Abstract
Background: Palliative care (PC) and hospice care are underutilized for patients with end-stage liver disease, but factors associated with these patterns of utilization are not well understood.
Objective: We examined patient-level factors associated with both PC and hospice referrals in patients with decompensated cirrhosis (DC).
Design: Retrospective cohort study.
Setting/Subjects: Patients with DC hospitalized at a single tertiary center and followed for one year.
Measurements: We assessed PC and hospice referrals during follow-up and examined patient-level factors associated with the receipt of PC and/or hospice, as well as associated clinical outcomes. We also examined late referrals (within one week of death).
Results: Of 397 patients, 61 (15.4%) were referred to PC, 71 (17.9%) were referred to hospice, and 99 (24.9%) were referred to PC and/or hospice. Two hundred patients (50.4%) died during the one-year follow-up. In multivariable logistic regression, referral to PC was associated with increased comorbidity burden, ascites, increased MELD (Model for End-Stage Liver Disease)-Na score, lack of listing for liver transplant, and unmarried status. Hospice referral was associated with increased comorbidities, portal vein thrombosis, and hepatocellular carcinoma. PC referrals were late in 68.5% of cases, and hospice referrals were late in 62.7%. Late PC referrals were associated with younger age and married status. Late hospice referrals were associated with younger age and recent alcohol use.
Conclusions: PC and hospice is underutilized in patients with DC, and most referrals are late. Patient-level factors associated with these referrals differ between PC and hospice.
Keywords: hospice, liver cirrhosis, palliative care
Introduction
Liver cirrhosis represents the end-stage of hepatic fibrosis, caused by various chronic liver diseases. Patients with cirrhosis are at risk for multiple complications (e.g., ascites, hepatic encephalopathy [HE], variceal bleeding). Once these symptoms develop, patients are considered to have decompensated cirrhosis (DC). Patients with DC have high rates of mortality.1 They also have poor quality of life,2–4 high rates of health care utilization,5,6 and high symptom burden.7,8
The high rates of morbidity and mortality in DC underscore the need for palliative care (PC) services to address the physical, spiritual, and psychosocial aspects of patients' wellbeing and for hospice to provide end-of-life support for patients and families.9–11 Despite this need, however, utilization of both PC and hospice for patients with DC remains low.12,13 To improve the appropriate utilization of PC and hospice, it is important to understand the patient factors that have driven these referrals historically. In recent years, several studies have examined characteristics associated with PC and hospice referrals, but these studies have been limited by small samples of only patients who died,7,14 a focus on patients with hepatocellular carcinoma (HCC) only,15–17 exclusion of patients on the basis of transplant eligibility,11,13 and a focus on only either PC or hospice (or a lack of distinction between the two).12,18–21 Thus, there remains a gap in the literature with regard to referrals to both PC and hospice among patients with DC.
To address this gap, we performed a cohort study of patients admitted to the hospital with DC. We sought to identify patient-level factors associated with both PC and hospice referrals. To examine the broad range of patients with DC, we included all patients without regard to the presence of HCC or transplant candidacy. We also examined both PC and hospice separately to delineate differences between them.
Methods
Study design and patients
We performed a retrospective cohort study of patients with DC admitted to Indiana University Hospital between January 1, 2012 and December 31, 2012. Indiana University Hospital is a tertiary referral center that is the home of the state's only liver transplant program. Patients were followed for one year from the admission date of their first hospitalization during 2012. Patients were identified through screening of the electronic medical record for diagnostic codes for cirrhosis that have been previously validated.22 We then confirmed the diagnosis of cirrhosis through manual chart review based on clinical, laboratory, histologic, and radiologic features. DC was defined based on the presence of ascites, HE, or history of variceal bleeding.1 We excluded patients <18 years of age, with a prior liver transplant, admitted electively for liver transplant surgery, or lost to follow-up within one year of admission. The study was approved by the Indiana University Institutional Review Board.
