Abstract
Vitamin D deficiency is a worldwide pandemic. The aim of this study was to evaluate associations of plasma 25(OH)D levels with the likelihood of coronavirus disease 2019 (COVID‐19) infection and hospitalization. The study population included the 14 000 members of Leumit Health Services, who were tested for COVID‐19 infection from February 1st to April 30th, 2020, and who had at least one previous blood test for the plasma 25(OH)D level. ‘Suboptimal’ or ‘low’ plasma 25(OH)D level was defined as plasma 25‐hydroxyvitamin D, or 25(OH)D, concentration below the level of 30 ng/mL. Of 7807 individuals, 782 (10.02%) were COVID‐19‐positive, and 7025 (89.98%) COVID‐19‐negative. The mean plasma vitamin D level was significantly lower among those who tested positive than negative for COVID‐19 [19.00 ng/mL (95% confidence interval (CI) 18.41–19.59) vs. 20.55 (95% CI: 20.32–20.78)]. Univariate analysis demonstrated an association between the low plasma 25(OH)D level and increased likelihood of COVID‐19 infection [crude odds ratio (OR) of 1.58 (95% CI: 1.24–2.01, P < 0.001)], and of hospitalization due to the SARS‐CoV‐2 virus [crude OR of 2.09 (95% CI: 1.01–4.30, P < 0.05)]. In multivariate analyses that controlled for demographic variables, and psychiatric and somatic disorders, the adjusted OR of COVID‐19 infection [1.45 (95% CI: 1.08–1.95, P < 0.001)] and of hospitalization due to the SARS‐CoV‐2 virus [1.95 (95% CI: 0.98–4.845, P = 0.061)] were preserved. In the multivariate analyses, age over 50 years, male gender and low–medium socioeconomic status were also positively associated with the risk of COVID‐19 infection; age over 50 years was positively associated with the likelihood of hospitalization due to COVID‐19. We concluded that low plasma 25(OH)D levels appear to be an independent risk factor for COVID‐19 infection and hospitalization.
Keywords: COVID‐19, Israeli population study, low plasma 25(OH) vitamin D level, risk of infection, vitamin D
Vitamin D deficiency is recognized as a significant health concern in which there is heightened interest because of the potential impact on COVID‐19 risk. In a landmark study involving a large Israeli population, Milana Morgenstern and colleagues found that the plasma level of vitamin D is lower among individuals who have tested positive for COVID‐19 and have been hospitalized. This indicates that low vitamin D status is an independent risk factor for severe COVID‐19; male gender and low‐medium socioeconomic status were also shown to be positively associated with the risk of infection. Moreover, age (being > 50 years old) was associated with COVID‐19 hospitalization.

Abbreviations
- BMI
body mass index
- COVID‐19‐N
COVID‐19‐negative
- COVID‐19‐P
COVID‐19‐positive
- LHS
Leumit Health Services
- SES
socioeconomic status
Introduction
From its origin in Wuhan, China, in December 2019, the novel coronavirus disease, COVID‐19, caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) virus, has spread rapidly throughout the world [1]. In Israel, the first case of the COVID‐19 infection was reported on February 21st, 2020. On March 11th, 2020, the World Health Organization (WHO) declared COVID‐19 disease a global pandemic [2]. Immediate targeted actions were needed to identify risk factors of COVID‐19. The SARS‐CoV‐2 virus has high levels of transmissibility, estimated basic reproduction (Ro) ranging from 2.6 to 4.7, and an average incubation duration ranging from 2 to 14 days [3]. The main routes of transmission are respiratory droplets and direct contact with contaminated objects and surfaces [4].
The status of the immune system is determined by a multitude of factors that may contribute to the risk of a viral infection [5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15]. Vitamin D has been recognized as an important cofactor in several physiological processes linked to bone and calcium metabolism, and also in diverse nonskeletal outcomes, including autoimmune diseases, cardiovascular diseases, diabetes type 2, obesity and cognitive decline and infections [16, 17]. In particular, the pronounced impact of vitamin D metabolites on the immune system response, and on the development of COVID‐19 infection by the novel SARS‐CoV‐2 virus, has been described [5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15]. Vitamin D deficiency has been recognized as a worldwide pandemic [18, 19]. Therefore, we aimed to determine associations between low plasma 25(OH)D and the risk of COVID‐19 infection and hospitalization, using real‐world Israeli population‐based data. We hypothesized that the mean plasma level of 25(OH)D would be significantly lower and, accordingly, the rate of suboptimal plasma 25(OH)D levels would be found frequently among persons testing positive for COVID‐19 infection, and among persons subsequently hospitalized, in a large population‐based epidemiological study.
Results
Low vitamin D level and the likelihood of COVID‐19 infection
Of 14 022 subjects, aged 2 months to 103 years, who were tested for COVID‐19 infection, 1416 (10.1%) had at least one positive result; 12 606 (89.9%) had only negative results (Fig. 1). After excluding the 6215 individuals without data on plasma 25(OH)D levels, the study sample composed 7807 individuals (Fig. 1). Also for this sample, the proportion of infected individuals was 10.02% (782/7807) for COVID‐19‐positive (COVID‐19‐P), and 7025 (89.98%) for COVID‐19‐negative (COVID‐19‐N; Fig. 1). We run an univariate logistic regression analysis, assessing the odds ratio (OR) for COVID‐19 infections and different categories of plasma 25 (OH) D levels (Table 1). In a primary univariate analysis, COVID‐19‐P subjects were younger, and more likely to be males and to reside in a lower socioeconomic status (SES) area than were COVID‐19‐N subjects (Table 2). The mean plasma 25(OH)D level was significantly lower for COVID‐19‐P subjects (Table 2), and the proportion of individuals with low vitamin D levels was higher (89.90% vs. 84.91%, P < 0.001; Table 3). Interestinly, the prevalence of dementia, hypertension, cardiovascular disease and chronic lung disorders was greater among persons who were COVID‐19‐N than those who were COVID‐19‐P (P < 0.05, P < 0.001, P < 0.001, P < 0.001; Table 3).
