Abstract
Objective:
We evaluated the yield of systematic analysis and/or reanalysis of whole exome sequencing (WES) data from a cohort of well-phenotyped pediatric patients with epilepsy and suspected but previously undetermined genetic etiology.
Methods:
We identified and phenotyped 125 participants with pediatric epilepsy, the etiology of which was unexplained at the time of enrollment despite clinical testing, which included chromosomal microarray (57 patients), epilepsy gene panel (48), both (28), or WES (8). Clinical epilepsy diagnoses included developmental and epileptic encephalopathy (DEE), febrile-infection related epilepsy syndrome (FIRES), Rasmussen encephalitis, and other focal and generalized epilepsies. We analyzed WES data and compared the yield in participants with and without prior clinical genetic testing.
Results:
Overall, we identified pathogenic or likely pathogenic variants in 40% (50/125) of our study participants. Nine patients with DEE had genetic variants in recently published genes that had not been recognized as epilepsy-related at the time of clinical testing (FGF12, GABBR1, GABBR2, ITPA, KAT6A, PTPN23, RHOBTB2, SATB2), and eight patients in candidate epilepsy genes (CAMTA1, FAT3, GABRA6, HUWE1, PTCHD1). Ninety participants had concomitant or subsequent clinical genetic testing, which was ultimately explanatory for 26% (23/90). Of the 67 participants whose molecular diagnoses were ‘unsolved’ through clinical genetic testing, we identified pathogenic or likely pathogenic variants in 17 (25%).
Significance:
Our data argue for early consideration of WES with iterative re-analysis for patients with epilepsy, particularly those with DEE or epilepsy with ID. Rigorous analysis of WES data of well-phenotyped patients with epilepsy leads to a broader understanding of gene-specific phenotypic spectra as well as candidate disease gene identification. We illustrate the dynamic nature of genetic diagnosis over time, with analysis and in some cases re-analysis, of exome data leading to the identification of disease-associated variants among participants with previously non-diagnostic results from a variety of clinical testing strategies.
Keywords: Epilepsy, Genetics, Whole Exome Sequencing, Phenotype, Reanalysis
Introduction
In 2012, the parents of a 5-month-old boy were referred for epilepsy genetics consultation regarding the cause of their son’s epilepsy of infancy with migrating focal seizures (EIMFS), severe developmental delay, and microcephaly. The couple’s only other child was a similarly affected male infant who had passed away at 1 year of age and had not had genetic evaluation. Clinical evaluation of the 5-month-old boy included chromosomal microarray (CMA) and epilepsy gene panel, which were both non-explanatory. Whole exome sequencing (WES) was not available clinically at the time, and they enrolled in a study through which WES was pursued on a research basis. Initial WES research analysis did not reveal a definitive etiology, and the boy died at 18 months of age without a genetic diagnosis. Three years after his death, on reanalysis of his WES data, we identified a homozygous pathogenic variant in the gene ITPA. Variants in ITPA were first reported in 20151 in patients with infantile onset encephalopathy, progressive microcephaly, and early death, similar to our patient.
The case above illustrates the importance of revisiting a patient’s genetic data over time and illustrates the complex environment of clinical testing, in which not all modalities are available to patients in all settings due to variability in clinical provider awareness, institutional practices, and cost/insurance-related barriers. With the evolution of genetic testing modalities, continued identification of epilepsy genes, and improved cataloging of knowledge about specific gene variants, a patient whose testing was ‘negative’ months or years ago may have an identifiable genetic etiology upon re-analysis2,3. Given that approximately 70–80% of patients with otherwise unexplained epilepsy are thought to have an underlying genetic cause4, the genetic evaluation of patients with epilepsy is becoming more routine in clinical practice, particularly with the rationale that identifying genetic causes for epilepsy may guide treatment options for some patients and limit the need for on-going additional testing.
The advent of next-generation sequencing (NGS) has led to the detection of disease-associated gene variants at an unprecedented rate5. The reported yield of WES for patients with epilepsy is variable (28–70%), in part due to phenotypic differences among the analyzed cohorts6–10. NGS has led to the discovery of novel genes and expanded phenotypes of known genes. However, NGS also presents challenges, in particular with respect to variant interpretation and clinical correlation, both of which can be resource-intensive. Studies from control populations show that most individuals have more than 2500 non-synonymous variants, including approximately 150 loss-of-function variants and 20–40 variants predicted to be pathogenic11. Although some variants can be easily designated as pathogenic or benign, many variants identified by NGS are variants of unknown significance (VUS) or occur in genes of unknown clinical relevance.
We performed a systematic analysis of WES data from 125 pediatric patients with unexplained epilepsy and suspected genetic etiology. We demonstrate that an iterative process, combining on-going research analysis and re-analysis with clinically oriented interpretation, led to the identification of a genetic etiology for a substantial proportion of our cohort, including those for whom prior clinical genetic testing with a variety of available modalities had not yielded a conclusive result.
Methods
Phenotyping
From 2010 to 2017, we recruited patients and patients were referred with a diagnosis of epilepsy at Boston Children’s Hospital and through collaborating researchers and physicians, with approval from the Boston Children’s Hospital Institutional Review Board. Our team included board-certified pediatric epileptologists, neurologists, and genetic counselors with expertise in epilepsy genetics and variant interpretation. We reviewed phenotypic data, including seizure semiology, developmental history, electroencephalograms (EEGs), brain imaging reports, and clinical genetic testing reports. A summary epilepsy characterization was determined for each participant according to International League Against Epilepsy (ILAE) classification12.