Outcomes
The primary outcomes of interest were referral to PC or hospice at any point during the admission or follow-up. PC consults included multidisciplinary inpatient services staffed by a physician, nurse practitioner, social worker, and chaplain. Secondary outcomes included time spent in the hospital (cumulative days across admissions during follow-up, including the index admission), medical interventions (admission to intensive care, use of vasopressors, mechanical ventilation, cardiopulmonary resuscitation, and renal replacement therapy), limitations to code status (“do not resuscitate,” “do not intubate,” or both), liver transplant, and death. Medical interventions were specified as binary variables and were identified if they occurred at any point during follow-up (e.g., patients requiring mechanical ventilation for any duration). We also examined late referrals to PC and to hospice care, defined as occurring within one week of death.23,24 Outcomes were identified at any time during the one-year follow-up.
Variables
We collected patient characteristics at the time of admission that could impact outcomes. We recorded age, sex, race, marital status, social support (defined as living alone vs. living with others), alcohol and substance abuse (active use in preceding six months), health insurance, body mass index, and reason for hospital admission. Comorbidities of interest included psychiatric disease (any psychiatric diagnosis listed in the clinical notes) and acute kidney injury during the initial admission,25 as well as the Charlson Comorbidity Index (CCI).26 Liver disease categories were excluded from the CCI to avoid “double counting” the cirrhosis complications.27 Liver-specific variables included liver disease etiology, liver disease complications (ascites, HE, hepatic hydrothorax, portal vein thrombosis, HCC), and liver disease severity (Model for End-Stage Liver Disease (MELD)-Na and Child–Pugh scores).28,29 HCC was classified as being within or outside the Milan criteria for transplant eligibility.30
Statistical analyses
Categorical variables were specified as counts and percentages, and continuous variables were specified using mean and standard deviations when normally distributed, and as medians and ranges otherwise. Comparisons between categorical variables were performed with the Pearson χ2 test or Fisher's exact test where appropriate, and comparisons between continuous variables were made using Student's t-test for normally distributed variables or the Wilcoxon rank-sum test otherwise. We used logistic regression to examine factors associated with referrals to PC or hospice. Those factors associated with the outcomes with a p-value <0.2 in univariate analysis were included in the multivariable models, and the likelihood ratio test was used to eliminate nonsignificant variables. Final models consisted of variables that remained statistically significant after adjustment for other variables in the model. Variables were examined for collinearity before inclusion in the models. Two-sided tests were used, and were considered statistically significant when p-values were <0.05. Analyses were performed using STATA version 15 (College Station, TX).
