Abstract
Evidence is mounting to indicate that cancer patients may have more likelihood of having coronavirus disease 2019 (COVID‐19) but lack consistency. A robust estimate is urgently needed to convey appropriate information to the society and the public, in the time of ongoing COVID‐19 pandemic. We performed a systematic review and meta‐analysis through a comprehensive literature search in major databases in English and Chinese, and two investigators conducted publication selection and data extraction independently. A meta‐analysis was used to obtain estimates of pooled prevalence of cancer in patients with COVID‐19 and determine the association of cancer with severe events, after assessment of potential heterogeneity, publication bias, and correction for the estimates when necessary. Total 38 studies comprising 7094 patients with COVID‐9 were included; the pooled prevalence of cancer was estimated at 2.3% (95% confidence limit [CL] [0.018, 0.029]; P < .001) overall and 3.2% (95% CL [0.023, 0.041]; P < .001) in Hubei province; the corresponding estimates were 1.4% and 1.9% after correction for publication bias; cancer was significantly associated with the events of severe cases (odds ratio [OR] = 2.20, 95% CL [1.53, 3.17]; P < .001) and death (OR = 2.97, 95% CL [1.48, 5.96]; P = .002) in patients with COVID‐19, there was no significant heterogeneity and a minimal publication bias. We conclude that cancer comorbidity is associated with the risk and severe events of COVID‐19; special measures should be taken for individuals with cancer.
Keywords: cancer, comorbidity, COVID‐19, meta‐analysis, severe events
Short abstract
What's new?
There is mounting evidence that cancer patients have a greater likelihood of having coronavirus disease 2019 (COVID‐19), but consistency is still lacking. This study provides the first estimate after correction for publication bias of cancer prevalence in patients with COVID‐19 based on a comprehensive literature search and analysis. The results show that patients with cancer and cancer survivors are at an elevated risk of developing COVID‐19 and are also 2‐ to 3‐fold more likely of suffering severe events and death. These findings have implications for public health, calling for the establishment of appropriate measures to manage cancer patients with COVID‐19.
Abbreviations
- ACE‐2
angiotensin‐converting enzyme‐2
- CL
confidence limit
- COVID‐19
Coronavirus disease 2019
- CNKI
China National Knowledge Infrastructure
- ICU
intensive care unit
- OR
odds ratio
- PRISMA
preferred reporting items for systematic reviews and meta‐analysis
- PPE
personal protection equipment
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- WHO
World Health Organization
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is a new respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 Because the causative viral pathogen is highly infective and with human‐to‐human transmission, the COVOD‐19 is having a fast spread worldwide. On March 12, 2020, when there were over 20 000 confirmed cases and 1000 deaths in the European region, the World Health Organization (WHO) announced a pandemic of COVID‐19, which has caused the ongoing global public health emergency.
COVID‐19 is an infectious disease and almost all people lack specific immunity to the novel coronavirus. Individuals can be infected by contact with respiratory droplets containing SARS‐CoV‐2 virus or the surfaces contaminated. 2 Older people and individuals with underlying chronic health conditions may increase the risk of developing COVID‐19 and being with severe events, likely due to the aging‐associated dysregulation of immune systems. 3 The major symptoms of clinical manifestation include fever, cough, fatigue, myalgia or arthralgia, sore throat, headache, shortness of breath (anhelation or dyspnea) and sputum production. 4 A clinical report from one of the hospitals in Wuhan shows that patients who received Intensive Care Unit (ICU) services are more prevalent of respiratory failure (61.1%), arrhythmia (44.4%), and a sudden shock (30.6%). 5 In some severe cases, the coronavirus infection can lead to acute respiratory distress syndrome, acute cardiac or kidney injury, secondary infection, shock and high risk of death. 6 A nationwide report (n = 72 314) from China shows that the severe and critical cases of COVID‐19 account for 19% and the case‐fatality rate is about 49.0% in the critical cases 7 because of lacking effective treatment for the coronavirus. 8
In addition, multiple reports have shown that underlying chronic health conditions such as hypertension and diabetes are associated with severe patients with COVID‐19. 4 , 9 This is likely because the receptor of angiotensin‐converting enzyme‐2 (ACE‐2), which the spike proteins of the pathogen SARS‐CoV‐2 bind to enter the host cells, is associated with hypertension 10 and diabetes. 11 Also, there is a growing interest in the association of cancer with COVID‐19. 8 , 12
Cancer is a severe underlying condition in patients with COVID‐19. Patients with cancer are usually characterized by older age, compromised immune systems, and comorbid with chronic diseases, which might be partially caused by antitumor treatment. Individuals with cancer may be more likely to have COVID‐19. One hospital‐based study has noted a few cases with cancer, 13 but a robust estimate of cancer prevalence in patients with COVID‐19 is lacking. Here, we conducted a systematic review and meta‐analysis of cancer prevalence in patients with COVID‐19, and examined the association of cancer with severe events, which include severe cases judged by clinical symptoms (SJCS), utilization of ICU services and death.
2. METHODS
The meta‐analysis was conducted in accordance with the guidelines in the Preferred Reporting Items for Systematic Reviews and Meta‐analyzes (PRISMA) statement. 14 Conducting of this systematic review and meta‐analysis was based on existing literature, and therefore was not registered.
2.1. Search strategy
A literature search was first performed in major databases of PubMed, Elsevier and Web of Science to identify all published studies with full text, as of April 23, 2020, and the language was first limited to English. The search terms were as follows: “2019‐nCoV” [All fields] or “novel coronavirus” [All fields] or “SARS‐CoV‐2” [All fields] or “COVID‐19” [All fields]. Additional search was performed in the three major Chinese databases—China National Knowledge Infrastructure (CNKI) (https://www.cnki.net), WanFang (http://www.wanfangdata.com.cn), and VIP (http://www.cqvip.com). The above process was performed by two investigators (Y. T. and X. Q.) independently.
