Abstract
Hematopoietic cell transplantation (HCT) is a well-established treatment to control and/or cure many malignant and non-malignant diseases involving the hematopoietic system and some solid tumors. We report information about HCT procedures performed in the United States (US) in 2018 and analyze trends and outcomes of HCT as reported to the Center for International Blood and Marrow Transplant Research® (CIBMTR®). Overall, compared to 2017, the numbers of allogeneic transplant in the US increased by 1% and numbers of autologous transplants decreased by 5%. Key findings are fewer autologous transplants performed for non-Hodgkin lymphoma and increasing numbers of haploidentical transplants, nearly all of which use post-transplant cyclophosphamide for graft-versus-host disease prophylaxis. There is a continuing increase in transplantations in adults older than 70 years, particularly for acute myeloid leukemia and myelodysplastic syndromes. Survival rates by disease, disease stage, donor type and age are presented. This report, prepared annually by the CIBMTR, provides a snapshot of current transplant activity in the US.
Introduction
Hematopoietic cell transplantation (HCT) is an established therapy to control and/or cure many malignant and non-malignant hematologic diseases, congenital and acquired diseases of the immune system, some solid tumors, and some inherited disorders of metabolism.1 The Center for International Blood and Marrow Transplant Research® (CIBMTR®) database receives data reported by more than 550 centers in 56 countries worldwide, including 207 US active and follow up centers. More than 254,663 autologous, 251,053 allogeneic related and unrelated donor and 15,057 cord blood transplant procedures have been reported through 2018.
The Stem Cell Therapeutic and Research Act (Stem Cell Act) of 2005 (renewed 2010 and 2015) established a requirement to collect data for all allogeneic HCTs performed in the US for a national Stem Cell Therapeutic Outcomes Database (SCTOD). The CIBMTR holds the contract for the SCTOD. Autologous procedures in the U.S. are still reported on a voluntary basis but, currently, CIBMTR captures data for > 85% of US autologous transplants. Outcome data are prospectively collected. In this paper, we report and discuss current trends and outcomes of HCT as reported to the CIBMTR. While the focus is on HCT activity performed in the US in 2018, the trends and outcomes represent data reported from all countries.
Methods
Data collection:
Transplant centers submit data electronically to the CIBMTR into a web-based electronic data collection system called FormsNet. The CIBMTR assigns patients to either a Transplant Essential Data (TED) track, which collects core (“essential”) data, or a Comprehensive Report Form (CRF) track that captures detailed disease- and treatment-related data. Assignment to each track is done upon submission of the initial pretransplant TED form and uses a weighted randomization algorithm designed to produce a cohort that is representative of current practice but with adequate numbers of patients transplanted for rare conditions or with emerging transplant strategies. Data are collected at specific timepoints, including pre-transplant, 100 days, 6 months, one year, then annually six years posttransplant and then biannually until death. Repeat cellular infusions from the same donor and second transplants are also captured. The FormsNet system enforces allowable data and performs simple logic checking. Further quality checks are performed after data receipt using both computerized and manual inspection. Centers are audited on-site once within a four-year audit cycle where data submitted to CIBMTR are compared to source documents. Discrepancies are reviewed and centers may be required to submit a corrective action plan following audit. The current paper provides US transplant activity and outcomes, as of 2018. A slide set depicting the data summarized in this paper and additional details of HCT use and outcomes is available online at https://www.cibmtr.org.
Statistics:
Total transplant numbers are estimated based on data reported to CIBMTR in either reporting track. Estimates of overall and disease-specific autologous transplant activity are based on an assumption of 85% capture of autologous transplant data. Overall survival probabilities are presented according to disease, disease status, donor type, recipient age and conditioning regimen intensity. Estimates of survival and comparisons across survival curves are univariate and are not adjusted for potentially important contributing factors. Causes of death are reported by centers. Trends in total numbers of transplants conducted in the US from 2000 to 2018 were analyzed. Survival trends and cause of death represent data reported from the US and other countries. Survival analyses using Kaplan Meier estimators are performed and reported at a three-year time point with 95% confidence intervals.