Results
Patients
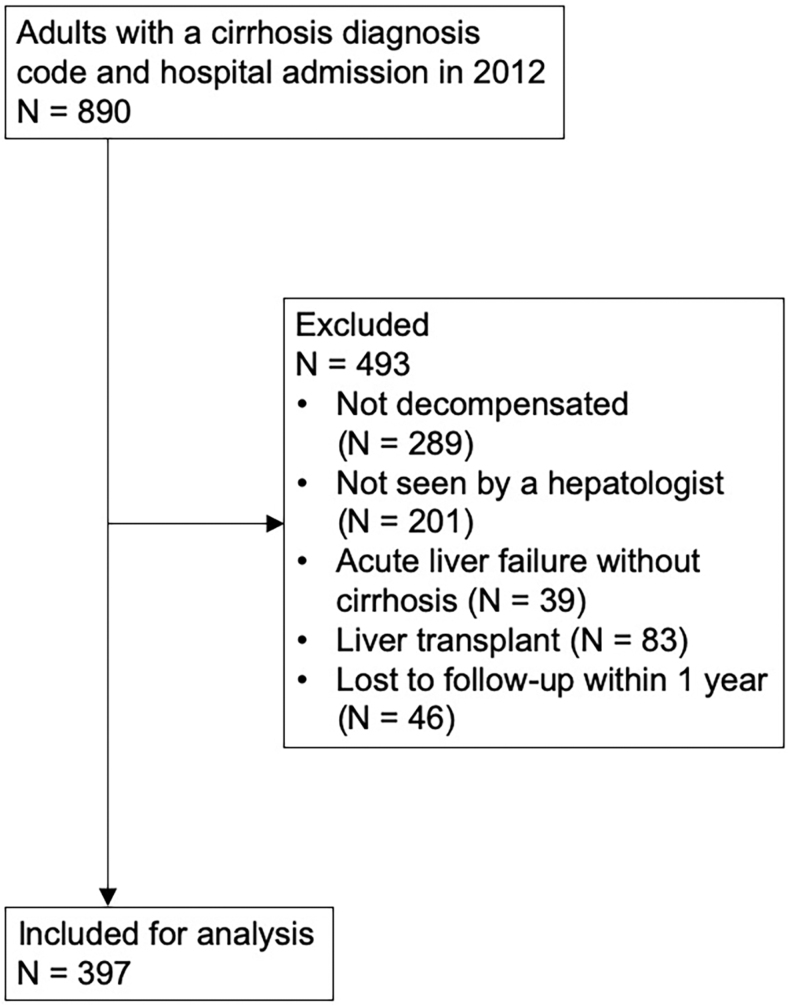
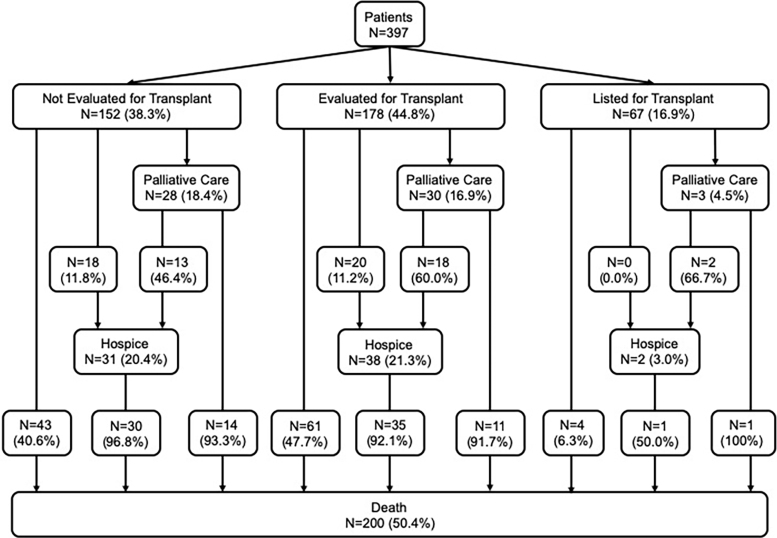
Eight hundred ninety patients with a code for cirrhosis were admitted to the hospital during the study period, of which 493 were excluded, leaving 397 patients for analysis (Fig. 1). The most common reasons for initial hospital admission were gastrointestinal bleeding (20.4%), HE (19.6%), acute kidney injury (12.3%), and ascites (12.1%). One hundred fifty-two patients (38.3%) were not evaluated for liver transplant, 178 (44.8%) were evaluated but not listed, and 67 (16.9%) were listed for transplant. Sixty-one patients (15.4%) were referred to PC after a median of 18 days (IQR 11–58 days), and 71 (17.9%) were referred to hospice after a median of 28 days (IQR 11–93 days). Thirty-three patients were referred to both PC and hospice (54.1% of patients who saw PC were later referred to hospice, and 46.5% of patients referred to hospice first saw PC). Ninety-nine patients were referred to either PC and/or hospice. Nearly all patients receiving PC and/or hospice were either not evaluated for transplant (46.5%) or were evaluated but not listed (50.5%). Patient flow is shown in Figure 2.
FIG. 1.
Cohort selection. Patients may have had more than one reason for exclusion.
FIG. 2.
Patient referrals to palliative care and hospice based on transplant evaluation status and relationships to mortality.
Characteristics of patients referred to PC or hospice
Baseline characteristics are shown in Table 1. Patients referred to PC and hospice were older than those who were not. Patients referred to PC were less likely to be married and more likely to live alone, but these factors were not associated with hospice referral. Psychiatric comorbidity was comprised predominantly of depression (44.8%), anxiety (16.4%), both depression and anxiety (10.4%), and bipolar disorder (20.9%). Psychiatric comorbidity was less common in those referred to hospice, but was not associated with PC referral. CCI was greater in both those receiving PC or hospice, as was the prevalence of acute kidney injury. Ascites was more common in those receiving PC and/or hospice, but hepatic hydrothorax and HE were similar between groups. Portal vein thrombosis and HCC were strongly associated with hospice referral but not with PC. Liver disease severity as measured by both MELD-Na and Child–Pugh was greater in those receiving either PC and/or hospice.
Table 1.