Exome sequencing and variant interpretation
DNA extracted from blood or saliva was subjected to WES capture using either the Agilent SureSelect XTHuman All Exon v4 or Illumina Rapid Capture Exome enrichment kit. Sequencing of 100bp paired end reads was obtained using Illumina HiSeq (Illumina, SanDiego, CA). WES was performed for 76 probands through the Epi25 project (http://epi-25.org, Epi25 Collaborative, AJHG, 2019, in press). Coverage was >90% or >80% meeting 20x coverage with the two methods, respectively. We utilized the BCH Connect Genomic Data Commons, which leverages WuXi NextCODE’s analysis platform13 for variant interrogation and analysis of FASTQ and BAM files from WES experiments, including secondary analysis and variant interpretation (tertiary analysis). The sequencing analysis pipeline, described previously,14 included alignment and quality score recalibration with Burrows Wheeler Aligner (BWA, http://bio-bwa.sourceforge.net/) and variant calling with GATK (www.broadinstitute.org/gatk/). All variants were classified according to ACMG guidelines for interpretation of sequence variants15. Sanger sequencing was performed to confirm variants in probands and in parents when parental DNA was available.
To aid in the prioritization of variants, we developed an algorithm based on the following parameters: (i) sequence quality (GT call ratio >0.3 and GT depth >20); (ii) maximum population allele frequency 0.0001 reported in the population database gnomAD16,17 (http://gnomad.broadinstitute.org/); (iii) conservation and predicted impact using the Variant Effect Predictor18 (VEP) classification from NextCODE (including HIGH, which refers to protein-truncating variants and MODERATE, which refers to missense mutations, inframe indels, and splice region variants outside the canonical splice site), and pathogenicity prediction from Polyphen219 and SIFT20; (iv) human neurological disease association(s); (v) segregation data when parental samples were sequenced, such that the presence of a de novo variant is considered evidence suggesting pathogenicity; (vi) representation in a list of epilepsy genes drawn from 6 clinical gene panels designed for patients with epilepsy and containing 513 putatively epilepsy-related genes (Supplementary Table 1); and (vii) pLI score (> 0.9) and missense Z-score (> 3.09) from the gnomAD database16,17, metrics that indicate that a gene is intolerant of loss-of-function or missense variants, respectively. Phenotypic data were re-reviewed in light of the variants under consideration for each case.
Categorization of genes
We classified genes into three categories: (1) established published genes associated with epilepsy compiled from 6 panels used by commercial genetic testing laboratories (Supplementary Table 1, note that these panels are intended for patients with epilepsy but include many genes also associated with ID); (2) published epilepsy genes only recently associated with epilepsy in the scientific literature (after 2016); and (3) candidate epilepsy genes selected based on biological, physiological, and functional relevance to epilepsy but for which no known human disease association yet exists.
Results
Participant demographics and phenotypes
Our cohort consisted of 125 participants with unexplained pediatric onset epilepsy referred to our Epilepsy Genetics Program from 2010 to 2017. The majority had DEE (n=88; 70%). For the remainder of the cohort, epilepsy diagnoses included focal epilepsy (n=12) and generalized epilepsy (n=14) without identifiable genetic syndromes, Rasmussen encephalitis (n=5), and FIRES (n=5). One participant had a history of a prolonged febrile seizure at 15 months and no subsequent seizures. Phenotypic details are presented in Supplementary Table 2 for all patients, with additional details regarding the patients with focal or generalized epilepsy without DEE in Supplementary Table 3. The majority of patients had a severe phenotype that included developmental delay, ID, or other neurodevelopmental abnormality, including all patients with DEE, 8/14 with generalized epilepsy, and 7/12 with focal epilepsy. Among the 12 patients with focal epilepsy without DEE or another diagnosis, subsequent review of imaging and records revealed a focal malformation of cortical development in 7.
Age at enrollment was 4 weeks to 25 years, with seizure onset between birth and 10 years of age (median = 4 months, 84% < 1 year). Fifty-three percent of participants were male (66/125). Eighty-three percent of participants were white (104/125). The ethnicity of the other participants was Asian (n=4, 3%), Black/African American (n=4, 3%), Hispanic (n=2, 2%), Asian/White (n=1, 1%) or unknown (n=10, 8%). Eleven participants died prematurely (10/11 under the age of 19 months) either prior to (n=3) or following enrollment (n=8).
For 84 of the 125 participants, research samples were available from the proband only. For the remaining 41 participants, samples were available from the proband as well as both biological parents (trios).
Overall yield of research exome analysis
The overall yield of pathogenic or likely pathogenic variants from our research analysis was 40% (50/125). The highest rate of diagnosis in our cohort was achieved among participants with DEE, with pathogenic or likely pathogenic variants in 44% (Table 1). Somewhat surprisingly to us, the overall rate of pathogenic or likely pathogenic variants did not differ significantly between participants initially sequenced as trios (39%, 16/41) and those with proband-only sequencing (40%, 34/84).
Table 1:
Diagnostic yield of research WES across epilepsy diagnoses
| Epilepsy diagnoses | n | P finding | LP finding | VUS | unsolved |
|---|---|---|---|---|---|
| DEE | 88 | 30 (34%) | 9 (10%) | 17 (19%) | 32 (36%) |
| Infantile spasms | 44 | 8 (18%) | 8 (18%) | 10 (23%) | 18 (41%) |
| Ohtahara syndrome | 26 | 12 (46%) | 1 (4%) | 4 (15%) | 9 (35%) |
| DEE, other | 18 | 10 (56%) | 0 (0%) | 3 (17%) | 5 (28%) |
| FIRES | 5 | 0 (0%) | 0 (0%) | 0 (0%) | 5 (100%) |
| Rasmussen encephalitis | 5 | 0 (0%) | 0 (0%) | 1 (20%) | 4 (80%) |
| Other focal epilepsies | 12 | 1 (8%) | 3 (25%) | 0 (0%) | 8 (67%) |
| Other generalized epilepsies | 14 | 6 (43%) | 3 (21%) | 1 (7%) | 4 (29%) |
| Prolonged febrile seizures | 1 | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) |
| Total | 125 | 35 (28%) | 16 (12%) | 19 (15%) | 56 (44%) |
Detection rate of WES analysis according to epilepsy syndrome. DEE = developmental and epileptic encephalopathy; FIRES = Febrile Infection-Related Epilepsy Syndrome; LP = likely pathogenic; n = number of patients; P = pathogenic; VUS = variant of unknown significance; WES = whole exome sequencing.