Results
Transplant Activity in the United States in 2018
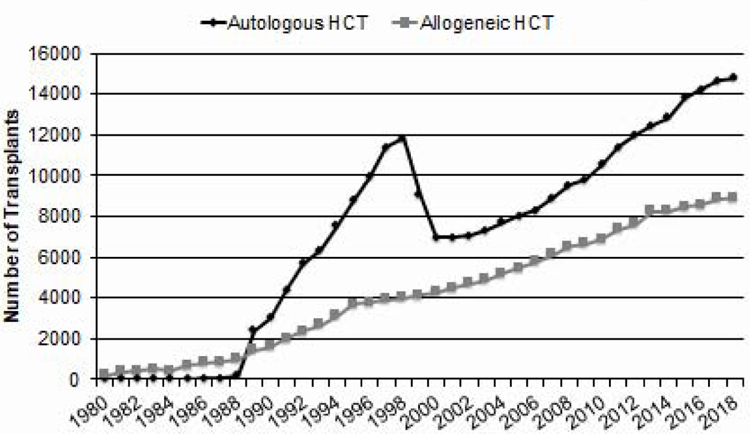
Figure 1 shows the estimated annual number of transplants in the US by year. In 2018, there were an estimated 13,924 autologous, 2,371 HLA-matched related donor, 4,309 unrelated adult donor, 1,503 haploidentical (defined as relative with 2 or more antigen mismatch) and 466 cord blood transplants performed in the US. Among adults (age >18 years), there were more autologous HCT (N=14,173) than allogeneic (N=7,543) HCTs. Among all autologous HCTs performed, 691 were second or subsequent transplants.
Figure 1.
Annual Number of HCT Recipients in the US by Transplant Type
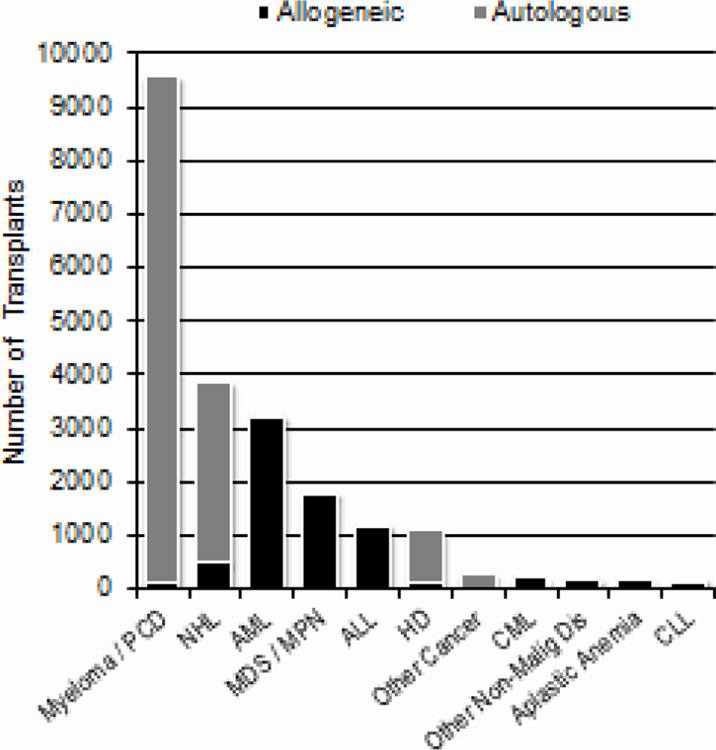
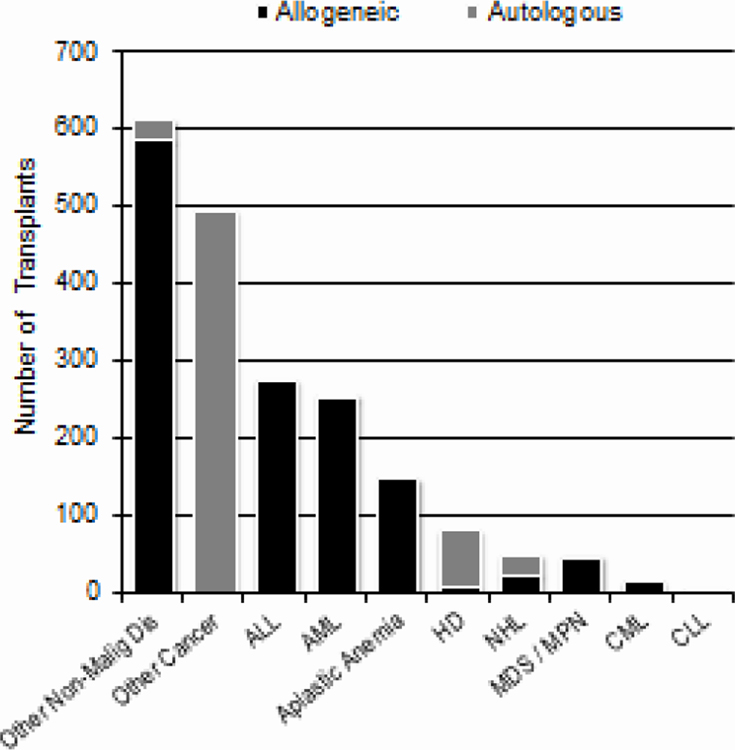
Figure 2 shows the number of transplants done in the US in 2018 (N=23,706) for various indications among adults (2A) and children (2B). Among 13,376 adults receiving autologous HCTs, there were 8,924 (67%) for multiple myeloma and related plasma cell disorders and 3,167 (24%) for non-Hodgkin lymphoma. Among 7,543 