Characteristics of Patients According to Palliative Care and Hospice Referrals
| Characteristic | Overall (N = 397) | No PC (N = 336) | PC (N = 61) | p | No hospice (N = 326) | Hospice (N = 71) | p |
|---|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 56.8 (9.8) | 56.2 (9.8) | 60.1 (9.7) | 0.005 | 56.0 (9.9) | 60.4 (8.5) | 0.001 |
| Males, % | 62.5 | 61.9 | 65.6 | 0.59 | 62.3 | 63.4 | 0.86 |
| Caucasian race, % | 90.5 | 90.6 | 90.0 | 0.88 | 91.6 | 85.7 | 0.13 |
| Married, % | 53.7 | 56.9 | 36.1 | 0.003 | 55.3 | 46.5 | 0.18 |
| Lives alone, % | 40.3 | 38.3 | 50.8 | 0.07 | 39.8 | 42.3 | 0.70 |
| Alcohol use within 6 months, % | 18.6 | 19.0 | 16.4 | 0.63 | 18.3 | 19.7 | 0.78 |
| Substance use within 6 months, % | 4.8 | 4.5 | 6.6 | 0.51 | 4.6 | 5.6 | 0.76 |
| Psychiatric comorbidity, % | 17.4 | 17.8 | 15.3 | 0.64 | 19.6 | 7.2 | 0.01 |
| Acute kidney injury, % | 46.9 | 42.9 | 68.9 | <0.001 | 44.8 | 56.3 | 0.08 |
| Charlson comorbidity index, mean (SD) | 3.4 (2.5) | 3.3 (2.4) | 4.1 (2.5) | 0.01 | 3.2 (2.3) | 4.6 (2.6) | <0.001 |
| Insurance, % | 0.53 | 0.91 | |||||
| Medicare | 40.3 | 39.9 | 42.6 | 39.6 | 43.7 | ||
| Medicaid | 16.6 | 15.8 | 21.3 | 16.6 | 16.9 | ||
| Private | 35.0 | 36.3 | 27.9 | 35.6 | 32.4 | ||
| Uninsured | 8.1 | 8.0 | 8.2 | 8.3 | 7.0 | ||
| Body mass index, kg/m2, mean (SD) | 30.3 (7.2) | 30.2 (7.2) | 30.7 (7.4) | 0.65 | 30.6 (7.2) | 28.8 (7.1) | 0.09 |
| Liver disease etiology, % | 0.48 | 0.45 | |||||
| Alcohol | 20.9 | 19.6 | 27.9 | 19.9 | 25.4 | ||
| Viral | 24.4 | 25.3 | 19.7 | 23.3 | 29.6 | ||
| Alcohol+Viral | 15.9 | 16.4 | 13.1 | 16.3 | 14.1 | ||
| NASH/cryptogenic | 25.7 | 26.2 | 23.0 | 26.4 | 22.5 | ||
| Other | 13.1 | 12.5 | 16.4 | 14.1 | 8.5 | ||
| Ascites, % | 87.9 | 86.0 | 98.4 | 0.006 | 86.5 | 94.4 | 0.06 |
| Hepatic hydrothorax, % | 15.6 | 15.8 | 14.8 | 0.84 | 15.6 | 15.5 | 0.98 |
| Hepatic encephalopathy, % | 71.7 | 70.1 | 80.3 | 0.10 | 72.0 | 70.4 | 0.79 |
| Portal vein thrombosis, % | 10.1 | 10.4 | 8.3 | 0.62 | 7.7 | 21.4 | 0.001 |
| Hepatocellular carcinoma, % | 15.9 | 15.2 | 19.7 | 0.38 | 12.0 | 33.8 | <0.001 |
| Outside Milan criteria | 43.4 | 40.5 | 54.5 | 0.50 | 27.6 | 62.5 | 0.01 |
| MELD-Na, mean (SD) | 22.8 (7.9) | 22.2 (7.9) | 26.3 (6.9) | <0.001 | 22.5 (8.0) | 24.3 (7.3) | 0.08 |
| Child–Pugh score, mean (SD) | 10.6 (2.0) | 10.4 (2.0) | 11.5 (1.9) | <0.001 | 10.4 (2.0) | 11.2 (2.0) | 0.006 |
| Child–Pugh class, % | 0.007 | 0.07 | |||||
| A | 3.4 | 4.0 | 0 | 4.2 | 0 | ||
| B | 23.8 | 26.2 | 11.5 | 25.2 | 17.4 | ||
| C | 72.8 | 69.8 | 88.5 | 70.6 | 82.6 | ||
| Transplant status, % | 0.02 | <0.001 | |||||
| Not evaluated | 38.3 | 36.9 | 45.9 | 37.1 | 43.7 | ||
| Evaluated, but not listed | 44.8 | 44.0 | 49.2 | 42.9 | 53.5 | ||
| Listed | 16.9 | 19.0 | 4.9 | 19.9 | 2.8 |
MELD, Model for End-Stage Liver Disease; PC, palliative care; SD, standard deviation.