Comparing yields with and without concomitant genetic testing
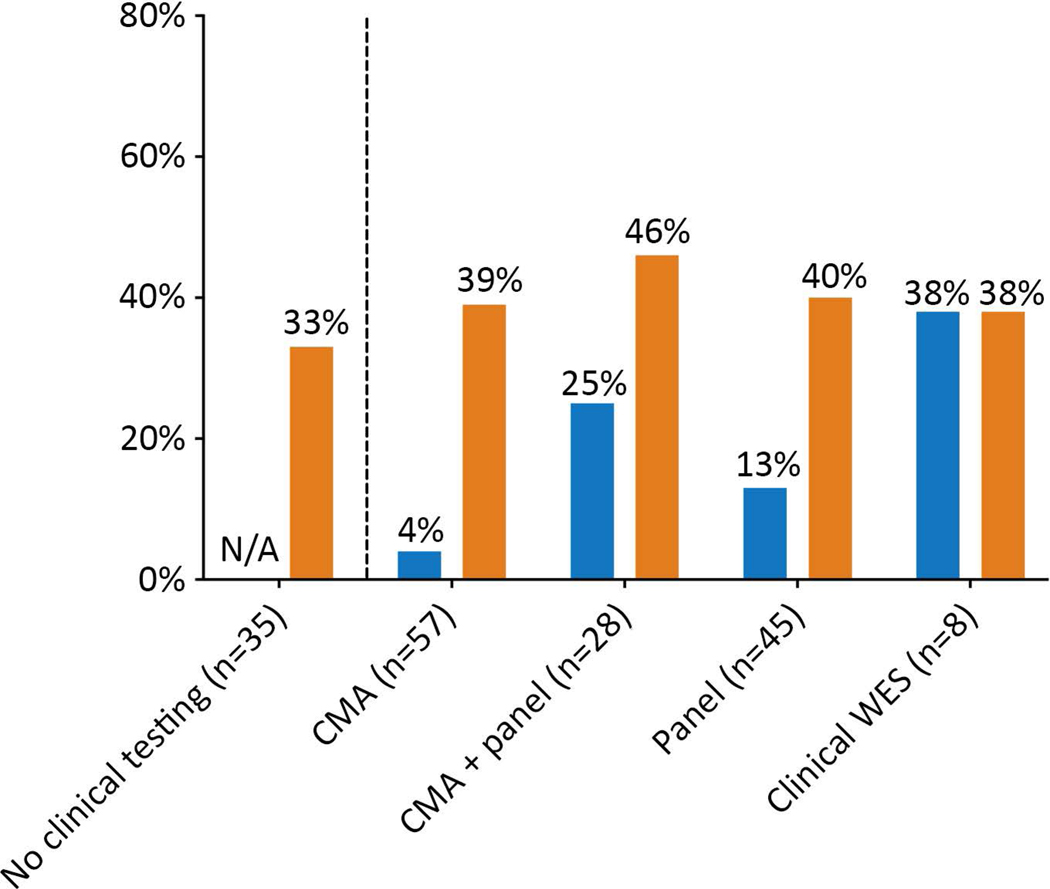
Clinical genetic evaluation, conducted either before or after research enrollment, varied widely across the cohort. At our institution, CMA was first available as a clinical test in 2006, and epilepsy panels in 2012. The epilepsy panels used included range in the number of genes included from 87 to 458. Thirty-five participants had no clinical genetic testing. The 90 patients who had some form of clinical testing had karyotype (n=17), CMA (n=57), Fragile X testing (n=9), multi-gene panel (n=45), CMA and multi-gene panel (n=28), and clinical WES (n=8) (Fig 1, Supplementary Table 4). Fifty-three participants had research WES data previously analyzed by our research team during the time period of 2012–2013.
Figure 1: Diagnostic yield of research WES.
Diagnostic yield of research WES compared with diagnostic yield of prior/concomitant clinical genetic testing. CMA = chromosomal microarray; WES = whole exome sequencing. Blue represents the diagnostic yield from clinical testing and orange the diagnostic yield from research WES. Overall, 90 participants had at least one clinical genetic test. Since the type of clinical genetic testing that each participant had was variable and some participants had more than one genetic test, the total n represented by the categories in the figure does not add up to 90.
We divided participants into 3 subcategories: (a) participants with concomitant clinical testing and/or previous research analysis that had identified an etiology by the time of the present analysis (n=23), (b) participants with concomitant clinical testing and/or research analysis that was inconclusive (n=67), and (c) participants with no concomitant clinical or previous research genetic testing (n=35).
(a) Participants with concomitant “positive” clinical testing
Among the 90 participants who had concomitant clinical genetic testing and/or research analysis, there were 23 who eventually received diagnostic results from clinical testing (3 by clinical WES, 18 by multi-gene panel and 2 by CMA). Our research WES analysis (conducted without knowledge of clinical test results) also identified the 21 pathogenic/likely pathogenic variants identified through clinical panels and WES. The two pathogenic findings on CMA (del 1p36 and dup 15q11.2-13) were present in patients with clinical phenotypes consistent with these copy number variants, and these were not detected by research WES analysis.
(b) Participants with concomitant “negative” clinical testing
Among the 67 participants with concomitant clinical testing that had not revealed a genetic diagnosis, our research analysis identified a pathogenic or likely pathogenic variant in 17 (25%). This included 1 of 5 participants who had non-explanatory clinical WES. No patient had a previously identified VUS that we reclassified as pathogenic or likely pathogenic. In this group, the yield from our WES analysis was similar among those who had had prior CMA only (10/45, 22%), both CMA and panel (6/30, 20%) and panel only (7/34, 21%). Finally, among patients with negative clinical WES testing, our WES yield was 20% (1/5). Among the participants who had prior/concomitant negative clinical testing, we were more likely to identify variants in recently published (6 cases with variants in GABBR1, ITPA, KAT6A, PCDH11X, PTPN23, RHOBTB2 and SATB2) and/or candidate genes (5 cases with variants in FAT3, GABRA6 (n=2), NRXN1/NRXN2 and PTCHD1) than established epilepsy genes (4 cases with variants in KCNA2, KCNH1, NR2F1, NPRL3).