adults receiving allogeneic HCTs, there were 3,177 (42%) for acute myeloid leukemia (AML), 1,754 (23%) for myelodysplastic syndromes (MDS)/myeloproliferative neoplasms (MPN) and 1,154 (15%) for acute lymphoblastic leukemia (ALL). Among 586 children receiving autologous HCTs, 466 (80%) were for solid tumors, 70 (12%) for Hodgkin lymphoma, and 25 (4%) for non-Hodgkin lymphoma. Among 1,351 children receiving allogeneic HCTs, 586 (43%) were for non-malignant disorders and 527 (39%) were for acute leukemia.
Figure 2a.
Indications for HCT in the US, 2018 (Adult)
Trends in HCT utilization
Autologous HCT:
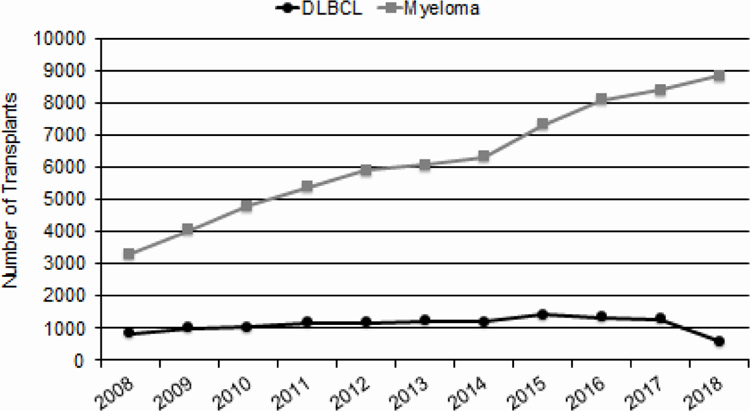
There was a decline in the number of autologous HCTs in 2018 (Figure 1). This appears to be related to a decrease in the number of autologous HCTs performed for diffuse large B cell lymphomas in 2018 compared to prior years (Figure 3). However, the total number of autologous is relatively stable over the last 4 years and is still greater than the number of allogeneic HCTs, accounting for 65% of all HCTs in the US.
Figure 3.