2.2. Inclusion and exclusion criteria
A study was included when met all the following criteria: (a) reporting information on clinical characteristics and epidemiology of the patients with a laboratory‐confirmed diagnosis of COVID‐19, (b) containing information on cancer and clinical subtypes, or outcomes of the clinically validated death, severe cases, ICU care or disease progression and (c) an original study.
A study was excluded when met one of the following criteria: (a) case report, animal research, review, guideline, expert consensus, letter, protocol, news or comment, (b) with incomplete information on related data, (c) only on special populations such as children, pregnancy, older people or (d) sample size of fewer than 20 individuals and overlapped study samples. Also, we excluded two studies that were conducted in the populations of the United States and Iran.
2.3. Data extraction
Two investigators (X. J. and X. Q.) screened and extracted data according to the predesigned form of Excel spreadsheet independently. Questions or inconsistencies were resolved through discussion or consultation with a third expert (J. H.) to make a final decision. Information extracted from the included literature includes the name of the first author, year of publication, region, sample size, age, gender, the status of severe cases, utilization of ICU and event of death, where appropriate.
2.4. Statistical analysis
The Stata (special edition version 14.0) for Windows (Stata Corp, College Station, Texas) was used to obtain the estimates of cancer prevalence in patients with COVID‐19. A pooled odds ratio (OR) was used to measure the strength of association, and 95% confidence limit (CL) measured the precision of the estimates. Heterogeneity was measured by , a statistic that measures the proportion of variance in the estimates is due to the heterogeneity between studies, where Q is Cochran's statistic, a classical measure of heterogeneity, which can provide a statistical test based on Chi‐square statistic. 15 A threshold of P‐value was set at .05. In the presence of a significant heterogeneity, a random‐effect meta‐analysis was performed. Also, cumulative meta‐analysis, sensitivity analysis, subgroup analysis was performed to assess the systematic bias, and meta‐regression modeling was performed to adjust for potential covariates.
Publications bias was assessed using the Begg's rank correlation test and Egger's linear regression test. A threshold of P‐value was set at .05. In the presence of publication bias, “trim and fill” method was used to estimate the number of expected missing studies and to obtain a corrected estimate. 16
Study power was calculated using PS (Power and Sample Size Calculations, version 3.0) software.
3. RESULTS
3.1. Characteristics of included studies
Through a comprehensive literature search, 5724 relevant articles were identified. Duplications were found by carefully scanning the titles and abstracts, and then removed. Thirty‐eight studies met the inclusion and exclusion criteria were included in the meta‐analysis, and 22 of which were from the Hubei province. Of the included studies, 7094 patients with COVID‐19, and 206 (2.90%) patients had cancer. The search process is shown in Figure 1, and the characteristics of all included studies are presented in Table 1.
FIGURE 1.
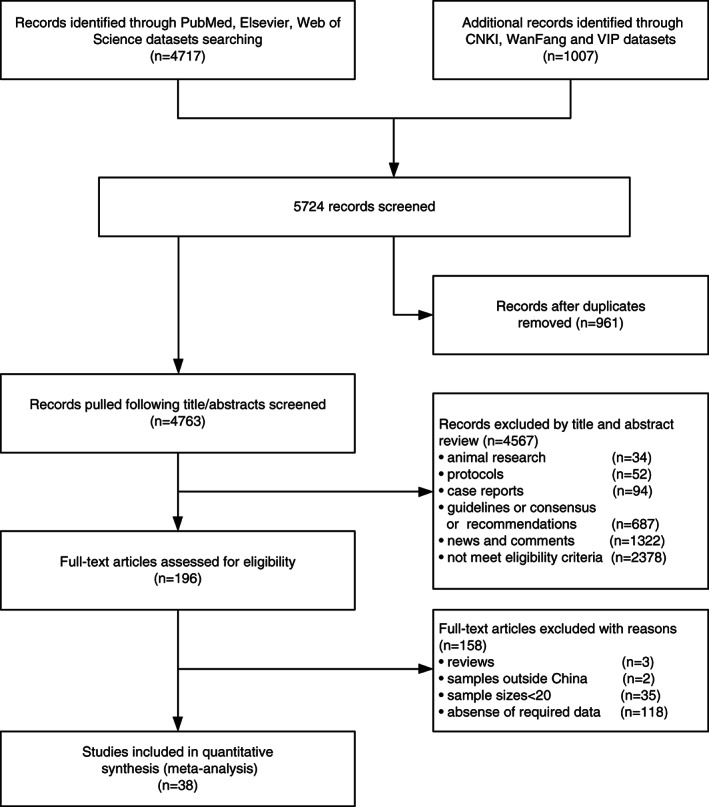
Flowchart of the literature search and selection process
TABLE 1.