(c) Participants without concomitant clinical testing/previous research analysis
Among the 35 participants who had no concomitant clinical genetic testing or previous research analysis, the yield of our WES analysis was 34% (12/35). It should be noted that almost all participants without clinical genetic testing had exome sequencing for the proband only (34/35, 97%) rather than as a trio. Among participants with no concomitant clinical testing or previous research analysis, we were more likely to identify variants in established epilepsy genes (DEPDC5, GNAO1, GRIN2A, KCNB1, KCNH1, MECP2, NF1, SCN8A, WWOX) than in recently published/candidate genes. Our analysis identified only one participant in this group with a variant in a recently published gene (FGF12), and 3 participants with variants in candidate genes (CAMTA1, HUWE1 and LPPR4).
Research findings in established, recently published, and candidate epilepsy genes
We identified a total of 50/125 participants in our cohort with pathogenic or likely pathogenic findings in well-established, recently published, or candidate epilepsy genes. These were most often de novo variants associated with autosomal dominant phenotypes, but there were 4 instances of autosomal recessive inheritance and 6 instances of X-linked inheritance (Table 2). Genetic and clinical details for each participant are available in the Supplemental data.
Table 2:
Distribution of identified gene variants
| Category | Inheritance | Gene |
|---|---|---|
| Established epilepsy genes n=33 | AD | COL4A1, DEPDC5, DYNC1H1, GNAO1, GRIN2A, KCNA2, KCNB1, KCNH1, KCNQ2 (n=8), MECP2, NF1, NPRL3, NR2F1, NRXN1, SCN1A, SCN2A (n=4), SCN8A, SPTAN1, STXBP1 (n=2), WWOX |
| AR | SZT2 | |
| X-linked | CASK, IQSEC2, PIGA | |
| Recently published epilepsy genes n=9 |
AD | FGF12, GABBR1, GABBR2, KAT6A, PTPN23, RHOBTB2, SATB2 |
| AR | ITPA | |
| X-linked | PCDH11X | |
| Candidate epilepsy genes n=8 | AD | CAMTA1, FAT3, LPPR4, NRXN2 |
| AR | DNAH7, GABRA6 | |
| X-linked | HUWE1, PTCHD1 |
AD = autosomal dominant; AR = autosomal recessive. The inheritance pattern of phenotypes associated with variants in candidate epilepsy genes was determined with the DOMINO tool41.
(a) Established epilepsy-associated genes
Of the 125 participants in our cohort, 33 had pathogenic/likely pathogenic variants in established epilepsy-associated genes. Variants in genes coding for channelopathies accounted for 55% (18/33) of the variants identified: KCNQ2 (n=8) most frequently, followed by SCN2A (n=4), SCN8A (n=2), KCNH1 (n=2), KCNA2 (n=1), and KCNB1 (n=1). Among the 15 participants without channelopathies, we identified pathogenic/likely pathogenic variants in STXBP1 (n=2) and 1 case each for the following genes: CASK, DEPDC5, GNAO1, GRIN2A, IQSEC2, MECP2, NF1, NPRL3, NR2F1, PIGA, SPTAN1, SZT2, and WWOX. Almost all variants identified were absent in control databases, with the exception of two KCNQ2 variants (c.793G>A, present only once in gnomAD, and c.1405G>A, present only 4 times in gnomAD). It should be noted that many of the 33 participants with pathogenic variants in established epilepsy-associated genes identified through our WES analysis had concomitant clinical testing that was non-explanatory (20/33). For example, a patient with a history of infantile spasms, intellectual disability, severe hypotonia and optic atrophy had prior clinical testing (CMA and multi-gene panel) which was non-explanatory. Our WES analysis revealed a pathogenic variant in NR2F1, which has been described as the gene associated with Bosch-Boonstra-Schaaf optic atrophy syndrome21 and more recently as being associated with a broader phenotype including epilepsy22. Additional Sanger sequencing of parental DNA revealed that the variant in NR2F1 was de novo. This gene had not been included on the multi-gene panel performed clinically at the time. A second participant with neonatal onset epileptic encephalopathy, global developmental delay, hypotonia, laryngomalacia, and brain atrophy had negative prior testing, including CMA and multi-gene epilepsy panel. We identified a likely de novo KCNH1 missense variant affecting the S6 transmembrane domain. Variants in KCNH1, including in this protein domain, are linked to Zimmermann-Laband and Temple-Baraitser syndrome23,24. Again, in this case, the multi-gene panel utilized clinically had not included KCNH1 at the time.
(b) Recently published epilepsy-associated genes
Nine participants in our cohort had a pathogenic/likely pathogenic variant in a recently identified epilepsy-associated gene (one case each): FGF12, GABBR1, GABBR2, ITPA, KAT6A, PCDH11X, PTPN23, RHOBTB2, and SATB2. These variants were absent from control databases, with the exception of ITPA c.452G>A (16 heterozygous alleles in gnomAD with allele frequency 0.00006, none homozygous) and SATB2 c.754C>G (1 allele in gnomAD). Interestingly, 7 of these 9 participants had prior non-explanatory clinical testing by multi-gene panel (n=3) and/or WES (n=5), as illustrated in the introduction by the patient with a homozygous variant in ITPA.
(c) Identification of variants in candidate epilepsy genes
When no clear pathogenic/likely pathogenic variant was found in the analysis of established or recently identified epilepsy genes, we assessed genes for a putative role in disease etiology. We identified 8 participants with variants in what we ultimately considered to be candidate genes for epilepsy (Table 3): CAMTA1, FAT3, LPPR4, DNAH7, GABRA6, HUWE1, PTCHD1, and NRXN2 (published with a concomitant variant in NRXN1, with each variant inherited from a different parent)25.
