge on this issue. For mild cases of COVID‐19, skin lesions can be a diagnostic hint; for severe cases, due to multiorganic involvement interdisciplinary approach is of outmost importance.
Conflict of interest
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
NN was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy—EXC2151—390873048. Some of the clinical cases are part of the PhD degree of Alba Català, Department of Medicine, Dermatology area, Universidad Autónoma de Barcelona, with special thanks to her supervisor V. García‐Patos. Open access funding enabled and organized by Projekt DEAL.
Novak N, Peng W, Naegeli MC, et al. SARS‐CoV‐2, COVID‐19, skin and immunology – What do we know so far?. Allergy.2021;76:698–713. 10.1111/all.14498
Novak Natalijia, Peng Wenming, Mirjam C. Naegeli, Christina Galvan contributed equally to this work.
REFERENCES
- 1. Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID‐19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363‐374. 10.1038/s41577-020-0311-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong X, Cao Y‐Y, Lu X‐X, et al. Eleven faces of coronavirus disease 2019. Allergy 2020;75(7):1699‐1709. 10.1111/all.14289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li L‐Q, Huang T, Wang Y‐Q, et al. COVID‐19 patients' clinical characteristics, discharge rate, and fatality rate of meta‐analysis. J Med Virol. 2020;92(6):577‐583. 10.1002/jmv.25757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS‐CoV‐2. Nat Med. 2020;26(4):450‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bourgonje AR, Abdulle AE, Timens W, et al. Angiotensin‐converting enzyme‐2 (ACE2), SARS‐CoV‐2 and pathophysiology of coronavirus disease 2019 (COVID‐19). J Pathol. 2020: 10.1002/path.5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karamloo F, König R. SARS‐CoV‐2 immunogenicity at the crossroads. Allergy 2020;75(7):1822‐1824. 10.1111/all.14360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Riggioni C, Comberiati P, Giovannini M, et al. A compendium answering 150 questions on COVID‐19 and SARS‐CoV‐2. Allergy 2020;75:2503‐2541. 10.1111/all.14449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Azkur AK, Akdis M, Azkur D, et al. Immune response to SARS‐CoV‐2 and mechanisms of immunopathological changes in COVID‐19. Allergy 2020;75(7):1564‐1581. 10.1111/all.14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wrapp D, Wang N, Corbett KS, et al. Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science 2020;367(6483):1260‐1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor‐binding domain complexed with receptor. Science 2005;309:1864‐1868. [DOI] [PubMed] [Google Scholar]
- 13. Li W, Zhang C, Sui J, et al. Receptor and viral determinants of SARS‐coronavirus adaptation to human ACE2. EMBO J. 2005;24:1634‐1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dujif PHG. (2020). Baseline pulmonary levels of CD8+ T cells and NK cells inversely correlate with expression of the SARS‐Cov‐2 entry receptor ACE2. 10.1101/2020.05.04.075291 [DOI]
- 15. Zhou G, Chen S, Chen Z. Advances in COVID‐19: the virus, the pathogenesis, and evidence‐based control and therapeutic strategies. Front Med. 2020;14(2):117‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS‐CoV‐2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sungnak W, Huang N, Bécavin C, et al. SARS‐CoV‐2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681‐687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS‐CoV‐2 receptor ACE2 is an interferon‐stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020;181(5):1016‐1035.e19. 10.1016/j.cell.2020.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu R, Cui B, Duan X, Zhang P, Zhou X, Yuan Q. Saliva: potential diagnostic value and transmission of 2019‐nCoV. Int J Oral Sci. 2020;12(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010;10(7):514‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID‐19: current state of the science. Immunity 2020;52(6):910‐941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilk AJ, Rustagi A, Zhao NQ, et al. A single‐cell atlas of the peripheral immune response in patients with severe COVID‐19. Nat Med. 2020;26(7):1070‐1076. 10.1038/s41591-020-0944-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kruglikov IL, Scherer PE. The role of adipocytes and adipocyte‐like cells in the severity of COVID‐19 infections. Obesity (Silver Spring). 2020;28(7):1187‐1190. 10.1002/oby.22856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oberfeld B, Achanta A, Carpenter K, et al. SnapShot: COVID‐19. Cell 2020;181(4): 10.1016/j.cell.