Fig. 1.
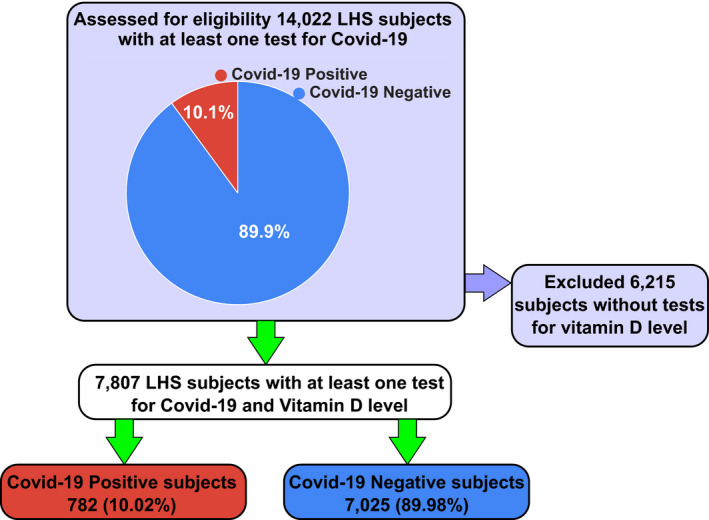
Flow chart of the study design. Of 14 022 subjects, aged 2 months to 103 years, who were tested for COVID‐19 infection, 1416 (10.1%) had at least one positive result; 12 606 (89.9%) had only negative results. After excluding the 6215 individuals without data on plasma 25(OH)D levels, the study sample composed of 7807 individuals. Also for this sample, the proportion of infected individuals was 10.02% (782/7807) for COVID‐19‐P, and 7025 (89.98%) for COVID‐19‐N.
Table 1.
Univariate logistic regression analysis, assessing the odds ratio for COVID‐19 infections and different categories of plasma 25(OH)D levels.
| Variable |
COVID‐19‐P n = 782 (10.02%) |
COVID‐19‐N n = 7025 (89.98%) |
Crude OR (95% CI) for COVID‐19 | P‐value |
|---|---|---|---|---|
| Plasma 25(OH) D level categories | ||||
| Sufficiency ≥ 30 ng/mL | 79 (10.1%) | 1060 (15.1%) | 1.00 | |
| Insufficiency 29–20 ng/mL | 598 (76.5%) | 5050 (71.8%) | 1.59 (1.24–2.02) | 0.0053 |
| Deficiency < 20 ng/mL | 105 (13.4%) | 915 (13.1%) | 1.58 (1.13–2.09) | 0.0002 |
Table 2.
Demographic characteristics of the study sample stratified by COVID‐19 test results.
| Demographics |
COVID‐19‐P n = 782 (10.02%) |
COVID‐19‐N n = 7025 (89.98%) |
P‐value |
|---|---|---|---|
| Mean age, (years, 95% CI) | 35.58 (34.49‐36.67) | 47.35 (46.87–47.85) | 0.001 |
| Age categories N (%) | |||
| 0–5 years | 3 (0.38%) | 18 (0.26%) | 0.023 |
| 5–20 years | 79 (10.10%) | 381 (5.42%) | 0.001 |
| 20–40 years | 249 (31.84%) | 2504 (35.64%) | 0.036 |
| 40–60 years | 266 (34.02%) | 2082 (29.64%) | 0.001 |
| 60–80 years | 152 (19.44%) | 1378 (19.62%) | 0.082 |
| 80+ years | 33 (4.22%) | 662 (9.42%) | 0.001 |
| SES | |||
| Low–medium | 601 (83.70%) | 4418 (67.73%) | 0.001 |
| High–medium | 117 (16.30%) | 2105 (32.27%) | 0.001 |
| Gender N (%) | |||
| Male | 385 (49.23%) | 2849 (40.56%) | 0.001 |
| Female | 397 (50.77%) | 4176 (59.44%) | 0.001 |
| Smoking N (%) | 127 (18.70%) | 1136 (19.39%) | 0.056 |
| Mean BMI, (95% CI) | 27.32 ( 26.88–27.77) | 27.36 (27.22–27.52) | 0.432 |
| Mean vitamin D (ng/mL; 95% CI) | 19.00 (18.41–19.59) | 20.55 (20.32–20.78) | 0.026 |
Table 3.
Clinical characteristics of the study sample stratified by COVID‐19 test results.
| Variable N (%) |
COVID‐19‐P n = 782 (10.02%) |
COVID‐19‐N n = 7025 (89.98%) |
P‐value |
|---|---|---|---|
| Low vitamin D level a | 703 (89.90%) | 5965 (84.91%) | 0.001 |
| Smoking b | 127 (16.24%) | 1136 (16.17%) | 0.669 |
| Depression/Anxiety | 73 (9.34%) | 817 (11.63%) | 0.055 |
| Schizophrenia | 15 (1.92%) | 141 (2.01%) | 0.866 |
| Dementia | 27 (3.45%) | 427 (6.08%) | 0.025 |
| Diabetes mellitus | 154 (19.69%) | 1578 (22.46%) | 0.055 |
| Hypertension | 174 (22.25%) | 1962 (27.93%) | 0.046 |
| Cardiovascular disease | 78 (9.97%) | 1172 (16.68%) | 0.001 |
| Chronic lung disorders | 66 (8.44%) | 935 (13.31%) | 0.001 |
| Obesity c | 235 (30.05%) | 1900 (27.05%) | 0.350 |
Low plasma 25(OH)D level – the total plasma levels < 30 ng/mL
Missing data: 13.1%
Missing data: 9.8%
The significant values (P‐value < 0.05) were shown in bold.
