Abstract
Background
COVID‐19 outbreak profoundly affected health systems and people's daily life worldwide. Parkinson's disease (PD) patients lost their normal routine and interrupted regular physical activity, either as physiotherapy or sport, with inevitable consequence on their daily‐life and well‐being.
Objectives
To evaluate the changes in physical activity due to COVID‐19 emergency, including self‐management strategies or technology‐assisted activities, and the subsequent clinical implications in PD patients.
Methods
Seventy‐four patients from an Italian center have been remotely examined during the lockdown (April–May 2020) by an e‐mail structured survey, including self‐administered scales. We collected and analyzed data on changes, modalities and amount of physical active practice, on the use of technology‐based tools, and on self‐perceived clinical condition.
Results
Sixty percent of patients reported a significant worsening of their general conditions during the lockdown, the reduction of physical activity being the main risk factor for such change. However, patients found ways to practice physical activity, using satisfactorily technology assistance in 50% of cases (mostly women).
Conclusions
The COVID‐19 emergency has been an ordeal for PD patients. Nevertheless, patients adapted their habits to continue practicing physical activity that resulted a main determinant of their well‐being; as well, they successfully approached technology‐based assistance. Education, communication, and networking emerge as critical for a constructive reaction to the emergency's challenges.
Keywords: Parkinson's disease, COVID‐19, Sars‐CoV‐2, technology, physical activity
Since March 2020, the world has been struck by SARS‐CoV‐2‐related disease (COVID‐19). The infection caused a terrible global health emergency and a high number of deaths. Many countries had to impose severe rules to limit the virus' diffusion, such as social distancing and the lockdown of all unnecessary activities. Health systems had to prioritize their resources and efforts in the cure of COVID‐19, and many clinical services had been suddenly interrupted. 1
Daily routine has profoundly changed in a short time for patients with chronic diseases, including Parkinson's disease (PD). Indeed, PD patients missed their follow‐up visits and quit regular activities such as physiotherapy or trained sport, with inevitable consequences on their daily life and well‐being. 2 , 3
COVID‐19 emergency thus obliged PD patients to adopt self‐management strategies to deal with the challenges related to this crisis and to overtake distress due to routine clinical care interruption. Furthermore, they had the unique opportunity to test in real life, in time of need, the available home‐based or technology‐assisted techniques for rehabilitation and physical exercise (e.g. video/web sessions, video games, and smartphone applications), whose use has been encouraged in last years, with promising results. 4 , 5 , 6
Here, we used a structured survey to remotely investigate the impact of COVID‐19 emergency on the daily life of a cohort of Italian PD patients, specifically focusing on the relationship between physical activity changes and the self‐perceived health. In addition, the use of available technology‐based tools (TBT) to assist physical exercise and its implications in such a critical period has been explored.
Methods
A cohort of 109 PD patients was enrolled at the Neurology Unit of Tor Vergata University Hospital (Rome, Italy). Patients were previously diagnosed according with current diagnostic criteria and followed‐up for at least 2 years to avoid misdiagnosis with atypical parkinsonism. All patients had to be contactable via e‐mail and able to fill out the forms (demented and severely affected patients, as reported in clinical charts, were not included).
A standard introductive message was transmitted by e‐mail or phone to explain the study. Then, a survey was sent by e‐mail and returned within 1 week. The study covered the Italian lockdown period between April 20, 2020 and May 2, 2020.
A structured questionnaire was set up to collect data on demographics including age at PD onset and disease duration; motor activity habits before COVID‐19 emergency: physiotherapy/rehabilitation practice, sports practice (type and weekly frequency); motor activity habits during lockdown: physiotherapy/rehabilitation practice, physical exercise practice (indoor/outdoor, type of activity); use of TBTs (specifically free web video lessons, web video course organized by institutions/associations, video games, and smartphone applications), previous experience, frequency of current use, opinion on the usefulness; use of wearable devices (eg pedometer); perception of own health during COVID‐19 emergency (specifically, we asked: “Do you feel that your global health was worsened or remained stable during the lockdown?”). Three self‐administered scales were also included in the survey: the International Physical Activity Questionnaires‐Short Form (IPAQ–SF), 7 , 8 a self‐reported questionnaire to quantify the intensity of physical activity as metabolic equivalent (MET) min/week; the Parkinson's Well‐Being Map (PWBM), a self‐reported score of motor and non‐motor symptoms divided into 8 items 9 to assess clinical severity; and the Beck Depression Index (BDI) to estimate depression. All responses were transformed in categorical or continuous variables as appropriate for statistical analysis.