Characteristics of the included studies
| First author | Province | COVID‐19 | Severe events | |||||
|---|---|---|---|---|---|---|---|---|
| Sex | Age(year) | Cancer | Covid19 | Cancer | ||||
| All | (M/F) | (Med/Mean) | n (%) | Type | n (%) | n (%) | ||
| Cao, J. L. 17 | Hubei | 102 | 53/49 | 54 (37‐67) | 4 (3.9%) | Death | 17 (16.7%) | 1 (1%) |
| Chen, J. 18 | Shanghai | 249 | 126/123 | 51 (36‐64) | 1 (0.4%) | ICU | 22 (8.8%) | NA |
| Chen, L. 19 | Wuhan | 29 | 21/8 | 56 (26‐79) | 1 (3.4%) | Death | 2 (6.9%) | 1 (3.4%) |
| Chen, L. 19 | Wuhan | 29 | 21/8 | 56 (26‐79) | 1 (3.4%) | SJCS | 14 (48.3%) | 1 (3.4%) |
| Chen, N. S 20 | Hubei | 99 | 76/32 | 55.5 ± 13.1 | 1 (1%) | Death | 11 (11.1%) | NA |
| Chen, N. S 20 | Hubei | 99 | 76/32 | 55.5 ± 13.1 | 1 (1%) | ICU | 23 (23.2%) | NA |
| Chen, T. 21 | Hubei | 274 | 171 /103 | 51 (44‐70) | 7 (2.6%) | Death | 113 (41.2%) | 5 (1.8%) |
| Deng, Y. 22 | Hubei | 225 | 124/101 | NA | 8 (3.6%) | Death | 109 (48.4%) | 6 (2.7%) |
| Du, R. H. 23 | Hubei | 109 | 74/35 | 70.7 ± 10.9 | 8 (7.3%) | ICU | 51 (46.8%) | 2 (1.8%) |
| Fang, X.W. 24 | Anhui | 79 | 45/34 | 45.1 ± 16.6 | 1 (1.3%) | SJCS | 24 (30.4%) | 0 |
| Feng, Y. 25 | Multi‐PR | 476 | 271/205 | 53 (40‐64) | 12 (2.5%) | SJCS | 124 (26.1%) | 7 (1.5%) |
| Guan, W. J. 26 | Multi‐PR | 1590 | 904/674 | 48.9 ± 16.3 | 18 (1.1%) | Death | 50 (3.1%) | 3 (0.2%) |
| Guan, W. J. 26 | Multi‐PR | 1590 | 904/674 | 48.9 ± 16.3 | 18 (1.1%) | ICU | 99 (6.2%) | 5 (0.3%) |
| Huang, C.L. 6 | Hubei | 41 | 30/11 | 49 (41‐58) | 1 (2.4%) | ICU | 13 (31.7%) | 0 |
| Huang, Y. H 27 | Hubei | 34 | 14/20 | 56.24 ± 17.14 | 3 (8.8%) | NA | NA | NA |
| Li, D. 28 | Hunan | 80 | 40/40 | 47.5 (3‐90) | 1 (1.3%) | SJCS | 17 (21.3%) | 1 (1.3%) |
| Li, X. C 29 | Hubei | 548 | 279/269 | 60 (48‐69) | 24 (4.4) | SJCS | 269 (49.1%) | 14 (2.6%) |
| Li, Y.L. 30 | Anhui | 49 | 28/21 | 45 (32‐60) | 1 (2.0%) | NA | NA | NA |
| Liu, C. 31 | Multi‐PR | 32 | 20/12 | 38.5 (26.5‐5.75) | 2 (6.3%) | NA | NA | NA |
| Liu, K. 32 | Hubei | 137 | 61/76 | 57 (20‐83) | 2 (1.5%) | Death | 16 (11.7%) | NA |
| Liu, W. 33 | Hubei | 78 | 39/39 | 38 (33‐57) | 4 (5.1%) | SJCS | 7 (9%) | 2 (2.6%) |
| Mao, L. 34 | Hubei | 214 | 87/127 | 52.7 ± 15.5 | 13 (6.1%) | SJCS | 88 (41.1%) | 5 (2.3%) |
| Mo, P. Z. 35 | Hubei | 155 | 86/69 | 54 (42‐66) | 7 (4.5%) | SJCS | 85 (54.8%) | 5 (3.2%) |
| Shi, H. S. 36 | Hubei | 81 | 42/39 | 49.5 ± 11.0 | 4 (4.9%) | NA | NA | NA |
| Sun, D.F. 37 | Zhejiang | 30 | 15/15 | 49 (30‐71) | 1 (3.3%) | NA | NA | NA |
| Wan, S. X 38 | Chongqing | 135 | 72/63 | 47 (36‐55) | 4 (3.0%) | SJCS | 40 (29.6%) | 3 (2.2%) |
| Wang, D. W. 5 | Hubei | 138 | 75/63 | 56 (42‐68) | 10 (7.2%) | ICU | 36 (26.1%) | 4 (2.9%) |
| Wang, F. 39 | Ningxia | 29 | 19/10 | NA | 1 (3.4%) | NA | NA | NA |
| Wang, L. Z. 40 | Shandong | 26 | 15/11 | 42.0 (33.5‐53.3) | 1 (3.8%) | NA | NA | NA |
| Wang, R. R 41 | Anhui | 125 | 71/54 | 38.76 ± 13.79 | 1 (0.8%) | Death | 0 | NA |
| Wang, Z. L 42 | Hubei | 69 | 32/37 | 42.0 (35.0‐62.0) | 4 (5.8%) | SJCS | 14 (20.3%) | NA |
| Wang, Z. L 42 | Hubei | 69 | 32/37 | 42 (35‐62) | 4 (5.8%) | Death | 5 (7.2%) | NA |
| Wu, C. M 43 | Hubei | 201 | 128/73 | 51 (43‐60) | 1 (0.5%) | Death | 44 (21.9%) | NA |
| Wu, C. M 43 | Hubei | 201 | 128/73 | 51 (43‐60) | 1 (0.5%) | ICU | 53 (26.4%) | NA |
| Wu, J. 44 | Jiangsu | 80 | 39/41 | 46.10 ± 15.42 | 1 (1.3%) | Death | 0 | NA |
| Wu, J. 44 | Jiangsu | 80 | 39/41 | 46.10 ± 15.42 | 1 (1.3%) | SJCS | 3 (3.8%) | NA |
| Xu, X. 45 | Guangdong | 90 | 39/51 | 50 (18‐86) | 2 (2.2%) | SJCS | 38 (42.2%) | NA |
| Yang, W.J 46 | Zhejiang | 149 | 81/68 | 45.11 ± 13.35 | 2 (1.3%) | NA | NA | NA |
| Yang, X. B. 9 | Hubei | 52 | 35/17 | 59.7 ± 13.3 | 2 (3.8%) | Death | 32 (61.5%) | 1 (1.9%) |
| Zhang, G. Q 47 | Hubei | 221 | 108/113 | 55.0 (39.0‐66.5) | 9 (4.1%) | SJCS | 55 (24.9%) | 3 (1.4%) |
| Zhang J. X. 48 | Hubei | 663 | 321/342 | 55.6 (44‐69) | 14 (2.1%) | SJCS | 409 (61.7%) | 4 (0.6%) |
| Zhang J. X. 48 | Hubei | 663 | 321/342 | 55.6 (44‐69) | 14 (2.1%) | Death | 25 (3.8%) | 11 (1.7%) |
| Zhang, J. J. 49 | Hubei | 140 | 71/69 | 57 (25‐87) | 6 (4.3%) | SJCS | 58 (41.4%) | 0 |
| Zhang, W. 50 | Beijing | 74 | 45/34 | 52.7 ± 19.1 | 2 (2.7%) | SJCS | 18 (24.3%) | 2 (2.7%) |
| Zhou F. 51 | Hubei | 191 | 119/72 | 56 (46‐67) | 2 (1.0%) | Death | 54 (28.3%) | 0 |
Note: M, male; F, female; ICU, intensive care unit; Multi‐PR (multi‐province), means the sample source including both Hubei and one or more other provinces; SJCS, severity judged by clinical symptoms; NA, not available.
