To the Editor,
We read with great interesting of the findings from Mak et al., 1 and the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) is indeed a significant health challenge for countries worldwide. Furthermore, biologics, which are also associated with increased risk of viral infections, are primary treatment methods for controlling the disease in many inflammatory bowel disease (IBD) patients. 2 While evidence from the SARS and Middle East Respiratory Syndrome (MERS) outbreaks suggest that immunosuppression is unlikely to increase the individual's susceptibility to infection, 3 whether this holds true in the context of SARS‐CoV‐2 is uncertain.
To this end, a systematic review was conducted (CRD42020195943), and PubMed, Embase, and China National Knowledge Infrastructure were searched for articles discussing the incidence of COVID‐19 infection in IBD patients. Proportions of IBD patients diagnosed with SARS‐CoV‐2 were extracted, with a subsequent sensitivity analysis including only those from reverse transcriptase‐polymerase chain reaction (RT‐PCR). Subsequently, a meta‐analysis was undertaken with R (https://www.r-project.org).
A total of 183 articles were retrieved, and 43 full‐text articles were reviewed, of which 10 studies were included in the final analysis. Three hundred nine IBD patients tested positive for SARS‐CoV‐2 out of a total of 249 095 patients, and four out of 5098 IBD patients under biologics treatment tested positive for the infection. This gives an average pooled incidence of 1.93 infections per 1000 IBD patients (confidence interval [CI]: 0.48 to 7.77, Figure 1) and 0.68 infections per 1000 IBD patients taking biologics (CI: 0.26 to 1.82). When a sensitivity analysis was performed to include RT‐PCR diagnosis only, the rate of infections was increased to 4.24 per 1000 IBD patients (CI: 3.82 to 4.70) for overall infections and 1.14 (CI: 0.01 to 94.22) for patients on biologics.
FIGURE 1.
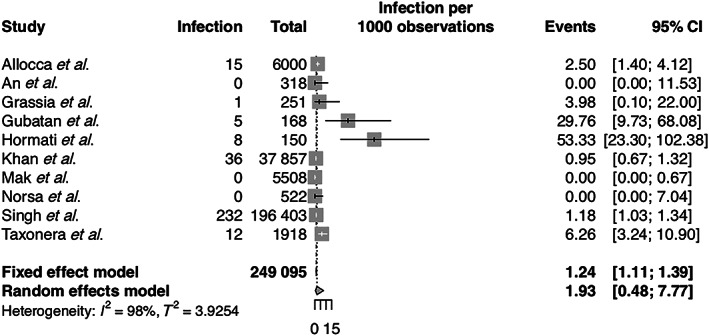
Incidence of COVID‐19 infection in inflammatory bowel disease. CI, confidence interval.
Existing guidelines on the clinical management of IBD patients in the COVID‐19 pandemic recommend that IBD patients treated with anti‐tumor necrosis factor, tofacitinib, ustekinumab, and vedolizumab should continue with their existing therapy although it is unclear if these biologics increase the risk of SARS‐CoV‐2 infection, 4 due to the possible risks of flares and disease exacerbation from biologics withdrawal in IBD patients with quiescent disease. Our analysis lends further support to this recommendation with the findings of a low incidence of SARS‐CoV‐2 in IBD, even in the population having biologics therapies such as Hong Kong or Taiwan. 1 Additional sensitivity analysis based on solely from RT‐PCR diagnoses found an elevation in rate of infections, though still relatively rare in occurrence. Therefore, it appears that the benefits of biologics to the IBD population outweigh the risk of discontinuation of these therapies.
SARS‐CoV‐2 infects its host by binding to angiotensin‐converting enzyme 2 (ACE2), and its subsequent cleavage by host transmembrane serine protease 2 (TMPRSS2) facilitates the fusion of the virus with the host. 5 Both ACE2 and TMPRSS2 are highly expressed by cells in the gastrointestinal tract. 5 However, reports on whether ACE2 and TMPRSS2 expression are upregulated in the gastrointestinal tract of IBD patients with active disease are conflicting. 6 Still, it has been suggested that elevated levels of soluble ACE2 lacking the membrane anchor in the peripheral blood of IBD patients may help limit SARS‐CoV‐2 infectivity in these patients. 7 Additionally, it was reported that biologics used in the treatment of IBD do not influence the levels of expression of both genes. 5 This suggests that biologics treatment may not lead to unfavorable effects on the promotion of viral entry into the host and may explain the low levels of incidence of SARS‐CoV‐2 infection in IBD patients having biologic therapy.
However, there are some limitations in our incidence estimates. Underreporting is a possibility in many countries, especially those facing a shortage of test kits or manpower. Individuals may also face significant institutional barriers in access to SARS‐CoV‐2 testing. There may also exist spatial variations in SARS‐CoV‐2 incidence across the globe, given that different nations have seen varied success in their management strategies for the pandemic.
Ultimately, the relatively low incidence of SARS‐CoV‐2 in IBD patients having biologic therapy may provide some reassurance to patients and clinicians for the continuation of these treatment methods. 1 Regardless, further studies on larger populations of IBD patients are needed to clarify the risks of biologics on SARS‐CoV‐2 incidence in IBD patients as well as in other medical conditions treated with biologics.
References
- 1. Mak JWY, Weng M‐T, Wei SC, Ng SC. Zero COVID‐19 infection in inflammatory bowel disease patients: findings from population‐based inflammatory bowel disease registries in Hong Kong and Taiwan. J. Gastroenterol. Hepatol. 2020. 10.1111/jgh.15164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersen NN, Jess T. World J. Gastroenterol. 2014; 20: 16014–16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thng ZX, de Smet MD, Lee CS et al. Br J Ophthalmol 2020. 10.1136/bjophthalmol-2020-316586 [DOI] [Google Scholar]
- 4. Rubin DT, Abreu MT, Rai V, Siegel CA. Gastroenterology 2020. 10.1053/j.gastro.2020.04.002 [DOI] [Google Scholar]
- 5. Rubin DT, Abreu MT, Rai V et al. Gut 2020; 69: 1010. [Google Scholar]
- 6. Burgueño JF, Reich A, Hazime H et al. Inflamm. Bowel. Dis. 2020; 26: 797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Monteleone G, Ardizzone S. J. Crohns. Colitis. 2020. 10.1093/ecco-jcc/jjaa061 [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee, M. H. , Ng, C. H. , Chin, Y. H. , Muthiah, M. , Foo, F. J. , and Chong, C. S. (2020) Incidence of SARS‐CoV‐2 infection in inflammatory bowel disease. Journal of Gastroenterology and Hepatology, 35: 2021–2022. 10.1111/jgh.15191.
Declaration of conflict of interest: Ming Hui Lee, Cheng Han Ng, Yip Han Chin, Mark Muthiah, Fung Joon Foo, and Choon Seng Chong declare that they have no conflict of interest.
Author contribution: Ming Hui Lee participated in the extraction and interpretation of data, as well as writing the manuscript. Cheng Han Ng planned and designed the study and participated in the extraction, analysis, and interpretation of data as well as reviewing the manuscript. Yip Han Chin planned and designed the study and participated in the extraction, analysis, and interpretation of data as well as reviewing the manuscript. Mark Muthiah, Fung Joon Foo, and Choon Seng Chong planned and designed the study and participated in the interpretation of data and review of the manuscript.
Financial support: No funding was received for this study.