Statistical Analysis
Distribution of continuous variables was assessed by Shapiro–Wilk test and those non‐normally distributed were log‐10 transformed to allow analysis. Descriptive static was performed for categorical variables. Mann–Whitney test and one‐way ANOVA were used for non‐parametric and parametric analysis respectively. For comparisons on the amount of physical activity (total MET), a one‐way MANCOVA model adjusted for main covariates, age, sex, depression (BDI score), and motor impairment (“movements” item score of PWBM) was used. For comparisons on the clinical outcomes (PWBM and BDI scores), a one‐way MANCOVA model adjusted for main covariates, age, sex, and disease duration was applied instead. To identify factors associated with different conditions, a binomial logistic regression analysis was performed adjusted for relevant covariates (as reported in results). SPSS 23.0 was used for all analyses.
Results
Study Population
From the initial cohort of 109 patients, 74 were finally included in the study (the remaining 35 did not respond, responded late, or incompletely). None of them were diagnosed with COVID‐19. A total of 50% were female. Age was 61.3 ± 9.3 years (mean ± SD). Disease duration was 6.5 ± 4.5 years, and age at PD onset was 55.5 ± 10.8 years. PWBM score was 48.6 ± 31.7, and BDI score was 8.5 ± 7.
Physical Activity during COVID‐19 Emergency
Because of the COVID‐19 emergency, the number of patients under physiotherapy/rehabilitation decreased from 32 (43%), 78% of which were treated in specialized centers, to 7 (9.7%; 78% reduction).
The total number of patients playing sports remained stable, 59 (80%) before and 60 (81%) during the emergency (53 patients continued, 7 instead started during the lockdown). The interruption of physiotherapy (n = 26) did not increase the number of sporting patients (reduced from 22 to 20, P > 0.05).
Before the emergency, 52% these patients played indoor sports (eg Pilates, gym, and swimming) and 69% outdoor (walking the most common); 31% used to have sport 1–2 times/week, 69% had higher frequency. The features of sportive activities during the emergency (place, type of sport, and motivation) are instead shown in Fig. 1. Overall, sporting patients had a shorter disease duration compared to inactive ones (5.8 ± 3.9 years vs. 9.8 ± 5.6, P = 0.004), whereas sex, age, age at onset, total PWBM, and BDI scores did not differ.
FIG 1.
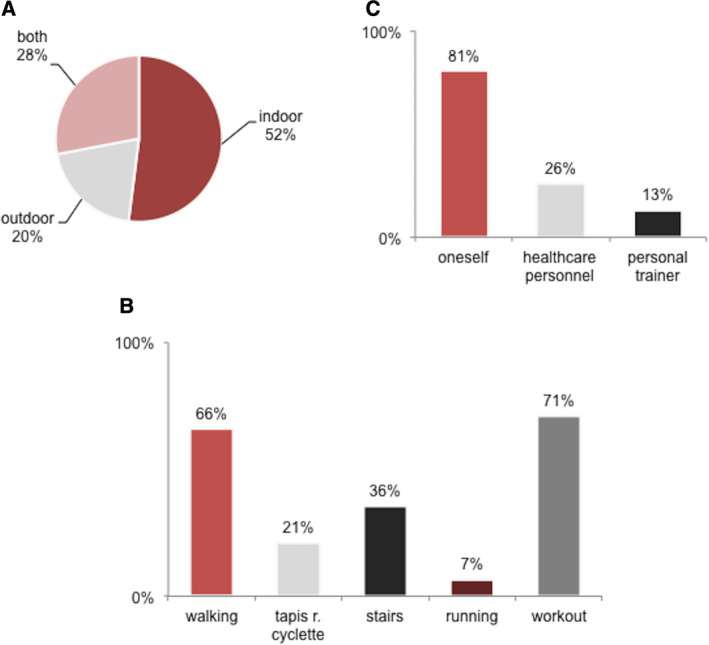
Sport activities during COVID‐19 emergency. (A) Prevalence of different places used to play sport. (B) Prevalence of different sports played by patients. (C) Prevalence of different source of initiative in motivating sport (who motivated sport).