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID‐19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 26. Morawska L, Cao J. Airborne transmission of SARS‐CoV‐2: The world should face the reality. Environ Int. 2020;139: 10.1016/j.envint.2020.105730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS‐CoV‐2 as compared with SARS‐CoV‐1. N Engl J Med. 2020;382(16):1564‐1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stadnytskyi V, Bax CE, Bax A, Anfinrud P. The airborne lifetime of small speech droplets and their potential importance in SARS‐CoV‐2 transmission. Proc Natl Acad Sci. 2020;117(22):11875‐11877. 10.1073/pnas.2006874117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID‐19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang D, Hu BO, Hu C, et al. Novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020;323(11):1061‐ 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schett G, Sticherling M, Neurath MF. COVID‐19: risk for cytokine targeting in chronic inflammatory diseases? Nat Rev Immunol. 2020;20(5):271‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Erener S. Diabetes, infection risk and covid‐19. Mol Metab. 2020; 10.1016/j.molmet.2020.101044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pettit NN, MacKenzie EL, Ridgway J, et al. Obesity is associated with increased risk for mortality among hospitalized patients with COVID‐19. Obesity. 2020; 10.1002/oby.22941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rubino F, Amiel SA, Zimmet P, et al. New‐onset diabetes in Covid‐19. N Engl J Med. 2020; 10.1056/NEJMc2018688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Froldi G, Dorigo P. Endothelial dysfunction in coronavirus disease 2019 (COVID‐19): gender and age influences. Med Hypoth. 2020;144: 10.1016/j.mehy.2020.110015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guzik TJ, Mohiddin SA, Dimarco A, et al. COVID‐19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res. 2020; 10.1093/cvr/cvaa106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nikolich‐Zugich J. Ageing and life‐long maintenance of T‐cell subsets in the face of latent persistent infections. Nat Rev Immunol. 2008;8(7):512‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maue AC, Yager EJ, Swain SL, Woodland DL, Blackman MA, Haynes L. T‐cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 2009;30(7):301‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen Y, Li L. SARS‐CoV‐2: virus dynamics and host response. Lancet Infect Dis. 2020;20(5):515‐516. 10.1016/S1473-3099(20)30235-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peters MC, Sajuthi S, Deford P, et al. COVID‐19 related genes in sputum cells in asthma: relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020; 10.1164/rccm.202003-0821OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Radzikowska U, Ding M, Tan G, et al. Distribution of ACE2, CD147, CD26 and other SARS‐CoV‐2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID‐19 risk factors. Allergy 2020;75:2828‐2844. 10.1111/all.14429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sama IE, Ravera A, Santema BT, et al. Circulating plasma concentrations of angiotensin‐converting enzyme 2 in men and women with heart failure and effects of renin‐angiotensin‐aldosterone inhibitors. Eur Heart J. 2020;41(19):1810‐1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dalpiaz PLM, Lamas AZ, Caliman IF, et al. Sex hormones promote opposite effects on ACE and ACE2 activity, hypertrophy and cardiac contractility in spontaneously hypertensive rats. PLoS One 2015;10(5):e0127515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. La Vignera S, Cannarella R, Condorelli RA, Torre F, Aversa A, Calogero AE. Sex‐specific SARS‐CoV‐2 mortality: among hormone‐modulated ACE2 expression, risk of venous thromboembolism and hypovitaminosis D. Int J Mol Sci. 2020;21(8):2948‐ 10.3390/ijms21082948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626‐638. [DOI] [PubMed] [Google Scholar]
- 47. Fish EN. The X‐files in immunity: sex‐based differences predispose immune responses. Nat Rev Immunol. 2008;8(9):737‐744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121(4):258‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ellinghaus D, Degenhardt F, Bujanda L, et al. Genomewide association study of severe covid‐19 with respiratory failure. N Engl J Med. 2020; 10.1056/NEJMoa2020283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hussain M, Jabeen N, Raza F, et al. Structural variations in human ACE2 may influence its binding with SARS‐CoV‐2 spike protein. J Med Virol. 2020; 10.1002/jmv.25832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cao Y, Li L, Feng Z, et al. Comparative genetic analysis of the novel coronavirus (2019‐nCoV/SARS‐CoV‐2) receptor ACE2 in different populations. Cell Discov. 2020;6: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Recalcati S. Cutaneous manifestations in COVID‐19: a first perspective. J Eur Acad Dermatol Venereol. 2020;34(5): 10.1111/jdv.16387 [DOI] [PubMed] [Google Scholar]