Table 3:
Candidate epilepsy genes
| Participant | Gene | Published phenotype (#OMIM or HGMD) | Participant’s phenotype |
|---|---|---|---|
| POD_0163 | CAMTA1 | Cerebellar ataxia with ID (#614765) | IS, refractory epilepsy, DD, CVI |
| LPPR4 | No human phenotype | ||
| POD_0030 | DNAH7 | Variable, one patient with dysmorphic features, seizures, hypotonia | OS, refractory seizures, DD, CVI, premature death |
| POD_0060 | FAT3 | Atypical Rett syndrome | Features of Rett syndrome |
| POD_0010 | GABRA6 | IGE, psychiatric disorders | Atypical absence epilepsy, focal abnormalities on EEG |
| POD_0184 | Temporal focal cortical dysplasia, refractory seizures, temporal lobe resection | ||
| POD_0081 | HUWE1 | X-linked ID (#300706) | OS |
| POD_0114 | IS, refractory seizures | ||
| POD_0130 | IS, refractory seizures | ||
| POD_0102 | NRXN1 | ASD, DD, epilepsy, neuropsychiatric disorders; Pitt-Hopkins-like syndrome 2 (#614325) | OS, dysregulated breathing, seizures, premature death |
| NRXN2 | ASD | ||
| POD_0073 | PTCHD1 | XL ID and/or autism (#300830) | West syndrome, refractory epilepsy, DD, CVI |
ASD = autism spectrum disorder; CVI = cerebrovisual impairment; DD = developmental delay; Het = heterozygous; FCD = focal cortical dysplasia; ID = intellectual disability; IGE = idiopathic generalized epilepsy; IS = infantile spasms; OMIM = Online Mendelian Inheritance in Man; OS = Ohtahara syndrome; PP-2 = PolyPhen2; SIFT = Sorting Intolerant from Tolerant; XL = X-linked. Transcript identification is provided in Supplementary Table 2.
Discussion
For most patients with epilepsy, particularly refractory epilepsy without identified cause, neurologists and patients’ families seek diagnostic precision in the form of genetic diagnosis in hope that this will lead to more specific approaches to treatment. A genetic diagnosis provides relief from uncertainty, which is important even if the diagnosis is associated with a poor prognosis. In addition, it may help patients avoid additional unnecessary clinical investigations and guide therapy selection. A genetic diagnosis enables accurate recurrence risk estimation and genetic counseling for future pregnancies and other family members. Besides prognosis and management changes, disease gene identification also serves to build cohorts for registries, natural history studies, and gene-specific drug trials. These implications are especially important for the evaluation and long-term management of patients with neonatal-onset severe epilepsies and other severe epilepsies and DEEs26.
Overall, we identified pathogenic or likely pathogenic variants in 40% of participants with suspected genetic epilepsy through research WES analysis with variant interpretation and participant specific phenotyping. This yield is comparable to that of recent NGS reports in pediatric epilepsy6–10. We report the findings in our cohort with mention of clinical testing, performed in tandem with our research process, since this reflects the reality of how prospective research participants present, some with clinical testing performed but often not explanatory, and some without access to clinical testing due to lack of insurance coverage or other barriers27. Importantly, of the 67 patients who had some form of clinical testing performed and remained ‘unsolved’ on a clinical basis, we identified pathogenic or likely pathogenic variants in 17 (25%), including the patient with the homozygous ITPA pathogenic variant in the vignette above. Research WES provides an option for evaluation in an era when there are no formal guidelines for clinical testing, which remains unavailable to many patients. We would have predicted a different yield from WES depending on what if any clinical testing was undertaken, but, somewhat surprisingly, we did not identify a difference in our research WES yield across the groups with different types of prior clinical testing.
The dynamic landscape of the genetic epilepsies
Our data demonstrate the importance of continued on-going analysis to incorporate current literature since gene panels from past years may not have included genes more recently established or identified as candidate genes for epilepsy. In addition, it is essential to continue to evaluate for a genetic etiology, especially for those patients who remain ‘unsolved’ after prior clinical testing or research analyses.
For a large proportion of our cohort (33/125, 26%) in whom we identified variants in established genes, diagnoses could have been made with clinical testing had it been available to these patients. Most patients presented with neonatal-onset encephalopathies and most frequently had variants in KCNQ2 (n=8) and SCN2A (n=4). In 9 patients, we identified variants in recently described genes such as GNAO128, NPRL329–31, NR2F132 and RHOBTB233. These variants were not identified nor reported clinically because of the time lag between research discovery and clinical testing incorporating new genes. Recently, we have observed that some testing laboratories report candidate gene findings, which would make such findings available for assessment and correlation with the clinical phenotype. Finally, for 8 patients we identified variants in candidate genes with a putative role in epilepsy etiology. It is not surprising that we identified more recently published and/or candidate genes in the group that had been previously tested on a clinical basis. Systems for data matching that connect researchers and clinicians who have patients with variants in the same gene, such as GeneMatcher, as well as collaboration within the broader epilepsy genetics community will allow us to re-evaluate these candidate variants over time.
We emphasize the relevance of implementing a practice that necessarily employs the reanalysis of NGS data for any ‘unsolved’ case with epilepsy who warrants sequencing34. The process of reanalysis requires the combined efforts of researchers, physicians, and genetic counselors with expertise in epilepsy to guide patients and families through what is often a clinical diagnostic odyssey alongside research enrollment. For healthcare providers, researchers, and clinical laboratories, it is imperative to stay up to date with expanding knowledge, to encourage participation in large consortia that may lead to the identification of novel epilepsy genes, and to communicate on a regular basis with patients and families as new technologies and understanding emerge.
While we did make progress in identifying the cause of several patients’ epilepsy with WES, these patients were predominantly in the category of DEE, which represented the largest group of patients referred and ascertained. Not surprisingly, given the small number of patients with generalized and focal epilepsies without ID or other features, we did not identify pathogenic variants for most of these patients, nor for patients with Rasmussen encephalitis or FIRES. These groups of patients continue to have severe and unexplained epilepsy that would benefit from a more precise molecular diagnosis, and additional attention is needed to elucidate their causes.