During the COVID‐19 emergency, total MET, a quantitative index of weekly physical activity, was 1994.7 ± 1971 min/week. Total MET level was significantly higher in patients playing sports (2195 ± 2045.8 min/week) than the inactive ones (872 ± 911.7 min/week; F(1,55) = 9,8, P = 0.003). Conversely, no differences resulted between TBT users (1845 ± 1552.7 min/week) and non‐users (2913.2 ± 2690.4).
Clinical Outcome
A total of 59.5% of patients referred a perception of worsening in their global health during COVID‐19 emergency. Worsening patients did not differ for age, age at onset, sex, and disease duration from those referring stable conditions. Conversely, they had higher scores of both PWBM (55.5 ± 30.6 vs. 39.8 ± 32.7; F(1,66) = 7.4, P = 0.008) and BDI (11.3 ± 8.2 vs. 5.9 ± 4.7; F(1,63) = 6.21, P = 0.012), and lower total MET (1714 ± 1570.5 min/week vs. 2399.6 ± 2412.3; F(1,53) = 4.5, P = 0.038). Binomial logistic regression model indicated that total MET was inversely associated with “worsening” (odds ratio [OR] = 0.2, P = 0.05), independently from age, age at onset, disease duration, BDI, and PWBM total scores. Likewise, none of the PWBM items score was associated with a change in clinical conditions. Three separate models corrected for age, age at onset, sex, and disease duration have been used to test the association between “worsening” and previous physiotherapy/rehabilitation, practice of sport during the lockdown, and use of TBTs, but no significant results emerged.
Use of TBTs
During the lockdown, patients based their training on known exercises in 86% of cases. Assistive TBTs were used by 37 patients (63% of sporting patients; 50% of the whole study population). Female sex was prevalent among TBT users rather than non‐users (65% vs. 27%, P = 0.005), whereas no significant differences emerged in age, age at onset, disease duration, total PWBM, and BDI scores. Binomial logistic regression model indicated that female sex was the only factor associated with “user” condition (OR = 7.14, P = 0.016), independently from age, age at onset, disease duration, BDI, and PWBM scores. Figure 2 shows the TBTs features of use during the lockdown (type of TBT, confidence and continuity of use, final opinion). No significant predictors of the final opinion resulted from binomial logistic analysis. Commercially available wearable devices (as pedometer) were used by 25% of patients.
FIG 2.
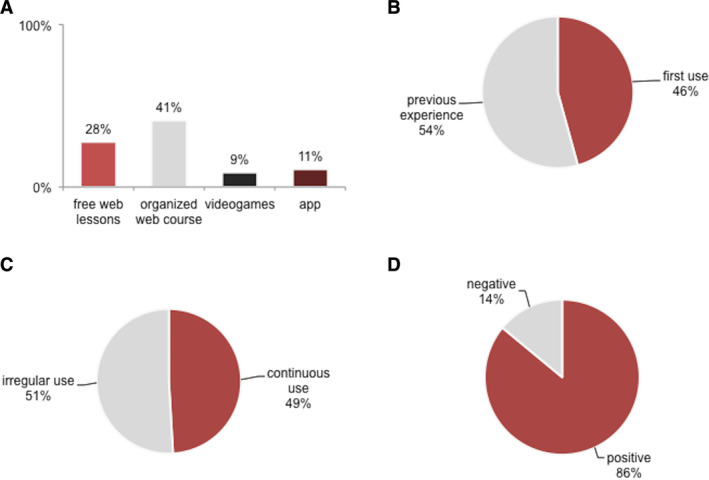
Use of TBT. (A) Prevalence of different TBTs used. (B) Confidence of TBTs' use (first use during the lockdown or previous experience). (C) Continuity of TBTs' use (continuous or irregular). (D) Final opinion on TBTs usefulness during the emergency (positive or negative).
Discussion
This survey on a selected PD population showed that COVID‐19 outbreak, and the subsequent restrictions, had a significant impact on PD patients' daily life.
Up to 60% of these patients referred a perception of clinical worsening during the lockdown period. Although the reasons of such decline could not be directly determined, we observed that the main identified risk factor associated with perceived worsening was the low total MET score. MET is an index of energy expenditure that results from IPAQ‐SF, a commonly used questionnaire to assess quantitatively physical activity, even in PD. 8 , 10 A higher rate of physical activity improves quality of life, mood, and cognitive performances, and even motor disturbances in PD patients, exerting beneficial effects either at brain or systemic level. 8 , 11 , 12 The reduction of such “protective” action might thus explain the perception of worsening in these patients, which also presented a greater burden of PD‐related disturbances (PWBM score) and depressive symptoms (BDI score) compared to those referring stable conditions.