When combining referrals to either PC and/or hospice, factors significantly associated with the outcome included older age, acute kidney injury, CCI, ascites, portal vein thrombosis, HCC, MELD-Na, Child–Pugh score, and transplant evaluation status (Table 2).
Table 2.
Characteristics of Patients Referred to Either Palliative Care and/or Hospice
| Characteristic | All patients |
Patients who died |
||||
|---|---|---|---|---|---|---|
| Neither PC or hospice (N = 298) | PC and/or hospice (N = 99) | p | Neither PC or hospice (N = 108) | PC and/or hospice (N = 92) | p | |
| Age, years, mean (SD) | 55.9 (10.0) | 59.6 (8.7) | <0.001 | 54.7 (10.0) | 59.8 (8.8) | <0.001 |
| Males, % | 61.4 | 65.7 | 0.45 | 63.0 | 66.3 | 0.62 |
| Caucasian race, % | 91.5 | 87.6 | 0.26 | 90.5 | 87.8 | 0.55 |
| Married, % | 56.1 | 46.5 | 0.10 | 52.3 | 47.8 | 0.53 |
| Lives alone, % | 38.5 | 45.5 | 0.22 | 38.7 | 43.5 | 0.49 |
| Alcohol use within 6 months, % | 17.7 | 21.2 | 0.44 | 19.8 | 21.7 | 0.74 |
| Substance use within 6 months, % | 4.1 | 7.1 | 0.28 | 5.7 | 6.5 | 0.80 |
| Psychiatric comorbidity, % | 19.4 | 11.3 | 0.07 | 21.4 | 10.0 | 0.03 |
| Acute kidney injury, % | 42.6 | 59.6 | 0.003 | 63.9 | 62.0 | 0.78 |
| Charlson comorbidity index, mean (SD) | 3.1 (2.4) | 4.3 (2.5) | <0.001 | 3.3 (2.6) | 4.4 (2.6) | 0.004 |
| Insurance, % | 0.92 | 0.71 | ||||
| Medicare | 39.9 | 41.4 | 35.2 | 40.2 | ||
| Medicaid | 16.1 | 18.2 | 22.2 | 17.4 | ||
| Private | 35.9 | 32.3 | 32.4 | 34.8 | ||
| Uninsured | 8.1 | 8.1 | 10.2 | 7.6 | ||
| Body mass index, kg/m2, mean (SD) | 30.3 (7.1) | 30.4 (7.6) | 0.90 | 29.7 (6.6) | 30.4 (7.7) | 0.51 |
| Liver disease etiology, % | 0.82 | 0.53 | ||||
| Alcohol | 19.5 | 25.3 | 21.3 | 27.2 | ||
| Viral | 24.8 | 23.2 | 31.5 | 22.8 | ||
| Alcohol+Viral | 16.1 | 15.2 | 19.4 | 16.3 | ||
| NASH/cryptogenic | 26.2 | 24.2 | 20.4 | 22.8 | ||
| Other | 13.4 | 12.1 | 7.4 | 10.9 | ||
| Ascites, % | 85.5 | 94.9 | 0.01 | 93.5 | 94.6 | 0.74 |
| Hepatic hydrothorax, % | 16.4 | 13.1 | 0.43 | 15.7 | 13.0 | 0.59 |
| Hepatic encephalopathy, % | 71.0 | 73.7 | 0.61 | 76.9 | 73.9 | 0.63 |
| Portal vein thrombosis, % | 8.4 | 15.3 | 0.049 | 8.3 | 16.5 | 0.08 |
| Hepatocellular carcinoma, % | 12.1 | 27.3 | <0.001 | 13.0 | 28.3 | 0.007 |
| Outside Milan criteria | 25.9 | 61.5 | 0.009 | 50.0 | 64.0 | 0.47 |
| MELD-Na, mean (SD) | 22.0 (8.0) | 25.1 (7.0) | <0.001 | 26.1 (7.6) | 25.6 (6.9) | 0.61 |
| Child–Pugh score, mean (SD) | 10.3 (2.0) | 11.3 (2.0) | <0.001 | 11.1 (1.7) | 11.4 (2.0) | 0.31 |
| Child–Pugh class, % | 0.001 | 0.59 | ||||
| A | 4.6 | 0 | 0 | 0 | ||
| B | 27.0 | 14.4 | 17.3 | 14.4 | ||
| C | 68.4 | 85.6 | 82.7 | 85.6 | ||