Myeloma and DLBCL Trends for Autologous HCT in the US
Age:
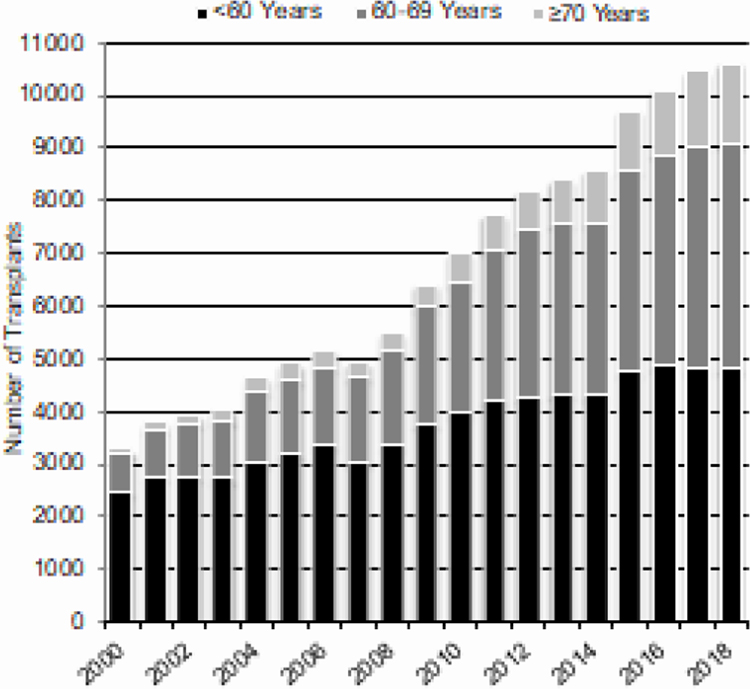
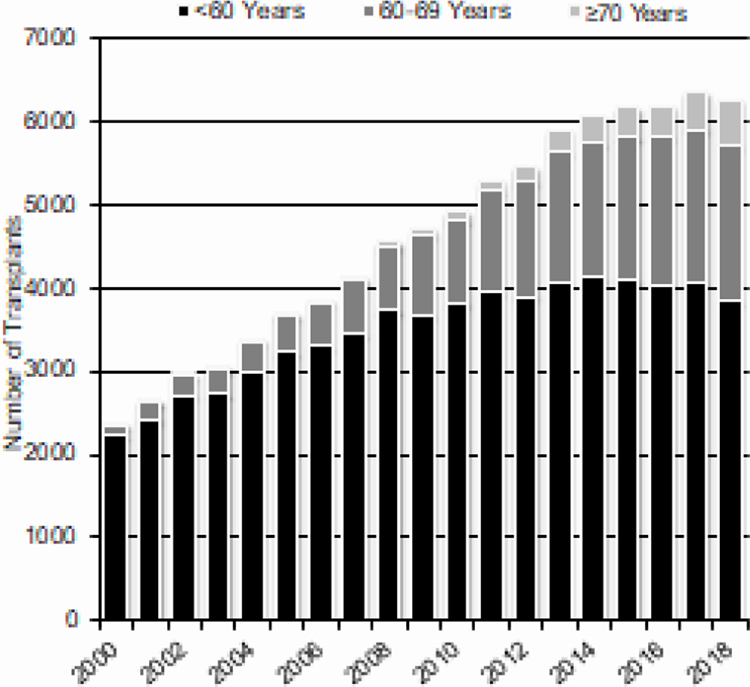
Increasing numbers of older adults undergo autologous and allogeneic HCT. Figure 4A (autologous HCT) and 4B (allogeneic HCT) shows increasing numbers of HCT in patients 70 years and older over time. The numbers of adults younger than 60 years who received allogeneic or autologous HCTS is stable since 2015.
Figure 4a.