Multivariate analysis, after controlling for the demographic variables, and psychiatric and somatic disorders, demonstrated an independent and significant association between the low 25(OH)D levels and the increased likelihood of COVID‐19 infection [adjusted OR of 1.50 (95% confidence interval (CI): 1.13–1.98, P < 0.001); Fig. 2A]. The risk of COVID‐19 infection was independently positively associated with being male [adjusted OR of 1.49 (95% CI: 1.24–1.79, P < 0.05)], aged older than 50 years [adjusted OR of 1.56 (95% CI: 1.26–1.92, P < 0.05)] and residing in a low–medium SES city or town [adjusted OR of 2.06 (95% CI: 1.65–2.59, P < 0.001)] (Fig. 2B). Independent negative associations were observed between the risk of COVID‐19 infection and having a diagnosis of dementia [adjusted OR of 0.56 (95% CI: 0.32–0.98, P < 0.05], of cardiovascular disease [adjusted OR of 0.59 (95% CI: 0.44–0.79 P < 0.001] and of a chronic lung disorder [adjusted OR of 0.58 (95% CI: 0.42–0.79 P < 0.001] (Table 4).
Fig. 2.
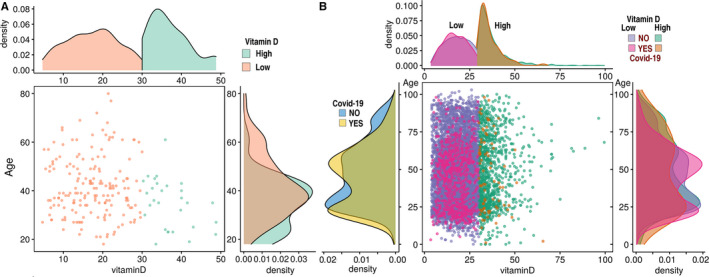
(A) Distribution densities of plasma 25(OH) vitamin D levels (horizontcal axis) and age (vertical axis) among persons infected (A) and not infected (B) with COVID‐19. The criterion for plasma vitamin D ‘suboptimal’ or ‘low’ status was < 30 ng/mL.
Table 4.
Multivariate logistic regression analysis of the odds ratio (OR) for infection with COVID‐19, controlling for multiple conditions, with 95% confidence interval (CI).
| Variable | Crude OR (95% CI) | P‐value | Adjusted OR (95% CI) | P‐value |
|---|---|---|---|---|
| Low vitamin D level a | 1.58 (1.24–2.01) | 0.001 | 1.50 (1.13–1.98) | 0.001 |
| Age over 50 years | 1.51 (1.21–1.89) | 0.001 | 1.56 (1.26–1.92) | 0.001 |
| Male | 1.42 (1.23–1.65) | 0.001 | 1.49 (1.24–1.79) | 0.001 |
| Low–medium – SES | 2.45 (1.99–3.01) | 0.001 | 2.13 (1.69–2.68) | 0.001 |
| Smoking | 0.95 (0.78–1.17) | 0.669 | 0.71 (0.56–0.91) | 0.05 |
| Depression/Anxiety | 0.78 (0.61–1.01) | 0.062 | 1.13 (0.84–1.51) | 0.423 |
| Schizophrenia | 0.95 (0.56–1.63) | 0.478 | 1.01 (0.54–1.86) | 0.991 |
| Dementia | 0.55 (0.29–0.84) | 0.001 | 0.56 (0.32–0.98) | 0.006 |
| Diabetes mellitus | 0.84 (0.71–1.01) | 0.07 | 0.91 (0.71–1.17) | 0.469 |
| Hypertension | 0.74 (0.62–0.88) | 0.001 | 0.86 (0.67–1.11) | 0.670 |
| Cardiovascular disease | 0.55 (0.43–0.71) | 0.001 | 0.58 (0.44–0.79) | 0.001 |
| Chronic lung disorders | 0.60 (0.46–0.78) | 0.001 | 0.58 (0.45–0.76) | 0.001 |
| BMI | 0.99 (0.98–1.011) | 0.857 | 0.99 (0.98–1.009) | 0.523 |
Low plasma 25(OH)D level Low vitamin D level – the total plasma levels less than 25‐(OH)D levels of 30 ng/mL
The significant values (P‐value < 0.05) were shown in bold.
Low vitamin D level and the likelihood of hospitalization due to COVID‐19 infection
Interestingly, the hospitalized COVID‐19‐P individuals were older [58.69 years (95% CI: 54.78‐62.61) vs. 46.88 (95% CI: 46.42–47.35)], and more likely to be male (47.8% vs. 41.3%, P < 0.001) and to reside in a city or town of low–medium SES (73.64% vs. 69.45%, P < 0.001). The hospitalized compared to nonhospitalized individuals had a significantly lower mean plasma 25 (OH) D level [18.38 ng/mL(95% CI: 16.79–19.96) vs. 20.45 ng·mL−1 (95% CI: 20.22–20.68), P < 0.001]. In a univariate analysis, a low plasma 25(OH)D level was associated with an increased likelihood of hospitalization for COVID‐19 infection [crude OR of 2.09 (95% CI: 1.01–4.31, P < 0.05)]. In a multivariate analysis that controlled for demographic variables and chronic disorders, the adjusted OR decreased slightly to 1.95 (95% CI: 0.98–4.84, P = 0.061). Therefore, in this analysis, only age over 50 years was statistically significant associated with the likelihood for hospitalization due to COVID‐19 [adjusted OR of 2.71(95% CI: 01.55‐ 4.78, P < 0.001); Table 5 and Fig. 3].