To WES or not to WES?
WES has the potential to provide an early diagnosis and drive therapeutic choices to include empirically proven therapy or trials of new or targeted treatments. A physician may opt to order WES when a patient has undergone a series of genetic tests without identifying an etiology. In contrast, WES is sometimes ordered in an attempt to expedite a possible diagnosis and reduce the costs incurred by multiple tests. Currently, there are no existing practice guidelines regarding the best approaches to clinical genetic testing for individuals with epilepsy. In our experience, the yield of WES is fairly high and provides the opportunity to identify candidate genes and to re-evaluate the data on a regular basis.
The diagnostic yield from revised epilepsy gene panels is often comparable to WES9. Since epilepsy panels are still less expensive than clinical WES, recent cost-effectiveness analysis supports the use of panels35. Thus, it may be advisable or more feasible to first repeat a (revised) gene panel instead of extending genetic testing to WES or WGS. For example, our patient with Bosch-Boonstra-Schaaf optic atrophy syndrome22 had testing with a commercial gene panel in 2015 that did not include NR2F1. She had research sequencing as well as clinical WES and received a clinical diagnosis in 2018 that could have been provided using a revised gene panel.
From a diagnostic point of view, it should be noted that some diagnoses cannot (yet) be made with WES. Copy number variations (CNVs) play a causative role in 5% of pediatric epilepsy patients36. While most clinical laboratories use CMA for CNV detection, WES and whole genome sequencing (WGS) can also be used to detect structural variation, allowing combined small and large variant analysis37. We predict that CNV detection using NGS data will eventually replace the CMA as a first-line assessment for CNVs, since WES can theoretically detect all forms of genetic variation in one single test. Until then, CMA still has a role clinically. For example, two patients in our cohort had multiple clinical genetic tests, including multi-gene panel, and research exome, but were eventually diagnosed by CMA with 1p36 deletion and 15q11.2-q13.1 duplication.
Apart from structural genetic variations, routine WES analysis, particularly in the clinical setting, may also not detect somatic variants in the brain. Somatic mutation is a recognized cause for neurodevelopmental disorders, both with and without evidence of a lesion on neuroimaging38–40. Somatic variants that have arisen early in post-zygotic development may be detectable in DNA obtained from blood leukocytes, but those that have arisen later will be undetectable in blood40 and rather require high-depth sequencing of DNA derived from affected brain tissue. This phenomenon is illustrated by one of our patients who presented with infantile spasms, severe intellectual disability, and a right frontotemporal lesion that was eventually resected. A pathogenic somatic SLC35A2 variant was identified using deep sequencing in brain tissue40; the variant was undetectable in our WES analysis of leukocyte-derived DNA.
Conclusion
We demonstrate a high yield from WES analysis when combining careful patient selection and phenotyping and a streamlined bioinformatics approach that incorporates clinically relevant criteria and new literature to patients with unexplained epilepsy. Even as clinical WES testing becomes more commonly employed, re-evaluation of non-explanatory exome data on a research basis, and research WES analysis for those unable to undergo clinical testing,will guide neurobiological research into mechanisms of epilepsy and future therapies. In the appropriate setting and with appropriate research and clinical consents, research results can be conveyed to patients in the clinical setting to provide diagnoses, in some cases to inform clinical management, and to identify patients eligible for gene-specific clinical trials. We advocate for a multidisciplinary approach to all patients with unexplained epilepsy and a partnership between clinicians and researchers to maximize the number of patients for whom we can achieve genetic diagnoses.
Supplementary Material
Supplementary Table 1: Compiled Epilepsy Gene List (n=513).
The list is comprised of genes from 6 multi-gene epilepsy panels utilized by clinical diagnostic laboratories: Prevention Genetics (n=153), Claritas ROI Epilepsy (n=458), Emorey Genetics Laboratory epilepsy and other seizures panel (n=109), Athena/Quest epilepsy advanced sequencing panel (n=234), Ambry complete (n=100), GeneDX comprehensive (n=87).
Supplementary Table 2: Clinical and molecular data of all identified gene variants.
GnomAD = Genome Aggregation Database; HGMD = Human Gene Mutation Database; OMIM = Online Mendelian Inheritance in Man; PP-2 = PolyPhen2; SIFT = Sorting Intolerant from Tolerant; VUS = variant of uncertain significance. Genes marked with * were clinically confirmed.
Supplementary Table 3: Clinical details of the patients with focal or generalized epilepsy without DEE.
ASD = autism spectrum disorder; F = female; FCD = focal cortical dysplasia; GDD = global developmental delay; ID = intellectual disability; M = male; y = years; *mixed seizure types, predominant type listed; **unknown seizure type.
Supplementary Table 4: Details of clinical genetic testing for all patients.
CES = clinical exome sequencing; CMA = chromosomal microarray; DEE = developmental and epileptic encephalopathy; F = female; FE = focal epilepsy; FIRES = febrile infection-related epilepsy syndrome; GE = generalized epilepsy; IS = infantile spasms; M = male; mo = months; OS = Ohtahara syndrome; RE = Rasmussen encephalitis; RES = research exome sequencing; Rett = Rett syndrome; VUS = variant of unknown significance; we = weeks; y = years
Bullet points:
-
-
We demonstrate a 40% yield of WES in patients referred for sequencing with a diagnosis of epilepsy using a streamlined bioinformatics platform and iterative phenotyping and exome analysis.
-
-
Analysis and re-analysis of WES data incorporating current literature identifies disease-associated variants among participants with previously non-diagnostic results.
-
-
Research sequencing and analysis provides an option for evaluation in an era when there are no formal guidelines for clinical testing, which remains unavailable to many patients.
Acknowledgements
The authors thank the patients and families who participated in our research and our many colleagues who have referred patients to the Epilepsy Genetics Program. Dr. Anne Rochtus is supported by a Fellowship of the Belgian American Educational Foundation, by a Fulbright Program grant sponsored by the Bureau of Educational and Cultural Affairs of the United States Department of State and administered by the Institute of International Education and by an internal fund of the University of Leuven (PDM/17/195. Dr. Annapurna Poduri is supported by the Boston Children’s Hospital Translational Research Program.