Trends in Autologous HCT in the US by Recipient Age^
^Transplants for NHL, Hodgkin Disease and Multiple Myeloma
Race:
The CIBMTR collects self-reported race information. There are increasing numbers of African Americans and Hispanics undergoing autologous HCT in the US. From 2010 to 2018, the number of autologous HCTs for multiple myeloma increased from 2,960 in 2010 to 4,770 in 2018 for Whites (61% increase), from 632 in 2010 to 1,372 in 2018 for African Americans (117% increase) and from 349 in 2010 to 722 in 2018 for Hispanics (107% increase). These are greater increases than seen for overall autologous HCTs during the same time frame (40% increase among all ethnic groups). The numbers of allogeneic HCTs increased from 10,577 in 2010 to 11,052 in 2018 with, again a more striking increase in African Americans. The number of allogeneic HCTs for all indications increased from 6,306 in 2010 to 6,748 in 2018 for Whites (7% increase), from 520 in 2010 to 846 in 2018 for African Americans (63% increase) and from 1,031 in 2010 to 1,251 in 2018 for Hispanics (21% increase). This is in part due to the increasing use of haploidentical transplants, which increased from 347 in 2010 to 1,444 in 2018 (316% increase); about 20% of haploidentical HCTs are in African Americans.
Changing Donor Types for Allogeneic HCT
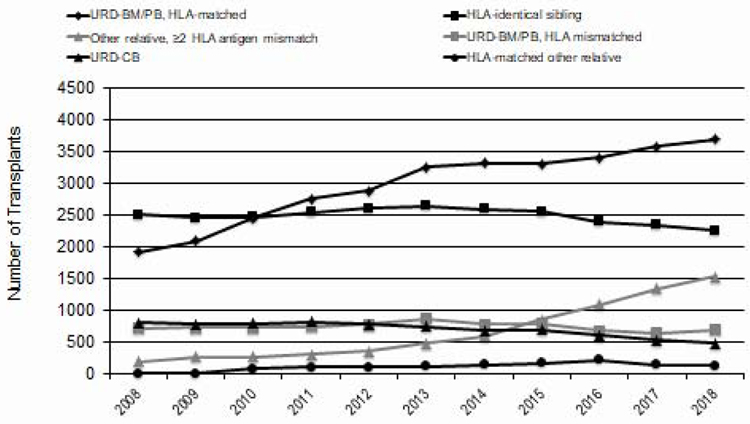
Figure 5 shows the trends in the distribution of graft sources for allogeneic HCT in the US over time. As noted above, the most striking change is in the use of haploidentical related donors. Most (80%) of these used post-transplant cyclophosphamide for graft-versus-host disease (GVHD) prophylaxis. Since 2015, these was a slight decline in the number of HLA-matched related transplants. Unrelated donor HCTs, which seemed to plateau in 2012–2016, increased in 2017 and 2018. Numbers of cord blood transplants declined from a high of 816 in 2011 to about 500 in 2018.
Figure 5.
Allogeneic HCT Recipients in the US, by Donor Type
Survival Rates:
The 100-day survival rate after autologous HCT in 2018 was 98% (95% Confidence Interval [CI], 98–99%). Disease relapse is the most common cause of early death after autologous HCT, accounting for 40% of deaths, followed by infection (18%), organ failure (22%), and other causes (20%). After day 100, disease relapse accounted for 80% of deaths, followed by organ failure (6%), infection (5%), second cancers (1%), and other causes (8%).
The 100-day survival rate after allogeneic HCT in 2018 was 92% (95% CI, 92–93%); 94% (95% CI, 94–95%) with matched related donors, 92% (95% CI, 91–92%) with unrelated donors, 91% (95% CI, 89–92%) with haploidentical donors, and 89% (95% CI, 86–92%) with cord blood grafts. Among allogeneic HCT recipients who died within 100 days, relapse was the most common cause of death (27%), followed by organ failure (25%), infection (20%), GVHD (10%), hemorrhage (5%), graft rejection (2%) and other causes (9%). After day 100, the most common cause of death was relapse (61%) followed by infection (11%), organ failure (10%), GVHD (9%), hemorrhage (1%), graft failure (1%), second malignancy (1%) and other causes (6%). Table 1 shows the 3-year survival rates in children and adults with various diseases treated with autologous and allogeneic HCT.
Table 1A.