| Transplant status, % | <0.001 | 0.49 | ||||
| Not evaluated | 35.6 | 46.5 | 39.8 | 47.8 | ||
| Evaluated, but not listed | 43.0 | 50.5 | 56.5 | 50.0 | ||
| Listed | 21.5 | 3.0 | 3.7 | 2.2 | ||
In multivariable logistic regression, PC referral was independently associated with greater CCI, presence of ascites, greater MELD-Na, lack of transplant listing, and unmarried marital status (Table 3). Hospice referral was also independently associated with greater CCI. In contrast, hospice was also only associated with the presence of portal vein thrombosis and HCC, but not with other markers of liver disease severity. The combined outcome of PC and/or hospice was associated with increased comorbidities, HCC, greater MELD-Na, and lack of transplant listing.
Table 3.
Factors Independently Associated with Palliative Care and Hospice Referrals
| All patients | Patients who died | |
|---|---|---|
| PC | ||
| Charlson comorbidity index | 1.12 (1.00–1.25) | |
| Ascites | 8.73 (1.10–69.4) | |
| MELD-Na | 1.06 (1.02–1.11) | |
| Not evaluated for transplant | 4.45 (1.27–15.7) | |
| Evaluated, but not listed | 3.89 (1.12–13.5) | |
| Listed for transplant | Ref. | |
| Married | 0.44 (0.24–0.80) | 0.39 (0.20–0.77) |
| Age | 1.07 (1.03–1.11) | |
| Child–Pugh score | 1.21 (1.01–1.46) | |
| Hospice | ||
| Charlson comorbidity index | 1.18 (1.06–1.32) | 1.13 (0.99–1.29) |
| Portal vein thrombosis | 2.90 (1.36–6.21) | 3.42 (1.33–8.80) |
| Hepatocellular carcinoma | 2.51 (1.29–4.90) | 2.53 (1.11–5.77) |
| PC and/or hospice | ||
| Charlson comorbidity index | 1.13 (1.02–1.26) | 1.18 (1.05–1.32) |
| Hepatocellular carcinoma | 2.36 (1.21–4.62) | |
| MELD-Na | 1.06 (1.02–1.09) | |
| Not evaluated for transplant | 10.1 (2.94–34.8) | |
| Evaluated, but not listed | 8.22 (2.42–28.0) | |
| Listed for transplant | Ref. | |
Data are presented as multivariable odds ratios with 95% confidence intervals.
Characteristics of patients who died
Two hundred patients died within one year (50.4%). Of these, 57 were referred to PC (28.5%), and 66 were referred to hospice (33.0%). Characteristics of these patients are shown in Table 4. Similar to the overall cohort, unmarried patients were more likely to be referred to PC, whereas patients with psychiatric comorbidity were less likely to be referred to hospice. CCI and the proportion with portal vein thrombosis or HCC were also greater among those referred to hospice. In contrast to the overall cohort, MELD-Na and Child–Pugh scores were no different in those referred to either PC or hospice. The combined outcome of PC and/or hospice was associated with only age, psychiatric comorbidity, CCI, portal vein thrombosis, and HCC (Table 2).
Table 4.