Table 5.
Multivariate logistic regression analysis of the odds ratio (OR) for hospitalization of patients with COVID‐19, controlling for multiple clinical conditions.
| Variable | Crude OR (95% CI) | P‐value | Adjusted OR (95% CI) | P‐value |
|---|---|---|---|---|
| Low vitamin D level | 2.09 (1.01–4.31) | 0.021 | 1.95 (0.99–4.78) | 0.056 |
| Age over 50 years | 2.51 (1.21–4.89) | 0.001 | 2.71 (1.55–4.78) | 0.002 |
| Male sex | 1.32 (0.76–2.11) | 0.223 | 1.35 (0.83–2.21) | 0.324 |
| Low–medium – SES | 1.24 (0.81–1.91) | 0.254 | 1.36 (0.83–2.21) | 0.222 |
| Smoking | 1.14 (0.68–2.17) | 0.669 | 1.22 (0.71–2.08) | 0.470 |
| Depression/Anxiety | 0.78 (0.61–1.01) | 0.662 | 0.94 (0.50–1.76) | 0.846 |
| Schizophrenia | 0.95 (0.56–1.63) | 0.478 | 1.24 (0.58–2.67) | 0.581 |
| Dementia | 1.65 (0.29–4.84) | 0.625 | 1.52 (0.46–4.98) | 0.489 |
| Diabetes mellitus | 2.04 (1.39–2.99) | 0.001 | 1.82 (0.41–2.36) | 0.696 |
| Hypertension | 1.81 (1.49–2.33) | 0.001 | 1.56 (0.91–2.71) | 0.113 |
| Cardiovascular disease | 1.54 (0.67–3.53) | 0.231 | 1.06 (0.44–2.58) | 0.896 |
| Chronic lung disorders | 1.44 (0.89–2.34) | 0.142 | 0.94 (0.52–1.71) | 0.726 |
| BMI | 1.17 (0.98–1.38) | 0.075 | 0.99 (0.98–1.011) | 0.804 |
The significant values (P‐value < 0.05) were shown in bold.
Fig. 3.
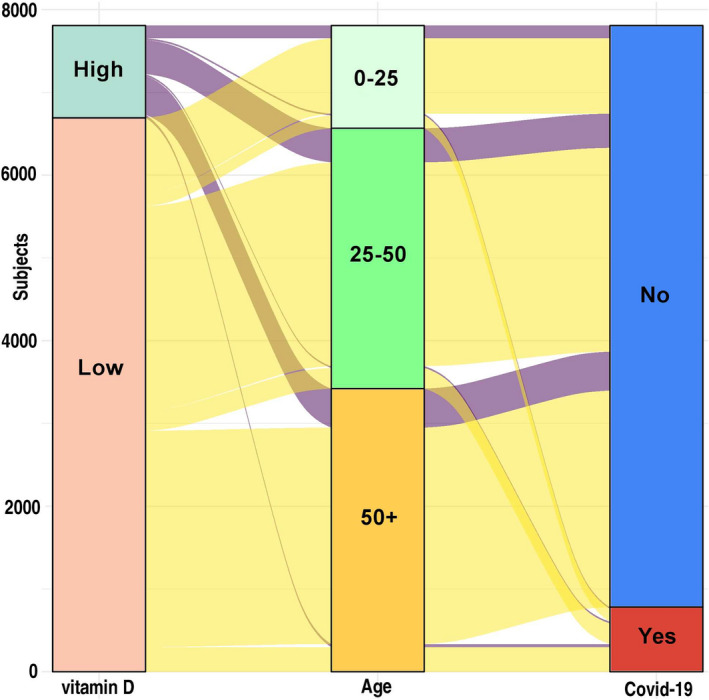
The likelihood of hospitalization due to COVID‐19 according to two‐risk factors: the low or high vitamin D levels and age groups, classified by: 0–25, 25–50, 50+ years. Most of the patients with the low vitamin D were COVID‐19‐P as shown on the scheme.
Discussion
The main finding of this study was the low plasma 25(OH)D level association with COVID‐19 hospitalization as a risk factor, particularly, for patients tested positively for COVID‐19, after adjusting for age, gender, SES and chronic, mental and physical disorders. Hence, low 25(OH)D level was identified as independently associated with the likelihood of COVID‐19 infection. This finding is in agreement with the results of other studies [5, 7, 9, 10, 11, 12, 13, 14, 15, 19, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42]. Further, reduced risk of acute respiratory tract infection following vitamin D supplementation has been reported [43, 44]. Notably, a recent study from the UK [31, 32, 33] that included 449 subjects (from the UK Biobank) with confirmed COVID‐19 infection did not find an association between vitamin D metabolite concentration and the risk of viral infections [45] as well as COVID‐19 infection [5, 7, 9, 10, 11, 12, 13, 14, 15, 19, 30, 31, 32, 33, 34, 35, 36, 37, 39, 40, 41, 42, 46]. Particularly, the participants were recruited by UK Biobank in the period 2006–2010 [31, 32, 33]. This time lag by itself may produce a bias the results obtained in 2020, for any participant. During the 10‐year period, there may be significant changes in the lifestyle and health factors associated with the vitamin D status of patients. Moreover, the discrepancy between those and our results may be explained by a sample size of less than half in that study, the older population and the inability to control for several confounders, like SES and chronic medical conditions.