Three-year survival probabilities with 95% confidence intervals for myeloid and lymphoid malignancies, and severe aplastic anemia, 2008–2018.
| Matched related donor | Unrelated donor | ||||||
|---|---|---|---|---|---|---|---|
| Early | Intermediate | Advanced | Early | Intermediate | Advanced | ||
| AML | Pediatric | 70 (67–74)% | 66 (59–72)% | 30 (22–39)% | 63 (59–67)% | 60 (55–65)% | 32 (26–39)% |
| Adult | 58 (57–59)% | 51 (49–54)% | 29 (27–31)% | 54 (53–55)% | 49 (47–51)% | 29 (27–30)% | |
| ALL | Pediatric | 76 (72–79)% | 62 (58–66)% | 48 (37–60)% | 72 (68–76)% | 60 (57–64)% | 55 (44–65)% |
| Adult | 62 (60–64)% | 40 (37–44)% | 30 (25–35)% | 61 (59–63)% | 40 (37–43)% | 31 (26–36)% | |
| MDS | Early | Advanced | Early | Advanced | |||
| 51 (48–54)% | 46 (43–48)% | 49 (47–52)% | 43 (41–45)% | ||||
| Myelofibrosis | 63 (59–67)% | 55 (52–59)% | |||||
| Other MPN | 52 (49–56)% | 50 (47–53)% | |||||
| CLL | 61 (58–65)% | 55 (52–57)% | |||||
| CML | Chronic | Accelerated | Blast | Chronic | Accelerated | Blast | |
| 66 (61–70)% | 65 (60–70)% | 33 (23–43)% | 62 (58–66)% | 59 (54–63)% | 39 (30–49)% | ||
| SAA | Pediatric | 93 (91–94)% | 84 (81–86)% | ||||
| Adult | 80 (78–82)% | 72 (69–75)% | |||||
Abbreviations: AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasms; CLL, chronic lymphoid leukemia; CML, chronic myeloid leukemia; SAA, severe aplastic anemia
Discussion
This report which summarizes transplant activity in the US in 2018 shows that the annual numbers of HCTs performed in the US continues to increase though we see a decline in autologous transplant activity for lymphomas. The latter may be due to the availability of several FDA-approved therapies including newer drugs e.g. Bruton tyrosine kinase (BTK) inhibitors, phosphatidylinositol-3-kinase (PI3K) inhibitors, antibodies, immunotoxins, in addition to approved cellular therapies in 2017 and 2018 for relapsed diffuse large B cell lymphoma and other non-Hodgkin lymphoma subtypes.2–4 Despite this drop, autologous HCT remains the predominant transplant type in adults accounting for approximately 65% of all transplants. This is mainly driven by autologous HCT performed for multiple myeloma as upfront and salvage treatment. With several new treatment approaches, including cellular therapies, in development for multiple myeloma, use of autologous HCT may continue to evolve over the next several years as more targeted therapies and cellular therapies are approved.
Transplant activity in older adults continues to increase for autologous and allogeneic HCT, in particular among patients 70 years and older.5,6 Given that the most common indication for autologous HCT in the US is multiple myeloma, a disease with higher incidence in African Americans, the historically low transplantation rates in African Americans have been surprising.7 Our data suggest that this disparity is being addressed somewhat, as transplant activity in multiple myeloma is increasing in minorities at a greater rate than among Whites. In allogeneic HCT, utilization of haploidentical transplants is increasing the number of minorities being transplants, with about 21.2% of such transplants being done in African Americans as compared to 4.6% of transplants using unrelated donors.
Increased use of haploidentical donors accounts for most of the increase in use of allogeneic HCTs overall. We also observed an increase in the use of unrelated donors after plateauing numbers over many years. Future activity among unrelated donor transplants may well increase if the post-transplant cyclophosphamide platform for GVHD prophylaxis, pioneered in haploidentical HCTs, also allows the use of unrelated donors with some degree of HLA mismatch. There was a slight decrease in use of HLA-identical sibling donors. Given that more patients have available haploidentical siblings than identical siblings, the former become may become the most common type of related donor.
Survival rates within 100 days of transplant in 2018 showed that autologous HCT is a safe therapy with 2% early mortality. After allogeneic HCT, day 100 mortality rates depend on donor type and ranges between 6% (matched related donor) and 11% (cord blood transplant). The most common cause of mortality, early and late, is relapse after both autologous and allogeneic HCT. Long-term survival rates depend on many factors, including age, disease, disease stage and donor type, though differences among donors types are relative small.