Up to 80% of patients doing physiotherapy interrupted treatments during COVID‐19 emergency. Although this was felt as a major issue, 3 we did not find a significant association with health self‐perception in patients. Indeed, regardless of physiotherapy, the majority of patients, especially those with shorter disease duration, continued sport activities. They had to adapt to the modalities allowed, practicing physical exercise mostly at home or outside nearby (as allowed). Sporting patients obviously had higher levels of METs compared to inactive ones. However, the latter ones did not result at risk for clinical worsening, probably because of the small number.
A previous study, assessing through traditional enrolment into a clinic setting the relationship between physical exercise and dementia risk factors, showed in PD patients a METs level of 1443 ± 2197.5 min/week. 8 Although proper comparisons with this previous group are not reliable, the relatively higher METs amount that we found here (1994.7 ± 1971 min/week) suggests some peculiarity in the present cohort, probably due to different educational level or clinical severity. In fact, in this study, we had to rely just on e‐mail communication that effectively could exclude more advanced, cognitively impaired or uneducated patients. Actually, higher educational level and mild motor impairment are significant predictors of daily physical activity in PD patients 10 that, in turn, might explain the awareness of these patients on the importance of physical activity and their resilient response to COVID‐19‐related changes. Indeed, 80% of patients decided by themselves to get active and exercise during the lockdown, just repeating those exercises previously learned or playing simple activities, as walking, running, or climbing the stairs.
Half of our cohort (63% of sporting patients), mostly women, used TBTs for training during the lockdown. In 45.5% of cases, patients approached TBTs for the first time in this occasion, confirming their intention to react to the emergency's challenges. Web video training sessions promoted by patients associations or other social communities resulted as the preferred TBT (41%). One‐third of TBT users took advantage of free web video lessons for self‐managed training. Other TBTs, including video games or smartphone apps, were less used (~10%), probably because they required expensive devices or fee‐paying services. Similarly, wearable biometric devices (eg pedometers or heart rate sensors) have been used by only 25% of the cohort. TBTs mostly provided assistance for mild activity exercises (eg stretching, postural gym), rather than intensive workout. Accordingly, levels of MET did not differ between users and non‐users, such as the self‐perception of their own condition. Overall, patients used regularly TBTs in half of cases, but up to 90% of them gave a positive opinion on the usefulness.
Our survey was essentially focused on physical activity, however, many other factors could have contributed to patients' perception of worsening during COVID‐19 emergency. Definitely, worries about the pandemic and its consequences on health, economy, and society might have caused anxiety and stress, which is known to precipitate symptomatology in PD. 3 , 13 Although we roughly assessed depression by the self‐administered BDI, we could not provide a comprehensive neuropsychological evaluation. In general, this study has been limited by the lack of objective and accurate measurement of clinical features; similarly, the clinical outcome consisted just in the individual perception of patients' own condition and self‐reported scores. Moreover, it should be considered that the enrollment and participation in the study was carried out by e‐mail, which inevitably restricted the sample and caused a selection bias that prevents the generalization of findings to the whole PD population.
Despite these limitations, our study evaluated the negative impact of COVID‐19 emergency on PD patients, showing, once more, that physical activity is critical for their well‐being. On the other hand, we noticed an unexpected resilient response to the outbreak's challenges. In fact, patients found self‐management strategies to continue physical activity at home, in spite of imposed restrictions and interruption of clinical services, which resulted fundamental to preserve global health. Actually, physical exercise and, specifically, home‐based training, now represent promising strategies in the therapeutic scenario of PD. 12
Previous education and awareness of patients on the importance of physical activity in PD might have a role, because a high percentage of this cohort was active before the emergency and maintained active during the lockdown. The emergency also gave the opportunity to test in daily‐life TBTs for physical exercise. Although the use involved 50% of the cohort and was often promoted by social initiatives and patient associations, the final opinion on TBTs usefulness was largely positive.