3.2. Cancer prevalence of patients with COVID‐19
The pooled prevalence of cancer in patients with COVID‐19 (Figure 2) was estimated at 2.3% (95% CL [0.018, 0.029]; I 2 = 52.6%, P < .001), which was obtained with a random‐effect meta‐analysis. Cumulative analysis showed the estimates of early studies were fluctuating and with larger variance. However, by the last few studies, the estimates and precision approached a constant (Figure S1).
FIGURE 2.
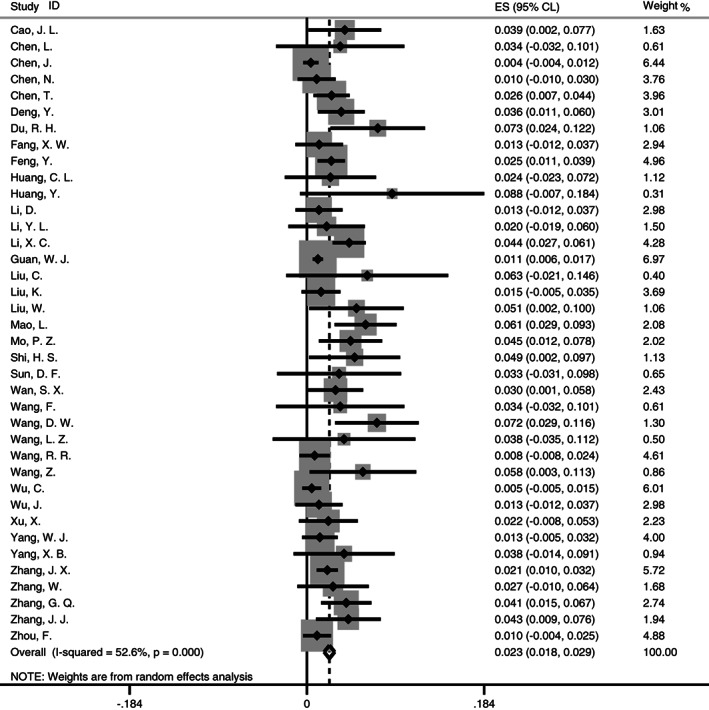
Forest plot for meta‐analysis of cancer prevalence in patients with COVID‐19
Separate analysis by sample location estimated that the pooled cancer prevalence in Hubei (Figure 3) was at 3.2% (95% CL [0.023, 0.041]; I 2 = 59.3%; P < .001) with a random‐effect meta‐analysis, while it was at 1.0% in the samples outside Hubei and 1.8% in the samples from multiple provinces.
FIGURE 3.
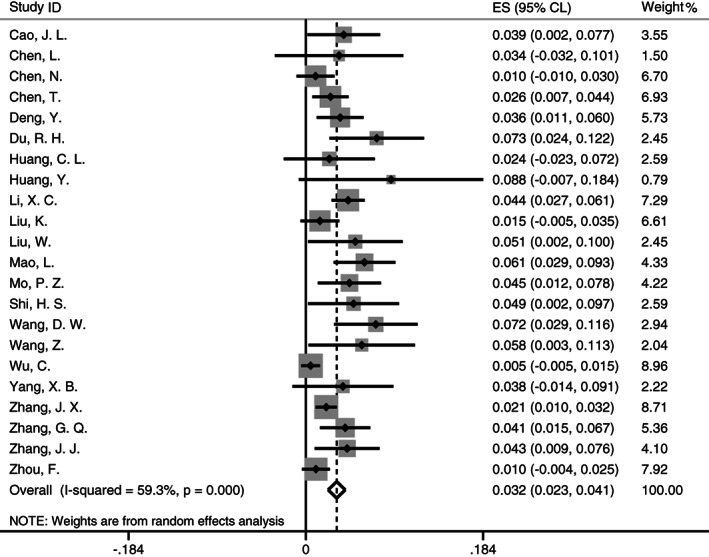
Forest plot for meta‐analysis of cancer prevalence in patients with COVID‐19 from Hubei
3.3. Assessment of the source of heterogeneity
To determine additional source of heterogeneity in the studies, we performed a sensitivity and subgroup analysis. Sensitivity analysis showed the effect size had no noted deviation when an individual study was omitted at a time (Figure S2). Subgroup analysis indicated that the estimates were not significantly different by gender and sample size, but were affected by age and sample location (Table 2). Meta‐regression analyses showed that the cancer prevalence in patients with COVID‐19 was significantly affected by sample location (P = .016), but not gender (P = .403), age (P = .257) and sample size (P = .740), which were also confirmed by multiple meta‐regression analysis that only sample location (P = .036) was a significant factor causing the overall heterogeneity, supporting our analysis performed above by Hubei‐only.