- 53. Wollina U, Karadağ AS, Rowland‐Payne C, Chiriac A, Lotti T. Cutaneous signs in COVID‐19 patients: a review. Dermatol Ther. 2020; 10.1111/dth.13549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Matar S, Oulès B, Sohier P, et al. Cutaneous manifestations in SARS‐CoV‐2 infection (COVID‐19): a French experience and a systematic review of the literature. J Eur Acad Dermatol Venereol. 2020; 10.1111/jdv.16775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gisondi P, PIaserico S, Bordin C, Alaibac M, Girolomoni G, Naldi L. Cutaneous manifestations of SARS‐CoV‐2 infection: a clinical update. J Eur Acad Dermatol Venereol. 2020; 10.1111/jdv.16774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Galván Casas C, Català A, Carretero Hernández G, et al. Classification of the cutaneous manifestations of COVID‐19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol. 2020; 10.1111/bjd.19163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Joob B, Wiwanitkit V. COVID‐19 can present with a rash and be mistaken for dengue. J Am Acad Dermatol. 2020;82(5):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tammaro A, Adebanjo GAR, Parisella FR, Pezzuto A, Rello J. Cutaneous manifestations in COVID‐19: the experiences of Barcelona and Rome. J Eur Acad Dermatol Venereol. 2020;34(7): 10.1111/jdv.16530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Marzano AV, Genovese G, Fabbrocini G, et al. Varicella‐like exanthem as a specific COVID‐19‐associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83(1):280‐285. 10.1016/j.jaad.2020.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Guan W‐J, Ni Z‐Y, Hu YU, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Genovese G, Colonna C, Marzano AV. Varicella‐like exanthem associated with COVID‐19 in an 8‐year‐old girl: a diagnostic clue? Pediatr Dermatol. 2020;37(3):435‐436. 10.1111/pde.14201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1‐13. 10.1016/j.trsl.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Landa N, Mendieta‐Eckert M, Fonda‐Pascual P, Aguirre T. Chilblain‐like lesions on feet and hands during the COVID‐19 pandemic. Int J Dermatol. 2020;59(6):739‐743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kolivras A, Dehavay F, Delplace D, et al. Coronavirus (COVID‐19) infection‐induced chilblains: a case report with histopathologic findings. JAAD Case Rep. 2020;6(6):489‐492. 10.1016/j.jdcr.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fernandez‐Nieto D, Jimenez‐Cauhe J, Suarez‐Valle A, et al. Characterization of acute acro‐ischemic lesions in non‐hospitalized patients: a case series of 132 patients during the COVID‐19 outbreak. J Am Acad Dermatol. 2020; 10.1016/j.jaad.2020.04.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Drago F, Ciccarese G, Gasparini G, et al. Contemporary infectious exanthems: an update. Future Microbiol. 2017;12:171‐193. [DOI] [PubMed] [Google Scholar]
- 67. Keighley CL, Saunderson RB, Kok J, Dwyer DE. Viral exanthems. Curr Opin Infect Dis. 2015;28(2):139‐150. [DOI] [PubMed] [Google Scholar]
- 68. Zhang J‐J, Cao Y‐Y, Dong X, et al. Distinct characteristics of COVID‐19 patients with initial rRT‐PCR‐positive and rRT‐PCR‐negative results for SARS‐CoV‐2. Allergy 2020;75(7):1809‐1812. 10.1111/all.14316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Molina‐Ruiz AM, Santonja C, Rütten A, Cerroni L, Kutzner H, Requena L. Immunohistochemistry in the diagnosis of cutaneous viral infections–part I. Cutaneous viral infections by herpesviruses and papillomaviruses. Am J Dermatopathol. 2015;37(1):1‐14. [DOI] [PubMed] [Google Scholar]
- 70. Molina‐Ruiz AM, Santonja C, Rütten A, Cerroni L, Kutzner H, Requena L. Immunohistochemistry in the diagnosis of cutaneous viral infections‐ part II: cutaneous viral infections by parvoviruses, poxviruses, paramyxoviridae, picornaviridae, retroviruses and filoviruses. Am J Dermatopathol. 2015;37(2):93‐106. [DOI] [PubMed] [Google Scholar]