According to our analysis, persons with COVID‐19‐P were younger than noninfected ones. Two‐peak distributions for age groups were demonstrated to confer increased risk for COVID‐19: ages 25 years old and 50 years old (Fig. 4). The first peak may be explained by high social gathering habits at the young age. The peak at age 50 years may be explained by continued social habits, in conjunction with various chronic diseases (Fig. 4). Other clinical characteristics that were significantly linked to the likelihood of COVID‐19 infection included male gender and low residential SES. Despite its being discussed as a risk factor in prior publications [47, 48, 49], obesity had not been significantly associated with either an increased risk for COVID‐19 infection or with hospitalization due to COVID‐19 in this study.
Fig. 4.
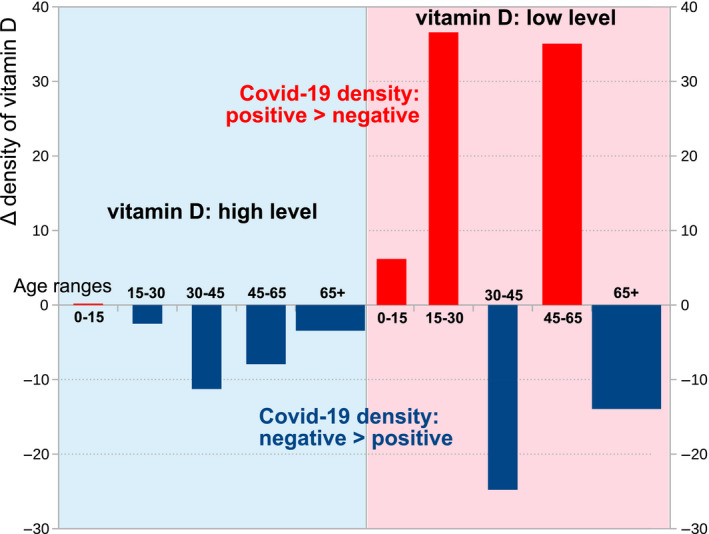
Two‐peak age groups as a high risk for COVID‐19: ages 25 and 50 years old (red bars). Both age groups were included in the subset of vitamin D‐deficient patients (the area highlighted pink). In the subset of persons with low vitamin D, the age range of 30–45 years old peaked (shown in the area highlighted in blue). The delta for vitamin D was calculated by the formulas described in Methods.
Surprisingly, chronic medical conditions, like dementia, cardiovascular disease and chronic lung disease that were considered to be very risky in previous studies [50, 51], were not found as increasing the rate of infection in our study. Particularly, this finding was highly biased by the severe social contact restrictions that were imposed on all the population and were even more emphasized in this highly vulnerable population. Therefore, we assume that following the Israeli Ministry of Health instructions, patients with chronic medical conditions significantly reduced their social contacts. This might indeed minimize the risk of COVID‐19 infection in that particular group of patients. The negative association with the current smoking status was unclear and should be further investigated, since recent studies had provided conflicting data on smoking [36, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63]. Finally, in a subset analysis of only COVID‐19‐P subjects, hospitalized patients were significantly older (58.69 vs. 46.88 years). Thus, multivariate analyses showed that being older than 50 years old was the single statistically significant risk factor for hospitalization. To conclude, the plasma 25(OH)D level under 20 ng/mL almost doubled the risk for hospitalization due to the COVID‐19 infection in the Israeli studied cohort.
Conclusions, strengths and limitations
The main strength of the study was its being large, real‐world, and population‐based. An additional strength was the analysis of a multitude of variables that may affect the risk of COVID‐19 infection, independent of plasma 25(OH)D levels. However, the major weakness of the study was the retrospective database design. Data regarding COVID‐19 symptoms and the hospitalization due to COVID‐19 infection, and also adverse clinical outcomes (for example, mechanical ventilation) should be further assessed. Moreover, a possible selection bias arises in that vitamin D levels were tested according to the presentation of symptoms, and not according to population‐wide testing. Interestingly, our previous study showed that the health functional status cannot predict low 25(OH)D levels [45]. Therefore, our study found that suboptimal plasma vitamin D levels may be a potential risk factor for COVID‐19 infection, particularly, for the high hospitalization risks, independent of demographic characteristics and medical conditions. The finding is important, since it could guide healthcare systems in identifying populations at risk, and contribute to interventions aimed to reduce the risk of the COVID‐19 infection. More studies are required to assess the effects of vitamin D3 supplements on the risk of hospitalizations due to COVID‐19 infection.
Methods
We conducted a population‐based study utilizing data from the Leumit Health Services (LHS) database, a large health maintenance organization in Israel that provides services to around 730 000 members nationwide. The comprehensive computerized database of LHS is continuously updated with regard to demographics, medical visits, laboratory tests and hospitalizations. The validity of the diagnoses in the registry is high for important medical diagnoses and laboratory data [20, 21, 22]. The study period was from February 1st to April 30th, 2020. The study population included all members of LHS who were tested for COVID‐19 infection during the study period and who had at least one previous test for plasma 25(OH)D level (7807 subjects). Referrals for viral tests were according to Israeli Ministry of Health guidelines (March 2020). COVID‐19 testing was done only by physician referral (based on clinical criteria of exposure to confirmed COVID‐19 patients or symptoms suggesting COVID‐19) using the AllplexTM 2019‐nCoV Assay (Seegene Inc., Seoul, Korea) [23]. According to LHS guidelines, blood was collected from fasting persons and transported on ice to the Center Laboratory for processing within 4 hours of collection using DiaSorin Chemiluminescence assay [24, 25, 26, 27]. Data of each subject were collected from the LHS computerized database and included age, gender, SES, weight, height, BMI, current smoking status, psychiatric and somatic comorbidities, and hospitalizations as a result of the COVID‐19 infection.