TABLE 2.
Meta‐analysis of cancer prevalence in patients with COVID‐19 by subgroup
| Group | No. Study | n | ES | 95% CL | P | I 2 | P |
|---|---|---|---|---|---|---|---|
| Overall | |||||||
| Prevalence | 38 | 7094 | 0.023 | 0.018‐0.029 | <.001 | 52.60% | <.001 |
| Subgroup | |||||||
| Gender | |||||||
| M/F < 1 | 9 | 1534 | 0.028 | 0.017‐0.039 | <.001 | 33.00% | <.001 |
| M/F ≥ 1 | 29 | 5560 | 0.022 | 0.016‐0.028 | <.001 | 53.50% | <.001 |
| Age group | |||||||
| <51 | 16 | 2734 | 0.013 | 0.009‐0.018 | <.001 | 0 | <.001 |
| ≥51 | 22 | 4360 | 0.028 | 0.020‐0.036 | <.001 | 67.00% | <.001 |
| Location | |||||||
| Within Hubei | 22 | 3801 | 0.032 | 0.023‐0.041 | <.001 | 59.30% | <.001 |
| Outside Hubei | 13 | 1195 | 0.010 | 0.004‐0.015 | <.001 | 0 | .001 |
| Multi‐province | 3 | 2098 | 0.018 | 0.004‐0.032 | <.001 | 56.70% | .01 |
| Sample size | |||||||
| <106 | 19 | 1154 | 0.022 | 0.014‐0.031 | <.001 | 0 | <.001 |
| ≥106 | 19 | 5940 | 0.023 | 0.016‐0.030 | <.001 | 71.70% | <.001 |
Note: 95% CL, 95% confidence limit; ES, effect size; F, female; M, male; Multi‐PR (multi‐province), means the sample source including both Hubei and one or more other provinces.
3.4. Association of cancer with the severe events of COVID‐19
Seventeen studies, including 11 of which were from Hubei province, reported the information on severe cases defined by clinical symptoms and ICU experience. No significant heterogeneity (I 2 = 19.2%; P = .230) was presented between the studies (Figure 4A). A fixed‐effect meta‐analysis showed that cancer was significantly associated with the severe cases of COVID‐19 (OR = 2.20, 95% CL [1.53, 3.17]; P < .001). The power to detect this significant association was estimated to be 85.9%.
FIGURE 4.
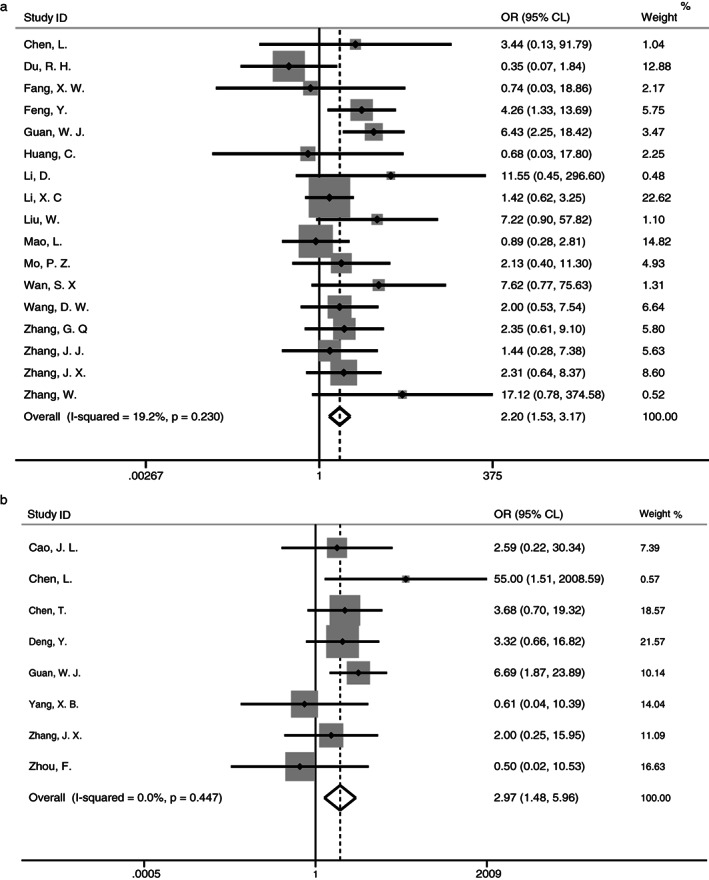
Forest plots of the meta‐analysis of the association of cancer with severe events of COVID‐19 (A, severe cases; B, death)
In addition, eight studies reported the outcome of death, and all were from Hubei province. No heterogeneity (I 2 = 0%, P = .447) was observed (Figure 4B). A fixed‐effect meta‐analysis showed that cancer was associated with the risk of death (OR = 2.97, 95% CL [1.48, 5.96]; P = .002) in patients with COVID‐19, with the estimated power to detect this association at 80.1%.
3.5. Publication bias and correction
Assessment of publication bias is a crucial step to present the findings from a meta‐analysis objectively. We noted a significant (P < .01) publication bias in the meta‐analysis of 38 studies; correction for the publication bias was made with the “filled” studies for overall sample (Figure 5A) and Hubei‐only (Figure 5B), separately. The random‐effect meta‐analysis was then used to obtain an adjusted estimate of pooled prevalence of cancer was at 1.4% (95% CL [0.8, 2.0]; heterogeneity P < .01) overall and at 1.9% (95% CL [1.0, 2.8], heterogeneity P < .01) in the Hubei‐only.
FIGURE 5.