- 71. Jimenez‐Cauhe J, Ortega‐Quijano D, Carretero‐Barrio I, et al. Erythema multiforme‐like eruption in patients with COVID‐19 infection: clinical and histological findings. Clin Exp Dermatol. 2020; 10.1111/ced.14281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Janah H, Zinebi A, Elbenaye J. Atypical erythema multiforme palmar plaques lesions due to Sars‐Cov‐2. J Eur Acad Dermatol Venereol. 2020; 10.1111/jdv.16623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Torrelo A, Andina D, Santonja C, et al. Erythema multiforme‐like lesions in children and COVID‐19. Pediatr Dermatol. 2020;37(3):442‐446. 10.1111/pde.14246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Robustelli Test E, Vezzoli P, Carugno A, et al. Acute generalized exanthematous pustulosis with erythema multiforme‐like lesions in a COVID‐19 woman. J Eur Acad Dermatol Venereol. 2020; 10.1111/jdv.16613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Poonawalla T, Kelly B. Urticaria: a review. Am J Clin Dermatol. 2009;10(1):9‐21. [DOI] [PubMed] [Google Scholar]
- 76. Sharlala H, Adebajo A. Virus‐induced vasculitis. Curr Rheumatol Rep. 2008;10(6):449‐452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Millikan LE, Flynn TC. Infectious etiologies of cutaneous vasculitis. Clin Dermatol. 1999;17(5):509‐514. [DOI] [PubMed] [Google Scholar]
- 78. Belizna CC, Hamidou MA, Levesque H, Guillevin L, Shoenfeld Y. Infection and vasculitis. Rheumatology (Oxford) 2009;48(5):475‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zulli A, Burrell LM, Buxton BF, Hare DL. ACE2 and AT4R are present in diseased human blood vessels. Eur J Histochem. 2008;52(1):39‐44. [DOI] [PubMed] [Google Scholar]
- 81. Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki‐like disease at the Italian Epicentre of the SARS‐CoV‐2 epidemic: an observational cohort study. Lancet 2020;395(10239):1771‐1778. 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Andina D, Noguera‐Morel L, Bascuas‐Arribas M, et al. Chilblains in children in the setting of COVID‐19 pandemic. Pediatr Dermatol. 2020;37(3):406‐411. 10.1111/pde.14215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Locatelli AG, Robustelli Test E, Vezzoli P, et al. Histologic features of long lasting chilblain‐like lesions in a pediatric COVID‐19 patient. J Eur Acad Dermatol Venereol. 2020; 10.1111/jdv.16617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Santonja C, Heras F, Núñez L, Requena L. COVID‐19 chilblain‐like lesion: immunohistochemical demonstration of SARS‐CoV‐2 spike protein in blood vessel endothelium and sweat gland epithelium in a PCR‐negative patient. Br J Dermatol. 2020; 10.1111/bjd.19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Herman A, Peeters C, Verroken A, et al. Evaluation of chilblains as a manifestation of the COVID‐19 pandemic. JAMA Dermatol. 2020; 10.1001/jamadermatol.2020.2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Roca‐Ginés J, Torres‐Navarro I, Sánchez‐Arráez J, et al. Assessment of acute acral lesions in a case series of children and adolescents during the COVID‐19 pandemic. JAMA Dermatol. 2020; 10.1001/jamadermatol.2020.2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Shipley D, Ormerod AD. Drug‐induced urticaria. Recognition and treatment. Am J Clin Dermatol. 2001;2(3):151‐158. [DOI] [PubMed] [Google Scholar]
- 88. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta‐analysis of prospective studies. JAMA 1998;279(15):1200‐1205. [DOI] [PubMed] [Google Scholar]
- 89. Bigby M, Jick S, Jick H, Arndt K. Drug‐induced cutaneous reactions. JAMA 1986;256(24):3358‐3363. 10.1001/jama.1986.03380240052027 [DOI] [PubMed] [Google Scholar]
- 90. Rosell‐Díaz AM, Mateos‐Mayo A, Nieto‐Benito LM, et al. Exanthema and eosinophilia in Covid‐19 patients: has viral infection a role in drug induced exanthemas? J Eur Acad Dermatol Venereol. 2020; 10.1111/jdv.16709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Singh S, Khandpur S, Arava S, et al. Assessment of histopathological features of maculopapular viral exanthem and drug‐induced exanthem. J Cutan Pathol. 2017;44(12):1038‐1048. [DOI] [PubMed] [Google Scholar]
- 92. Martínez‐Cabriales SA, Rodríguez‐Bolaños F, Shear NH. Drug reaction with eosinophilia and systemic symptoms (DReSS): how far have we come? Am J Clin Dermatol. 2019;20(2):217‐236. [DOI] [PubMed] [Google Scholar]
- 93. ten Holder SM, Joy MS, Falk RJ. Cutaneous and systemic manifestations of drug‐induced vasculitis. Ann Pharmacother. 2002;36(1):130‐147. [DOI] [PubMed] [Google Scholar]
- 94. Caputo V, Schroeder J, Rongioletti F. A generalized purpuric eruption with histopathologic features of leucocytoclastic vasculitis in a patient severely ill with COVID‐19. J Eur Acad Dermatol Venereol. 2020; 10.1111/jdv.16737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gómez E, Ruano M, Somoza ML, Fernández J, Blanca‐López N. Role of T cells in non‐immediate drug allergy reactions. Curr Opin Allergy Clin Immunol. 2019;19(4):294‐301. [DOI] [PubMed] [Google Scholar]
- 96. Khan DA. Cutaneous drug reactions. J Allergy Clin Immunol. 2012;130(5):1225. 10.1016/j.jaci.2012.08.007 [DOI] [PubMed] [Google Scholar]