We thank the Epi25 principal investigators and all of the patients with epilepsy who participated in the study for making possible this global collaboration and resource to advance epilepsy genetics research. This work is part of the Centers for Common Disease Genomics (CCDG) program, funded by the National Human Genome Research Institute (NHGRI) and the National Heart, Lung, and Blood Institute (NHLBI). CCDG-funded Epi25 research activities at the Broad Institute, including genomic data generation in the Broad Genomics Platform, are supported by NHGRI grant UM1 HG008895 (PIs: Eric Lander, Stacey Gabriel, Mark Daly, Sekar Kathiresan). The Genome Sequencing Program efforts were also supported by NHGRI grant 5U01HG009088-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank the Stanley Center for Psychiatric Research at the Broad Institute for supporting the genomic data generation efforts. We also thank the Genome Aggregation Database (gnomAD) and the groups that provided exome and genome variant data to this resource. A full list of contributing groups can be found at https://gnomad.broadinstitute.org/about.
Footnotes
Conflicts of Interest
Nothing to report. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Kevelam SH, Bierau J, Salvarinova R, et al. Recessive ITPA mutations cause an early infantile encephalopathy. Ann Neurol. 2015; 78(4):649–58. [DOI] [PubMed] [Google Scholar]
- 2.Ewans LJ, Schofield D, Shrestha R, et al. Whole-exome sequencing reanalysis at 12 months boosts diagnosis and is cost-effective when applied early in Mendelian disorders. Nat Publ Gr [Internet]. 2018; 00(January):1–11. Available from: 10.1038/gim.2018.39 [DOI] [PubMed] [Google Scholar]
- 3.Nambot S, Thevenon J, Kuentz P, et al. Clinical whole-exome sequencing for the diagnosis of rare disorders with congenital anomalies and / or intellectual disability : substantial interest of prospective annual reanalysis. Nat Publ Gr [Internet]. 2017; 20(6):645–54. Available from: 10.1038/gim.2017.162 [DOI] [PubMed] [Google Scholar]
- 4.Hildebrand MS, Dahl H-HM, Damiano JA, et al. Recent advances in the molecular genetics of epilepsy. J Med Genet [Internet]. 2013; 50(5):271–9. Available from: http://jmg.bmj.com/lookup/doi/10.1136/jmedgenet-2012-101448 [DOI] [PubMed] [Google Scholar]
- 5.McTague A, Howell KB, Cross JH, et al. The genetic landscape of the epileptic encephalopathies of infancy and childhood. Lancet Neurol [Internet]. 2016; 15(3):304–16. Available from: 10.1016/S1474-4422(15)00250-1 [DOI] [PubMed] [Google Scholar]
- 6.Veeramah KR, Johnstone L, Karafet TM, et al. Exome sequencing reveals new causal mutations in children with epileptic encephalopathies. Epilepsia. 2013; 54(7):1270–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Michaud JL, Lachance M, Hamdan FF, et al. The genetic landscape of infantile spasms. Hum Mol Genet. 2014; 23(18):4846–58. [DOI] [PubMed] [Google Scholar]
- 8.Dyment DA, Tétreault M, Beaulieu CL, et al. Whole-exome sequencing broadens the phenotypic spectrum of rare pediatric epilepsy: A retrospective study. Clin Genet. 2015; 88(1):34–40. [DOI] [PubMed] [Google Scholar]
- 9.Berg AT, Coryell J, Saneto RP, et al. Early-life epilepsies and the emerging role of genetic testing. JAMA Pediatr. 2017; 171(9):863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helbig KL, Farwell Hagman KD, Shinde DN, et al. Diagnostic exome sequencing provides a molecular diagnosis for a significant proportion of patients with epilepsy. Genet Med. 2016; 18(9):898–905. [DOI] [PubMed] [Google Scholar]
- 11.Altshuler DM, Durbin RM, Abecasis GR, et al. An integrated map of genetic variation from 1,092 human genomes. Nature. 2012; 491(7422):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falco-Walter JJ, Scheffer IE, Fisher RS. The new definition and classification of seizures and epilepsy. Epilepsy Res. 2018; 139(July 2017):73–9. [DOI] [PubMed] [Google Scholar]
- 13.Gudbjartsson H, Georgsson GF, Gudjónsson SA, et al. GORpipe: A query tool for working with sequence data based on a Genomic Ordered Relational (GOR) architecture. Bioinformatics. 2016; 32(20):3081–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olson HE, Kelly M, LaCoursiere CM, et al. Genetics and genotype–phenotype correlations in early onset epileptic encephalopathy with burst suppression. Ann Neurol. 2017; 81(3):419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17(5):405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lek M, Karczewski KJ, Minikel E V., et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature [Internet]. 2016; 536(7616):285–91. Available from: 10.1038/nature19057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karczewski KJ, Francioli LC, Tiao G, et al. Variation across 141,456 human exomes and genomes reveals the spectrum of loss-of-function intolerance across human protein-coding genes. bioRxiv [Internet]. 2019; :531210. Available from: https://www.biorxiv.org/content/10.1101/531210v2 [Google Scholar]
- 18.Mclaren W, Gil L, Hunt SE, et al. The Ensembl Variant Effect Predictor. Genome Biol [Internet]. 2016; :1–14. Available from: 10.1186/s13059-016-0974-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sim N, Kumar P, Hu J, et al. SIFT web server : predicting effects of amino acid substitutions on proteins. 2012; 40(June):452–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosch DGM, Boonstra FN, Gonzaga-Jauregui C, et al. NR2F1 mutations cause optic atrophy with intellectual disability. Am J Hum Genet. 2014; 94(2):303–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CA, Bosch DGM, Cho MT, et al. The expanding clinical phenotype of Bosch-Boonstra-Schaaf optic atrophy syndrome: 20 new cases and possible genotype-phenotype correlations. Genet Med. 2016; 18(11):1143–50. [DOI] [PubMed] [Google Scholar]