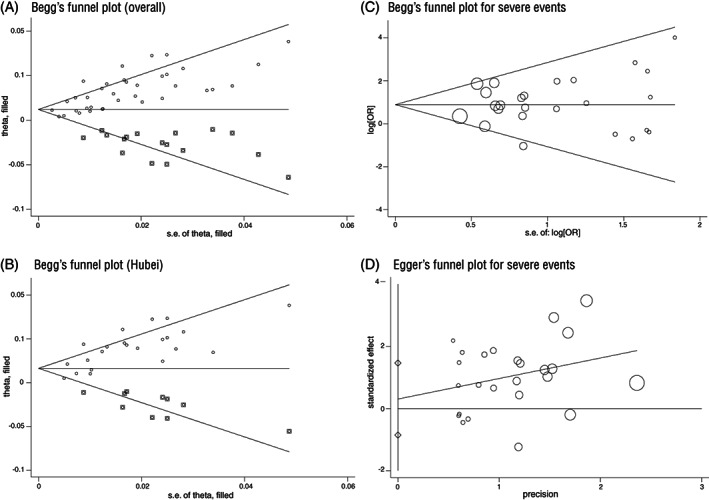
Plots for publication bias assessment (x‐axis, precision or SE; y‐axis, effect size; dot, individual study; circle size represents the weight of individual study). A, Begg's plot with filled studies for meta‐analysis of cancer prevalence in all samples; B, Begg's plot with filled studies for Hubei only of cancer prevalence in Hubei; C, Begg's plot for meta‐analysis of association with severe events; D, Egger's publication plot for publication bias in studies of severe events
We did not find evidence for publication bias in the association of cancer with severe events of COVID‐19 based on Begg's test (P = .761) and Egger's test (P = .592) (Figure 5C,D), and no evidence for a significant publication bias (P > .5, n = 17) in the association of cancer with the severe cases, either.
4. DISCUSSION
To the best of our knowledge, this is a more comprehensive meta‐analysis that has investigated the association between cancer with COVID‐19 so far. The analysis of 38 eligible studies comprising 7094 patients with COVID‐19 showed that the pooled prevalence of cancer comorbidity was estimated at 2.3%, and 1.4% after correction for publication bias, which was more than 4‐fold high as the cancer prevalence (0.26%) in the general populations in China. 52 In addition, cancer significantly increased the risk for being severe cases or death. Our study provides a solid evidence that patients with cancer were more susceptible to the coronavirus infection and significantly associated with severe events.
The study provides the first estimate with correction for publication bias of the pooled prevalence of cancer in patients with COVID‐19. Desai et al 53 had estimated the pooled prevalence of cancer at 2.0%, based on publications of 11 studies that were noted with the presence of heterogeneity, but the estimate was obtained without assessing publication bias. The increased cancer prevalence in patients with COVID‐19 may be less likely caused by the difference in the exposure. On the contrary, patients with cancer may have limited social and daily activities, which could have reduced the chance of exposure to the pathogen compared to the general populations. We suspect that the higher prevalence of cancer is due to an increase in the susceptibility to the infection with the SARS‐CoV‐2 virus, which can be attributed to the underlying health conditions, including older age and dysregulated immune systems.
However, we observed a noted regional difference in the cancer prevalence, which is likely due to the chance of exposure. The sample location, whether or not a study sample was from the epicenter of COVID‐19, accounted for major heterogeneity. The estimates of pooled cancer prevalence (unadjusted 3.2%, n = 22) in patients with COVID‐19 from Hubei was 3.2‐fold high (unadjusted 1.0%, n = 13) in the samples outside Hubei. Because the prevalence of cancer in the general populations of central China, including Hubei, is at a middle level behind the east and southwest China, 54 the elevated prevalence of cancer in the patients with COVID‐19 in Hubei is less likely caused by the general prevalence of cancer. Of note, the mean age of the study samples in Hubei and non‐Hubei was similar around 50 years, and the corresponding cancer incidence at this age group is reported at 0.37% 55 and 0.39% 56 in Hubei and China, respectively. We can reasonably speculate that the regional difference is due to the chance of exposure, that is, lower awareness of personal protection in the early stage of the outbreak. When the outbreak of COVID‐19 first started in Wuhan and then Hubei, people, even medical professionals, might have had lower awareness of the high infectivity and had inadequate equipment to protect themselves from exposure. It is documented that there were 3387 medical and health professionals from 476 medical institutions or hospitals across the country of China, infected with COVID‐19, 3062 cases (91%) of whom were from Hubei, according to the Beijing Evening Daily 1 (Official news online in Chinese).
In contrast, people outside Hubei may have more time to acquire related knowledge and adequate personal protection equipment to avoid effective exposure to the pathogens. It is expected that individuals with cancer, later outside Hubei, may have taken more careful protection from being exposed. This is supported by a significant regional difference in other comorbid conditions such as hypertension (18.1% vs 10.5%; P = .003) and diabetes (8.8% vs 4.7%; P = .025) between Hubei and outside Hubei. 57
Besides cancer prevalence, our study shows that cancer is associated with severe cases and the risk of death in patients with COVID‐19. Liang et al 13 observed that patients with cancer tend to have a higher risk of severe events, a composite endpoint defined by patients in ICU that required invasive ventilation, or death, compared to patients of COVID‐19 without cancer. However, a meta‐analysis of a small number of studies (n = 4) indicated that malignance was not significantly associated with the risk of COVID‐19. 58 Less is known about the progression and survival outcome of the patients with cancer and CVOID‐19. Our analysis provides robust evidence that cancer is associated with the risk of severe cases and death, based on the samples from Hubei province, in which relevant information is available and collected from the published studies.