Definitions
All the somatic and psychiatric diagnoses were based on the International Classification of Disease, tenth revision codes and included chronic lung disorders (asthma, chronic obstructive pulmonary disease), diabetes, hypertension, depressive and anxiety disorders, schizophrenia and dementia.
SES
Socioeconomic status (SES) was defined according to a person's home address. The Israeli Central Bureau of Statistics classifies all cities and towns into 20 subgroups of SES. The classifications of one to nine were considered as a low–medium SES, and ten to twenty were considered as medium‐high SES.
Obesity
Obesity was considered as BMI = 30m2/kg.
According to Endocrine Society, National Osteoporosis Foundation and International Osteoporosis Foundation, the optimal 25(OH) D levels should be = 30 ng/mL (75 nmol/L), thus the plasma 25(OH)D level that is < 30 ng/mL (75 nmol/L) was considered as suboptimal and referred as ‘low’ in our study [28, 29] (Table 1).
Statistical analysis
Statistical analysis was conducted using stata 12 software (StataCorp LP, College Station, TX, USA). The initial analysis compared demographic characteristics between individuals who tested positive (COVID‐19‐P) and negative (COVID‐19‐N) for COVID‐19. Student's t‐test and Fisher's exact chi‐square test were used for continuous and categorical variables, respectively, based on a normal distribution (0,1) and variable characteristics. The categorical data were shown in counts and percentages. Data on continuous variables with normal distribution were presented as means and 95% confidence intervals (CIs). The assumptions were based on two‐sided tests with α of 0.05.
Preliminary evaluation of risk estimates was conducted by stratified analyses. Subsequently, multivariate logistic regression was used to estimate the OR and 95% CI for the independent association between low plasma 25(OH)D and a positive PCR test for the SARS‐CoV‐2 virus, while controlling for potential confounders. The association of low plasma 25(OH)D level with hospitalization due to COVID‐19 infection was assessed among those who tested positively for COVID‐19.
Formulas for calculating the density of vitamin D
is a set of the vitamin D3 levels;
is a set of age levels (boxes);
is positive and negative COVID‐19;
Visualization methods
Open source programs, particularly, (Plotly R Open Source Graphing Library, Plotly Technologies Inc., Quebec, Canada) were used. Plotly's R library [64] was used for the production of figures, including scatter plots, area charts, bar charts and 3D charts.
Authors' contribution
EM, MFM, DT, IG, AVG and SV designed the project and contributed the research questions, EM, DT, MFM and IG performed data mining and analysed results. EM performed statistical analysis. MFM, EM and DT wrote and edited the manuscript. MFM, AG and DT presented results in visual forms, MFM and EM supervised the project, and both contributed to the project design. All the authors contributed to editing of the manuscript. No honorarium, grant or other form of payment was given to any of the authors to produce the manuscript.
Conflict of interest
The authors declare no conflict of interest.
Ethical considerations
This is a data‐based study, and as such, has no clinical trial registration number. The study received approval from the Leumit Health Services research committee and the Shamir Medical Centre IRB.
Acknowledgements
The study was funded by COVID‐19 Data Sciences Institute (DSI) grant (for MFM, #247017). All authors have indicated they have no financial relationships relevant to this manuscript to disclose.
Contributor Information
Eugene Merzon, Email: emarzon@leumit.co.il.
Milana Frenkel‐Morgenstern, Email: milana.morgenstern@biu.ac.il.
References
- 1. Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY et al. (2020) A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arshad Ali S, Baloch M, Ahmed N, Arshad Ali A & Iqbal A (2020) The outbreak of Coronavirus Disease 2019 (COVID‐19)‐An emerging global health threat. J Infect Public Health 13, 644–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, Sun C, Sylvia S, Rozelle S, Raat H et al. (2020) Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: a scoping review. Infect Dis Poverty 9, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Di Gennaro F, Pizzol D, Marotta C, Antunes M, Racalbuto V, Veronese N & Smith L (2020) Coronavirus diseases (COVID‐19) current status and future perspectives: a narrative review. Int J Environ Res Public Health 17, 2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cao Z, Wu Y, Faucon E & Sabatier JM (2020) SARS‐CoV‐2 & Covid‐19: Key‐Roles of the ‘Renin‐Angiotensin’ System / Vitamin D Impacting Drug and Vaccine Developments. Infect Disord Drug Targets 20, 348–349. [DOI] [PubMed] [Google Scholar]
- 6. Carr AC (2020) A new clinical trial to test high‐dose vitamin C in patients with COVID‐19. Crit Care 24, 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carter SJ, Baranauskas MN & Fly AD (2020) Considerations for obesity, vitamin D, and physical activity amidst the COVID‐19 pandemic. Obesity (Silver Spring) 28, 1176–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cheng RZ (2020) Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID‐19)? Med Drug Discov 5, 100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garg M, Al‐Ani A, Mitchell H, Hendy P & Christensen B (2020) Editorial: low population mortality from COVID‐19 in countries south of latitude 35 degrees North ‐ supports vitamin D as a factor determining severity. Authors' reply. Aliment Pharmacol Ther 51, 1438–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL & Bhattoa HP (2020) Evidence that vitamin D supplementation could reduce risk of influenza and COVID‐19 infections and deaths. Nutrients 12, 988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jakovac H (2020) COVID‐19 and vitamin D‐Is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab 318, E589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCartney DM & Byrne DG (2020) Optimisation of vitamin D status for enhanced immuno‐protection against Covid‐19. Ir Med J 113, 58. [PubMed] [Google Scholar]