- 23.Simons C, Rash LD, Crawford J, et al. Mutations in the voltage-gated potassium channel gene KCNH1 cause Temple-Baraitser syndrome and epilepsy. Nat Genet. 2015; 47(1):73–7. [DOI] [PubMed] [Google Scholar]
- 24.Fukai R, Saitsu H, Tsurusaki Y, et al. De novo KCNH1 mutations in four patients with syndromic developmental delay, hypotonia and seizures. 2016; 61(5):381–7. Available from: 10.1038/jhg.2016.1 [DOI] [PubMed] [Google Scholar]
- 25.Rochtus AM, Trowbridge S, Goldstein RD, et al. Mutations in NRXN1 and NRXN2 in a patient with early-onset epileptic encephalopathy and respiratory depression. 2019; :1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shellhaas RA, Glass HC, Massey SL, et al. Profile of neonatal epilepsies Characteristics of a prospective US cohort. 2017; :893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poduri A, Sheidley BR, Shostak S, et al. Genetic testing in the epilepsies-developments and dilemmas. Nat Rev Neurol [Internet]. 2014; 10(5):293–9. Available from: 10.1038/nrneurol.2014.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly M, Park M, Mihalek I, et al. Spectrum of neurodevelopmental disease associated with the GNAO1 guanosine triphosphate–binding region. Epilepsia. 2019;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ricos MG, Hodgson BL, Med DB, et al. Mutations in the Mammalian Target of Rapamycin Pathway Regulators NPRL2 and NPRL3 Cause Focal Epilepsy. 2015;. [DOI] [PubMed] [Google Scholar]
- 30.Sim JC, Scerri T, Fanjul-fern M, et al. Familial Cortical Dysplasia Caused by Mutation in the Mammalian Target of Rapamycin Regulator NPRL3. 2015;. [DOI] [PubMed] [Google Scholar]
- 31.Weckhuysen S, Marsan E, Lambrecq V, et al. Involvement of GATOR complex genes in familial focal epilepsies and focal cortical dysplasia. Epilepsia. 2016; 57(6):994–1003. [DOI] [PubMed] [Google Scholar]
- 32.Dimassi S, Labalme A, Ville D, et al. Whole-exome sequencing improves the diagnosis yield in sporadic infantile spasm syndrome. Clin Genet. 2016; 89(2):198–204. [DOI] [PubMed] [Google Scholar]
- 33.Straub J, Konrad EDH, Grüner J, et al. Missense Variants in RHOBTB2 Cause a Developmental and Epileptic Encephalopathy in Humans, and Altered Levels Cause Neurological Defects in Drosophila. Am J Hum Genet [Internet]. 2017; 0(0):44–57. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0002929717304615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Initiative EG. The Epilepsy Genetics Initiative : Systematic reanalysis of diagnostic exomes increases yield. 2019; (March):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez Fernández I, Gaínza-Lein M, Abend NS, et al. Factors associated with treatment delays in pediatric refractory convulsive status epilepticus. Neurology. 2018;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson H, Shen Y, Avallone J, et al. Copy Number Variation Plays an Important Role in Clinical Epilepsy. 2014; :943–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hehir-kwa JY, Pfundt R, Veltman JA. Exome sequencing and whole genome sequencing for the detection of copy number variation. 2015;. [DOI] [PubMed] [Google Scholar]
- 38.Poduri A, Evrony GD, Cai X, et al. Somatic Activation of AKT3 Causes Hemispheric Developmental Brain Malformations. Neuron [Internet]. 2012; 74(1):41–8. Available from: 10.1016/j.neuron.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jamuar SS, Lam A-TN, Kircher M, et al. Somatic Mutations in Cerebral Cortical Malformations. N Engl J Med [Internet]. 2014; 371(8):733–43. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1314432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winawer MR, Griffin NG, Samanamud J, et al. Somatic SLC35A2 Variants in the Brain Are Associated with Intractable Neocortical Epilepsy. 2018; (May). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quinodoz M, Royer-bertrand B, Cisarova K, et al. REPORT DOMINO : Using Machine Learning to Predict Genes Associated with Dominant Disorders. Am J Hum Genet [Internet]. 2017; 101(4):623–9. Available from: 10.1016/j.ajhg.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Compiled Epilepsy Gene List (n=513).
The list is comprised of genes from 6 multi-gene epilepsy panels utilized by clinical diagnostic laboratories: Prevention Genetics (n=153), Claritas ROI Epilepsy (n=458), Emorey Genetics Laboratory epilepsy and other seizures panel (n=109), Athena/Quest epilepsy advanced sequencing panel (n=234), Ambry complete (n=100), GeneDX comprehensive (n=87).
Supplementary Table 2: Clinical and molecular data of all identified gene variants.
GnomAD = Genome Aggregation Database; HGMD = Human Gene Mutation Database; OMIM = Online Mendelian Inheritance in Man; PP-2 = PolyPhen2; SIFT = Sorting Intolerant from Tolerant; VUS = variant of uncertain significance. Genes marked with * were clinically confirmed.
Supplementary Table 3: Clinical details of the patients with focal or generalized epilepsy without DEE.
ASD = autism spectrum disorder; F = female; FCD = focal cortical dysplasia; GDD = global developmental delay; ID = intellectual disability; M = male; y = years; *mixed seizure types, predominant type listed; **unknown seizure type.
Supplementary Table 4: Details of clinical genetic testing for all patients.
CES = clinical exome sequencing; CMA = chromosomal microarray; DEE = developmental and epileptic encephalopathy; F = female; FE = focal epilepsy; FIRES = febrile infection-related epilepsy syndrome; GE = generalized epilepsy; IS = infantile spasms; M = male; mo = months; OS = Ohtahara syndrome; RE = Rasmussen encephalitis; RES = research exome sequencing; Rett = Rett syndrome; VUS = variant of unknown significance; we = weeks; y = years