Conclusions
In addition to investigating outcomes and identifying factors associated with outcomes, CIBMTR data provide valuable information about the overall activity and aggregate success rates of HCT as practiced in the US. CIBMTR data on outcomes for various diseases can also be used to inform the baseline success rates against which new approaches can be compared. These data reveal considerations for the evolving risk-balance assessments of the hematology/oncology and transplant communities between improvements in non-transplant therapies and HCT (e.g. imatinib in chronic myeloid leukemia, monoclonal antibodies, BTK inhibitors, PI3K inhibitors, small molecule inhibitors in chronic lymphocytic leukemia, gene therapies for immunodeficiencies and hemoglobinopathies). To address the recent development, growth, and approvals of non-HCT cellular therapies for some hematologic malignancies, the CIBMTR launched the National Cancer Institute-sponsored Cellular Immunotherapy Data Resource (CIDR) which is now actively capturing and tracking outcomes of recipients of these novel therapies for the treatment of cancer. As the CIDR develops, addition of information on cellular therapies will further enhance the understanding of outcomes of patients receiving both transplant and non-transplant cellular therapies, and the impact of introduction of cellular therapy as standard of care on transplant practices.
The CIBMTR is committed to collecting and disseminating high quality data to improve outcomes in patients undergoing HCT. This annual report is intended to present the “current state of HCT” activity in the US so that investigators, clinicians, patients, and payors have access to accurate and timely data.
Figure 2b.
Indications for HCT in the US, 2018 (Pediatric)
Figure 4b.
Trends in Allogeneic HCT in the US by Recipient Age^^
^^Transplants for AML, ALL, MDS, NHL, Hodgkin Disease, Multiple Myeloma
Table 1B.
Three-year survival probabilities and 95% confidence intervals for common lymphomas, 2008–2018.
| Autologous HCT | Allogeneic HCT | |||||
|---|---|---|---|---|---|---|
| Chemo-sensitive | Chemo-resistant | Chemo-sensitive | Chemo-resistant | |||
| MRD | URD | MRD | URD | |||
| HL | 86 (85–87)% | 74 (70–77)% | 66 (61–70)% | 62 (59–66)% | 47 (37–56)% | 53 (44–61)% |
| FL | 81 (79–83)% | 67 (59–75)% | 76 (72–79)% | 68 (64–71)% | 55 (44–65)% | 53 (43–63)% |
| DLBCL | 67 (66–68)% | 48 (45–52)% | 55 (52–59)% | 50 (47–54)% | 30 (23–36)% | 26 (21–32)% |
Abbreviations: HCT, hematopoietic cell transplantation; HL, Hodgkin lymphoma; FL, follicular lymphoma; DLBCL, diffuse large B cell lymphoma
Table 1C.
Three-year survival probabilities and 95% confidence intervals for multiple myeloma and AL amyloidosis, 2008–2018.
| First autologous HCT | Salvage autologous HCT | |
|---|---|---|
| Multiple myeloma | 76 (76–76)% | 58 (56–60)% |
| AL amyloidosis | 80 (79–81)% | Too few to analyze |
Abbreviation: HCT, hematopoietic cell transplantation; AL, amyloid light chain
Highlights.
The paper summarizes hematopoietic cell transplant activity in the United States in 2018.
Key findings include fewer autologous transplants performed for non-Hodgkin lymphoma and increasing numbers of haploidentical transplants.
There is a continuing increase in transplant activity in adults older than 70 years.
Acknowledgements
The coauthors acknowledge CIBMTR teams of Statistical Operations, Data Operations, Information Technology, and Biostatistical Support who contribute to the collection and use of quality data. We also acknowledge the Data Management teams from all reporting centers.