It is definite that an adequate level of physical activity is critical to ensure patients' well‐being, even during COVID‐19 emergency. It is necessary either to promote communication and education among patients, especially through media, networks, and organizations, or to increase availability and accessibility to TBTs and telemedicine. 14
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
T.S.: 1A, 1B, 2A, 3A
G.D.L.: 1C, 2B, 2C; 3A
C.S.: 1B, 1C
R.C.: 1B, 1C
C.L.: 1B, 1C
S.S.: 1B, 1C
M.A.: 1B, 1C
N.B.M.: 2C, 3B
M.P.: 2C, 3B
A.S.: 1B, 3B
A.P.: 1B, 2C, 3B
Disclosures
Ethical Compliance Statement: All the procedures accorded local ethical standards and principles of Helsinki declaration. All participants signed an informed consent. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: No specific funding was received for this work. The authors declare that there are no conflicts of interest relevant to this work.
Financial Disclosures for the Previous 12 Months: The authors declare that there are no additional disclosures to report.
References
- 1. Remuzzi A, Remuzzi G. Health Policy COVID‐19 and Italy: What Next? Lancet 2020;395(10231):1225–1228. https://doi:10.1016/S0140-6736(20)30627-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Papa SM, Brundin P, Fung VSC, Kang UJ, Burn DJ, Colosimo C, et al. Impact of the COVID‐19 Pandemic on Parkinson's Disease and Movement Disorders. Mov Disord Clin Pract. 2020;7(4):357–360. https://doi:10.1002/mdc3.12953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schirinzi T, Cerroni R, Di Lazzaro G, Liguori C, Scalise S, Bovenzi R, et al. Self‐reported needs of patients with Parkinson's disease during COVID‐19 emergency in Italy. Neurol Sci 2020;41(6):1373–1375. https://doi:10.1007/s10072-020-04442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helmich RC, Bloem BR. The impact of the COVID‐19 pandemic on Parkinson's disease: Hidden sorrows and emerging opportunities. J Parkinsons Dis 2020;10:351–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Di Lazzaro G, Ricci M, Al‐Wardat M, Schirinzi T, Scalise S, Giannini F, et al. Technology‐based objective measures detect subclinical axial signs in untreated, de novo Parkinson's disease. J Parkinsons Dis 2020;10(1):113–122. [DOI] [PubMed] [Google Scholar]
- 6. Summa S, Schirinzi T, Bernava GM, et al. Development of SaraHome: A novel, well‐accepted, technology‐based assessment tool for patients with ataxia. Comput Methods Programs Biomed 2020;188:105257. [DOI] [PubMed] [Google Scholar]
- 7. Mannocci A, Thiene DD, Del CA, Masala D, Boccia A, De VE, et al. International physical activity questionnaire: Validation and assessment in an Italian sample. Ital J Public Health 2010;7(4):369–376. 10.2427/5694. [DOI] [Google Scholar]
- 8. Alwardat M, Schirinzi T, Di Lazzaro G, Sancesario GM, Franco D, Imbriani P, et al. Association between physical activity and dementia's risk factors in patients with Parkinson's disease. J Neural Transm 2019;126(3):319–325. [DOI] [PubMed] [Google Scholar]
- 9. Skogar O, Nilsson M. Distribution of non‐motor symptoms in idiopathic parkinson's disease and secondary parkinsonism. J Multidiscip Healthc 2018;11:525–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gorzkowska A, Cholewa J, Małecki A, Klimkowicz‐Mrowiec A, Cholewa J. What determines spontaneous physical activity in patients with Parkinson's disease? J Clin Med 2020;9(5):1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dauwan M, Begemann MJH, Slot MIE, Lee EHM, Scheltens P, Sommer IEC. Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: a transdiagnostic systematic review and meta‐analysis of randomized controlled trials. J Neurol. 2019. https://doi:10.1007/s00415-019-09493-9. [Online ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Kolk NM, de Vries NM, Kessels RPC, Joosten H, Zwinderman AH, Post B, Bloem BR. Effectiveness of home‐based and remotely supervised aerobic exercise in Parkinson's disease: A double‐blind, randomised controlled trial. Lancet Neurol 2019;18(11):998–1008. [DOI] [PubMed] [Google Scholar]
- 13. Hemmerle AM, Herman JP, Seroogy KB. Stress, depression and Parkinson's disease. Exp Neurol 2012;233:79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cilia R, Mancini F, Bloem BR, Eleopra R. Telemedicine for Parkinsonism: a two‐step model based on the COVID‐19 Expeeience in Milan, Italy. Parkinsonism Relat Disord 2020;75:130–132. https:/doi:10.1016/j.parkreldis.2020.05.038/. [DOI] [PMC free article] [PubMed] [Google Scholar]