To speculate the possible mechanisms underlying the association of cancer with COVID‐19, we believe that both initial innate immune response and specific adaptive immune response determine the outcome after infection. In response to coronavirus infection, typically, the regulated inflammation and adaptive immune response can eradicate the pathogen, and develop antibody and protective immunity. Patients with cancer, likely with advanced age and on active treatment or even during watchful observation, may have dysregulated immune systems, including a reduced innate immunity due to age, 59 or compromised immune system by using immunosuppressive or immune‐stimulating drugs for antitumor treatments. 8 They may have reduced the ability to develop an effective immune response to eradicate the pathogen successfully. Therefore, patients with cancer are more susceptible and likely to develop COVID‐19 after exposure to the pathogen virus.
However, the mechanism underlying the association of cancer with severe events of COVID‐19 seem more complicated without a rigorous study. A pathological study of a single individual with COVID‐19 indicates that inflammation and cytokines‐associated lung injury may be associated with severe events of COVID‐19. 60 This observation is supported by an analysis of more clinical samples that severe events or death of COVID‐19 are significantly associated with an increase in innate immune cells such as neutrophils and inflammatory cytokines, and lymphopenia, 29 , 51 indicating immunopathology is involved. However, people argue that the “cytokine storm” seems not to explain the association of cancer with the severe event of COVID‐19, 61 as the development of cancer is caused by blunted immune status such as immunosuppression 62 ; and also, a case report showed that immunosuppression might be with favorable outcomes in cancer patients with COVID‐19. 63
4.1. Limitations
The study has limitations. First, most of the included studies were based on hospital‐based case‐series, selection bias may exist and affect the estimates, although the publication bias was assessed and corrected. Second, due to limited clinical information, we were unable to perform the study by the site of cancer. Finally, while we had carefully investigated all articles, the included studies were limited to the study samples in China to reduce the heterogeneity. Even though two studies in non‐Chinese populations met our criteria for inclusion and exclusion, one from Iran and one from the United States of American, they reported two extreme cases of cancer prevalence in patients with COVID‐19; they were excluded from the analysis as outliers.
5. CONCLUSION
Despite the limitations, our analysis provides the first objective findings after adjusted for heterogeneity and correction for publication bias. Our study indicates that patients with cancer or cancer survivors are at an elevated risk for infection with the SARS‐CoV‐2 and are associated with severe cases and risk of death. Special preventive measures should be taken to protecting individuals, especially cancer survivors, with compromised immune systems or underlying health conditions, from exposure to the SRAS‐CoV‐2 virus.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
6.
Supporting information
Appendix S1 Figures.
ACKNOWLEDGEMENTS
We thank the academic committee of Beijing University of Chinese Medicine and Third Affiliated Hospital for the support of our study, Key Research Project and National Natural Science Foundation of China (J. H.), Beijing Haiju Scholarship (F. Z.). The study supported by Key Research Project of Beijing University of Chinese Medicine (2020‐JYB‐ZDGG‐143‐1), National Natural Science Foundation of China (81573736) and Beijing Haiju Scholarship (BHTO201511097).
Tian Y, Qiu X, Wang C, et al. Cancer associates with risk and severe events of COVID‐19: A systematic review and meta‐analysis. Int. J. Cancer. 2021;148:363–374. 10.1002/ijc.33213
Yehong Tian and Xiaowei Qiu contributed equally to this work.
Funding information Beijing Haiju Scholarship, Grant/Award Number: BHTO201511097; Key Research Project of Beijing University of Chinese Medicine, Grant/Award Number: 2020‐JYB‐ZDGG‐143‐1; National Natural Science Foundation of China, Grant/Award Number: 81573736
Endnote
Contributor Information
Wenquan Niu, Email: niuwenquan_shcn@163.com.
Jinchang Huang, Email: zryhhuang@163.com.
Fengyu Zhang, Email: zhangfy@gcatresearch.org.
DATA AVAILABILITY STATEMENT
Only publicly available data were used in our study, and data sources and handling of these data are described in Table 1 and in the Materials and Methods, respectively. Further details are available from the corresponding author upon request.
REFERENCES
- 1. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382:1199‐1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Won J, Lee S, Park M, et al. Development of a laboratory‐safe and low‐cost detection protocol for SARS‐CoV‐2 of the coronavirus disease 2019 (COVID‐19). Exp Neurobiol. 2020;29:107‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saghazadeh A, Rezaei N. Immune‐epidemiological parameters of the novel coronavirus ‐ a perspective. Expert Rev Clin Immunol. 2020;16:465‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 8. Russell B, Moss C, George G, et al. Associations between immune‐suppressive and stimulating drugs and novel COVID‐19‐a systematic review of current evidence. Ecancermedicalscience. 2020;14:1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Luo Y, Liu C, Guan T, et al. Association of ACE2 genetic polymorphisms with hypertension‐related target organ damages in South Xinjiang. Hypertens Res. 2019;42:681‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu C, Li Y, Guan T, et al. ACE2 polymorphisms associated with cardiovascular risk in Uygurs with type 2 diabetes mellitus. Cardiovasc Diabetol. 2018;17:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Watson M, Lam WWT, Pirl W, Fielding R. Cancer and COVID‐19: a call for comments. Psychooncology. 2020;29:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang W, Guan W, Chen R, et al. Cancer patients in SARS‐CoV‐2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539‐1558. [DOI] [PubMed] [Google Scholar]
- 16. Duval S, Tweedie R. Trim and fill: a simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics. 2000;56:455‐463. [DOI] [PubMed] [Google Scholar]