- 13. Molloy EJ & Murphy N (2020) Vitamin D, Covid‐19 and children. Ir Med J 113, 64. [PubMed] [Google Scholar]
- 14. Panarese A & Shahini E (2020) Letter: Covid‐19, and vitamin D. Aliment Pharmacol Ther 51, 993–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Silberstein M (2020) Vitamin D: A simpler alternative to tocilizumab for trial in COVID‐19? Med Hypotheses 140, 109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Llewellyn DJ, Lang IA, Langa KM, Muniz‐Terrera G, Phillips CL, Cherubini A, Ferrucci L & Melzer D (2010) Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med 170, 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Oliveira LF, de Azevedo LG, da Mota Santana J, de Sales LPC & Pereira‐Santos M (2020) Obesity and overweight decreases the effect of vitamin D supplementation in adults: systematic review and meta‐analysis of randomized controlled trials. Rev Endocr Metab Disord 21, 67–76. [DOI] [PubMed] [Google Scholar]
- 18. Holick MF (2017) The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord 18, 153–165. [DOI] [PubMed] [Google Scholar]
- 19. Ilie PC, Stefanescu S & Smith L (2020) The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res 32, 1195–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pringle M, Ward P & Chilvers C (1995) Assessment of the completeness and accuracy of computer medical records in four practices committed to recording data on computer. Br J Gen Pract 45, 537–541. [PMC free article] [PubMed] [Google Scholar]
- 21. Rennert G & Peterburg Y (2001) Prevalence of selected chronic diseases in Israel. Isr Med Assoc J 3, 404–408. [PubMed] [Google Scholar]
- 22. Shalev V, Chodick G, Goren I, Silber H, Kokia E & Heymann AD (2011) The use of an automated patient registry to manage and monitor cardiovascular conditions and related outcomes in a large health organization. Int J Cardiol 152, 345–349. [DOI] [PubMed] [Google Scholar]
- 23. Sokouti M, Sadeghi R, Pashazadeh S, Eslami S, Ghojazadeh M & Sokouti B (2020) Comparative global epidemiological investigation of SARS‐CoV‐2 and SARS‐CoV diseases using meta‐MUMS tool through incidence, mortality, and recovery rates. Arch Med Res 51, 458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. French D, Gorgi AW, Ihenetu KU, Weeks MA, Lynch KL & Wu AH (2011) Vitamin D status of county hospital patients assessed by the DiaSorin LIAISON® 25‐hydroxyvitamin D assay. Clin Chim Acta 412, 258–262. [DOI] [PubMed] [Google Scholar]
- 25. Rosecrans R & Dohnal JC (2014) Seasonal vitamin D changes and the impact on health risk assessment. Clin Biochem 47, 670–672. [DOI] [PubMed] [Google Scholar]
- 26. Moure Z, Rando‐Segura A, Gimferrer L, Roig G, Pumarola T & Rodriguez‐Garrido V (2018) Evaluation of the novel DiaSorin LIAISON. Enferm Infecc Microbiol Clin 36, 293–295. [DOI] [PubMed] [Google Scholar]
- 27. Thuzar M, Young K, Ahmed AH, Ward G, Wolley M, Guo Z, Gordon RD, McWhinney BC, Ungerer JP & Stowasser M (2020) Diagnosis of primary aldosteronism by seated saline suppression test‐variability between immunoassay and HPLC‐MS/MS. J Clin Endocrinol Metab 105, e477–e483. [DOI] [PubMed] [Google Scholar]
- 28. Holick MiF, Binkley NC, Bischoff‐Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM; Endocrine Society (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96, 1911–1930. [DOI] [PubMed] [Google Scholar]
- 29. Vieth R (2006) What is the optimal vitamin D status for health? Prog Biophys Mol Biol 92, 26–32. [DOI] [PubMed] [Google Scholar]
- 30. Tian Y & Rong L (2020) Letter: Covid‐19, and vitamin D. Authors' reply. Aliment Pharmacol Ther 51, 995–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grant WB & McDonnell SL (2020) Letter in response to the article: Vitamin D concentrations and COVID‐19 infection in UK biobank (Hastie et al.). Diabetes Metab Syndr 14, 893–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roy AS, Matson M & Herlekar R (2020) Response to 'Vitamin D concentrations and COVID‐19 infection in UK Biobank'. Diabetes Metab Syndr 14, 777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hastie CE, Mackay DF, Ho F, Celis‐Morales CA, Katikireddi SV, Niedzwiedz CL, Jani BD, Welsh P, Mair FS, Gray SR et al. (2020) Vitamin D concentrations and COVID‐19 infection in UK Biobank. Diabetes Metab Syndr 14, 561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Torjesen I (2020) Covid‐19: Public health agencies review whether vitamin D supplements could reduce risk. BMJ 369, m2475. [DOI] [PubMed] [Google Scholar]
- 35. Panfili FM, Roversi M, D'Argenio P, Rossi P, Cappa M & Fintini D (2020) Possible role of vitamin D in Covid‐19 infection in pediatric population. J Endocrinol Invest 15, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Raisi‐Estabragh Z, McCracken C, Bethell MS, Cooper J, Cooper C, Caulfield MJ, Munroe PB, Harvey NC & Petersen SE (2020) Greater risk of severe COVID‐19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)‐vitamin D status: study of 1326 cases from the UK Biobank. J Public Health (Oxf) 19, fdaa095. 10.1093/pubmed/fdaa095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Quesada‐Gomez JM, Castillo ME & Bouillon R (2020) Vitamin D Receptor stimulation to reduce Acute Respiratory Distress Syndrome (ARDS) in patients with Coronavirus SARS‐CoV‐2 infections: Revised Ms SBMB 2020_166. J Steroid Biochem Mol Biol 11, 105719. 10.1016/j.jsbmb.2020.105719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V & Solway J. (2020) Association of vitamin D deficiency and treatment with COVID‐19 incidence. medRxiv 13. 10.1101/2020.05.08.20095893 [DOI] [Google Scholar]