The CIBMTR is supported primarily by Public Health Service grant/cooperative agreement U24CA076518 with the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); grant/cooperative agreement U24HL138660 with NHLBI and NCI; grant U24CA233032 from the NCI; grants OT3HL147741, R21HL140314, K23HL141445 and U01HL128568 from the NHLBI; contract HHSH250201700006C with Health Resources and Services Administration (HRSA); grants N00014–18-1–2888, N00014–17-1–2850 and N00014–20-1–2705 from the Office of Naval Research; BARDA HHS0100201800012C; subaward from prime contract award SC1MC31881–01-00 with HRSA; subawards from prime grant awards R01HL131731 and R01HL126589 from NHLBI; subaward from prime grant award 5R01CA2151343 from NIH Cancer Institute. P01CA111412, R01CA152108, R01CA218285; R01CA231141, R01HL126589, R01HL129472, R01HL131731 and U01AI126612 from the NIH, and corporate funds from; AbbVie; Actinium Pharmaceuticals, Inc.; Adaptive Biotechnologies; Adienne SA; Allovir, Inc.; Amgen, Inc.; Anthem, Inc.; Astellas Pharma US; Atara Biotherapeutics, Inc.; bluebird bio, Inc.; Bristol Myers Squibb Co.; Celgene Corp.; Chimerix, Inc.; CSL Behring; CytoSen Therapeutics, Inc.; Daiichi Sankyo Co., Ltd.; Dana Farber Cancer Institute; Enterprise Science and Computing, Inc.; Gamida-Cell, Ltd.; Genzyme; GlaxoSmithKline (GSK); HistoGenetics, Inc.; Immune Deficiency Foundation; Incyte Corporation; Janssen Biotech, Inc.; Janssen Pharmaceuticals, Inc.; Janssen Research & Development, LLC; Jazz Pharmaceuticals, Inc.; Kiadis Pharma; Kite, a Gilead Company; Kyowa Kirin; Magenta Therapeutics; Medac GmbH; Merck & Company, Inc.; Merck Sharp & Dohme Corp.; Mesoblast; Millennium, the Takeda Oncology Co.; Miltenyi Biotec, Inc.; Montefiore Medical Center; Novartis Oncology; Novartis Pharmaceuticals Corporation; Omeros Corporation; Oncoimmune, Inc.; Orca Biosystems, Inc.; Pfizer, Inc.; Phamacyclics, LLC; Regeneron Pharmaceuticals, Inc.; REGiMMUNE Corp.; Sanofi Genzyme; Seattle Genetics; Shire; Sobi, Inc.; Takeda Oncology; Takeda Pharma; Viracor Eurofins; Xenikos BV and; non-profit funds from; Be the Match Foundation; National Marrow Donor Program; St. Baldrick’s Foundation; The Medical College of Wisconsin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kanate AS, Majhail NS, Savani BN, et al. : Indications for Hematopoietic Cell Transplantation and Immune Effector Cell Therapy: Guidelines from the American Society for Transplantation and Cellular Therapy: Guidelines for Hematopoietic Transplantation and Cellular Therapy. Biol Blood Marrow Transplant, 2020 [DOI] [PubMed]
- 2.https://www.cancer.gov/news-events/cancer-currents-blog/2018/tisagenlecleucel-fda-lymphoma, 2018
- 3.https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-axicabtagene-ciloleucel-large-b-cell-lymphoma, 2017
- 4.Jain T, Bar M, Kansagra AJ, et al. : Use of Chimeric Antigen Receptor T Cell Therapy in Clinical Practice for Relapsed/Refractory Aggressive B Cell Non-Hodgkin Lymphoma: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant 25:2305–2321, 2019 [DOI] [PubMed] [Google Scholar]
- 5.Muffly L, Pasquini MC, Martens M, et al. : Increasing use of allogeneic hematopoietic cell transplantation in patients aged 70 years and older in the United States. Blood 130:1156–1164, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Munshi PN, Hari P, Vesole D, et al. : Breaking the Glass Ceiling of Age in Transplant in Multiple Myeloma Blood 134:782, 2019 [Google Scholar]
- 7.Schriber JR, Hari PN, Ahn KW, et al. : Hispanics have the lowest stem cell transplant utilization rate for autologous hematopoietic cell transplantation for multiple myeloma in the United States: A CIBMTR report. Cancer 123:3141–3149, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]