- 17. Cao J, Tu WJ, Cheng W, et al. Short‐term outcomes of 102 patients with Corona virus disease 2019 in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen J, Qi T, Liu L, et al. Clinical progression of patients with COVID‐19 in Shanghai, China. J Infect. 2020;80:e1‐e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen L, Liu W, Zhang Q, et al. RNA based mNGS approach identifies a novel human coronavirus from two individual pneumonia cases in 2019 Wuhan outbreak. Emerg Microbes Infect. 2020;9:313‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ (Clin Res Ed) 2020;368:m1091.1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID‐19) in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133:1261‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Du RH, Liu LM, Yin W, et al. Hospitalization and critical care of 109 decedents with COVID‐19 pneumonia in Wuhan, China. Ann Am Thorac Soc. 2020;17:839‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fang XW, Mei Q, Yang TJ, et al. Clinical characteristics and treatment analysis of 79 patients infected with SARS‐CoV‐2. Chin Pharmacol Bull. 2020;36:453‐459. [Google Scholar]
- 25. Feng Y, Ling Y, Bai T, et al. COVID‐19 with different severity: a multi‐center study of Clinical Features. Am J Respir Crit Care Med. 2020;201:1380‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J. 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang Y, Tu M, Wang S, et al. Clinical characteristics of laboratory confirmed positive cases of SARS‐CoV‐2 infection in Wuhan, China: a retrospective single center analysis. Travel Med Infect Dis. 2020;101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li D, Long YZ, Huang P, Guo WL, Wu Q, JL fU. Clinical characteristics of 80 patients with COVID‐19 in Zhozhou city. Chin J Infect Control. 2020;19:227‐233. [Google Scholar]
- 29. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li YL, Shan NB, Sun W, Wang BG, Li DF. Comparative study for clinical features between COVID‐19 patients with conventional type and heavy/critical type. Pract J Cardiac Cereb Pneumal Vasc Dis. 2020;28:14‐19. [Google Scholar]
- 31. Liu C, Jiang ZC, Shao CX, et al. Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study. Chin J Hepatol. 2020;28:107‐111. [DOI] [PubMed] [Google Scholar]
- 32. Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). 2020;133:1025‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133:1032‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mo P, Xing Y, Xiao Y, et al. Clinical characteristics of refractory COVID‐19 pneumonia in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun DF, Deng M, Lu XZ, Xie XS. Clinical analysis of 30 patients infected with SARS‐CoV‐2. Zhejiang Clin Med. 2020;22:354‐356. [Google Scholar]
- 38. Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID‐19 patients in Northeast Chongqing. J Med Virol. 2020;92:797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang F, Yang JL, Liu BF, Xu L, Zhao GX, Yang YJ. Clinical characteristics of 29 patients with COVID‐19 in Ningxia city. Ningxia Med J. 2020;42:263‐365. [Google Scholar]
- 40. Wang L, Duan Y, Zhang W, et al. Epidemiologic and Clinical characteristics of 26 cases of COVID‐19 arising from patient‐to‐patient transmission in Liaocheng, China. Clin Epidemiol. 2020;12:387‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang R, Pan M, Zhang X, et al. Epidemiological and clinical features of 125 hospitalized patients with COVID‐19 in Fuyang, Anhui, China. Int J Infect Dis. 2020;95:421‐428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;e200994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu J, Liu J, Zhao X, et al. Clinical characteristics of imported cases of COVID‐19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Xu X, Yu C, Qu J, et al. Imaging and clinical features of patients with 2019 novel coronavirus SARS‐CoV‐2. Eur J Nucl Med Mol Imaging. 2020;47:1275‐1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID‐19):a multi‐center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80:388‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhang G, Hu C, Luo L, et al. Clinical features and short‐term outcomes of 221 patients with COVID‐19 in Wuhan, China. J Clin Virol. 2020;127:104364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang J, Wang X, Jia X, et al. Risk factors for disease severity, unimprovement, and mortality in COVID‐19 patients in Wuhan, China. Clinical microbiology and infection: the official publication of the. Eur Soc Clin Microbiol Infect Dis. 2020;26:767‐772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730‐1741. [DOI] [PubMed] [Google Scholar]
- 50. Zhang W, Hou W, Li TZ, et al. Clinical characteristics of 74 hospitalized patients with COVID‐19. J Cap Med Univ. 2020;41:160‐167. [Google Scholar]
- 51. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi [Chin J Oncol]. 2019;41:19‐28. [DOI] [PubMed] [Google Scholar]
- 53. Desai A, Sachdeva S, Parekh T, Desai R. COVID‐19 and cancer: lessons from a pooled meta‐analysis. JCO Global Oncol. 2020;6(6):557‐559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun (Lond). 2019;39:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tuo JY, Zhang M, Chang J, et al. Cancer incidence and mortality in cancer registries in Hubei Province, 2012. Cancer Res Prev Treat. 2016;43:974‐979. [Google Scholar]
- 56. Zheng RS, Zhang SW, Wu L, et al. Report of incidence and mortality from China cancer registries in 2008. China Cancer. 2012;21:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liang WH, Guan WJ, Li CC, et al. Clinical characteristics and outcomes of hospitalised patients with COVID‐19 treated in Hubei (epicenter) and outside Hubei (non‐epicenter): a Nationwide analysis of China. Eur Respir J. 2020;55:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID‐19: evidence from meta‐analysis. Aging. 2020;12:6049‐6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shaw AC, Goldstein DR, Montgomery RR. Age‐dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13:875‐887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xia Y, Jin R, Zhao J, Li W, Shen H. Risk of COVID‐19 for patients with cancer. Lancet Oncol. 2020;21:e180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331:1565‐1570. [DOI] [PubMed] [Google Scholar]
- 63. Sereno M, Gutiérrez‐Gutiérrez G, Sandoval C, et al. A favorable outcome of pneumonia COVID 19 in an advanced lung cancer patient with severe neutropenia: is immunosuppression a risk factor for SARS‐COV2 infection? Lung Cancer (Amsterdam, Netherlands). 2020;145:213‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Figures.
Data Availability Statement
Only publicly available data were used in our study, and data sources and handling of these data are described in Table 1 and in the Materials and Methods, respectively. Further details are available from the corresponding author upon request.