- 39. Ekiz T & Pazarlı AC (2020) Relationship between COVID‐19 and obesity. Diabetes Metab Syndr 14, 761–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Weir EK, Thenappan T, Bhargava M & Chen Y. (2020) Does vitamin D deficiency increase the severity of COVID‐19? Clin Med (Lond) 20, e107–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ribeiro H, Santana KVS, Oliver SL, Rondó PHC, Mendes MM, Charlton K & Lanham‐New S (2020) Does Vitamin D play a role in the management of Covid‐19 in Brazil? Rev Saude Publica 54, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zemb P, Bergman P, Camargo CA, Cavalier E, Cormier C, Courbebaisse M, Hollis B, Joulia F, Minisola S, Pilz S et al. (2020) Vitamin D deficiency and the COVID‐19 pandemic. J Glob Antimicrob Resist 22, 133–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov‐Raz G, Esposito S, Ganmaa D, Ginde AA et al. (2017) Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta‐analysis of individual participant data. BMJ 356, i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martineau AR, Jolliffe DA, Greenberg L, Aloia JF, Bergman P, Dubnov‐Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC et al. (2019) Vitamin D supplementation to prevent acute respiratory infections: individual participant data meta‐analysis. Health Technol Assess 23, 1–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Golan‐Cohen A, Merzon E, Alhin O, Kitai E & Fogelman Y (2016) Blood levels of vitamin D and health‐functional status in asymptomatic individuals: a cross sectional study. J Eval Clin Pract 22, 946–951. [DOI] [PubMed] [Google Scholar]
- 46. Rhodes JM, Subramanian S, Laird E & Kenny RA (2020) Editorial: low population mortality from COVID‐19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther 51, 1434–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Almerie MQ & Kerrigan DD (2020) The association between obesity and poor outcome after COVID‐19 indicates a potential therapeutic role for montelukast. Med Hypotheses 143, 109883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gao F, Zheng KI, Wang XB, Sun QF, Pan KH, Wang TY, Chen YP, Targher G, Byrne CD, George J et al. (2020) Obesity Is a Risk Factor for Greater COVID‐19 Severity. Diabetes Care 43, e72–e74. [DOI] [PubMed] [Google Scholar]
- 49. Hussain A, Vasas P & El‐Hasani S (2020) Letter to the Editor: Obesity as a risk factor for greater severity of COVID‐19 in patients with metabolic associated fatty liver disease. Metabolism 108, 154256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Team CC‐R (2020) Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 ‐ United States, February 12‐March 28, 2020. MMWR Morb Mortal Wkly Rep 69, 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ganatra S, Hammond SP & Nohria A (2020) The novel coronavirus disease (COVID‐19) threat for patients with cardiovascular disease and cancer. JACC CardioOncol 2, 350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alqahtani JS, Oyelade T, Aldhahir AM, Alghamdi SM, Almehmadi M, Alqahtani AS, Quaderi S, Mandal S & Hurst JR (2020) Prevalence, severity and mortality associated with COPD and smoking in patients with COVID‐19: a rapid systematic review and meta‐analysis. PLoS One 15, e0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cattaruzza MS, Zagà V, Gallus S, D'Argenio P & Gorini G (2020) Tobacco smoking and COVID‐19 pandemic: old and new issues. A summary of the evidence from the scientific literature. Acta Biomed 91, 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chakladar J, Shende N, Li WT, Rajasekaran M, Chang EY & Ongkeko WM (2020) Smoking‐mediated upregulation of the androgen pathway leads to increased SARS‐CoV‐2 susceptibility. Int J Mol Sci 21, 3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Garassino MC, Whisenant JG, Huang LC, Trama A, Torri V, Agustoni F, Baena J, Banna G, Berardi R, Bettini AC et al. (2020) COVID‐19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry‐based, cohort study. Lancet Oncol 21, 914–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jj S, N A & E G (2020) Active smoking and severity of coronavirus disease 2019 (COVID‐19): differences in measurement of variables could cause errors in the results. Eur J Intern Med 77, 127–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kaur G, Lungarella G & Rahman I (2020) SARS‐CoV‐2 COVID‐19 susceptibility and lung inflammatory storm by smoking and vaping. J Inflamm (Lond) 17, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leung JM, Yang CX & Sin DD (2020) Reply to: "Current smoking is not associated with COVID‐19". Eur Respir J 55, 2001340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li J, He X, Yuan Y, Zhang W, Li X, Zhang Y, Li S, Guan C, Gao Z & Dong G(2020) Meta‐analysis investigating the relationship between clinical features, outcomes, and severity of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pneumonia. Am J Infect Control. 10.1016/j.ajic.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Perski O, Herbeć A, Shahab L & Brown J (2020) Influence of the SARS‐CoV‐2 outbreak on the uptake of a popular smoking cessation app in UK smokers: interrupted time series analysis. JMIR Mhealth Uhealth 8, e19494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rossato M, Russo L, Mazzocut S, Di Vincenzo A, Fioretto P & Vettor R (2020) Current smoking is not associated with COVID‐19. Eur Respir J 55, 2001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Russo P, Bonassi S, Giacconi R, Malavolta M, Tomino C & Maggi F (2020) COVID‐19 and smoking: is nicotine the hidden link? Eur Respir J 55, 2001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Saheb Sharif‐Askari N, Saheb Sharif‐Askari F, Alabed M, Temsah MH, Al Heialy S, Hamid Q & Halwani R (2020) Airways expression of SARS‐CoV‐2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol Ther Methods Clin Dev 18, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sievert C (2020) Interactive Web‐Based Data Visualization with R, plotly, and shiny. Chapman and Hall/CRC. ISBN 9781138331457, https://plotly‐r.com.