Characteristics of Patients Who Died According to Palliative Care and Hospice Referrals
| Characteristic | Overall (N = 200) | No PC (N = 143) | PC (N = 57) | p | No hospice (N = 134) | Hospice (N = 66) | p |
|---|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 57.1 (9.8) | 55.9 (9.5) | 60.1 (9.9) | 0.006 | 55.2 (9.8) | 60.8 (8.6) | <0.001 |
| Males, % | 64.5 | 63.6 | 66.7 | 0.69 | 64.2 | 65.2 | 0.89 |
| Caucasian race, % | 89.2 | 89.2 | 89.3 | 0.99 | 90.8 | 86.2 | 0.33 |
| Married, % | 50.3 | 55.6 | 36.8 | 0.02 | 51.1 | 48.5 | 0.73 |
| Lives alone, % | 40.9 | 36.9 | 50.9 | 0.07 | 41.7 | 39.4 | 0.76 |
| Alcohol use within 6 months, % | 20.7 | 22.0 | 17.5 | 0.49 | 21.2 | 19.7 | 0.80 |
| Substance use within 6 months, % | 6.1 | 5.7 | 7.0 | 0.75 | 6.8 | 4.5 | 0.75 |
| Psychiatric comorbidity, % | 16.1 | 16.7 | 14.5 | 0.72 | 20.9 | 6.3 | 0.009 |
| Acute kidney injury, % | 63.0 | 60.1 | 70.2 | 0.19 | 64.9 | 59.1 | 0.42 |
| Charlson comorbidity index, mean (SD) | 3.8 (2.7) | 3.6 (2.7) | 4.1 (2.5) | 0.27 | 3.3 (2.5) | 4.7 (2.7) | <0.001 |
| Insurance, % | 0.88 | 0.36 | |||||
| Medicare | 37.5 | 37.1 | 38.6 | 34.3 | 43.9 | ||
| Medicaid | 20.0 | 18.9 | 22.8 | 22.4 | 15.2 | ||
| Private | 33.5 | 35.0 | 29.8 | 32.8 | 34.8 | ||
| Uninsured | 9.0 | 9.1 | 8.8 | 10.4 | 6.1 | ||
| Body mass index, kg/m2, mean (SD) | 30.0 (7.1) | 29.8 (7.0) | 30.7 (7.4) | 0.43 | 30.6 (7.0) | 28.8 (7.3) | 0.12 |
| Liver disease etiology, % | 0.26 | 0.88 | |||||
| Alcohol | 24.0 | 21.7 | 29.8 | 22.4 | 27.3 | ||
| Viral | 27.5 | 30.1 | 21.1 | 26.9 | 28.8 | ||
| Alcohol+Viral | 18.0 | 19.6 | 14.0 | 19.4 | 15.2 | ||
| NASH/cryptogenic | 21.5 | 21.7 | 21.1 | 21.6 | 21.2 | ||
| Other | 9.0 | 7.0 | 14.0 | 9.7 | 7.6 | ||
| Ascites, % | 94.0 | 92.3 | 98.2 | 0.19 | 94.0 | 93.9 | 0.99 |
| Hepatic hydrothorax, % | 14.5 | 14.7 | 14.0 | 0.91 | 14.2 | 15.2 | 0.85 |
| Hepatic encephalopathy, % | 75.5 | 74.1 | 78.9 | 0.47 | 77.6 | 71.2 | 0.32 |
| Portal vein thrombosis, % | 12.1 | 13.3 | 8.9 | 0.40 | 6.7 | 23.1 | 0.001 |
| Hepatocellular carcinoma, % | 20.0 | 19.6 | 21.1 | 0.81 | 12.7 | 34.8 | <0.001 |
| Outside Milan criteria | 60.0 | 62.5 | 54.5 | 0.72 | 50.0 | 65.2 | 0.48 |
| MELD-Na, mean (SD) | 25.9 (7.3) | 25.6 (7.5) | 26.6 (6.8) | 0.34 | 26.3 (7.3) | 24.9 (7.2) | 0.21 |
| Child–Pugh score, mean (SD) | 11.2 (1.9) | 11.1 (1.8) | 11.5 (2.0) | 0.15 | 11.3 (1.8) | 11.2 (2.0) | 0.90 |
| Child–Pugh class, % | 0.36 | 0.75 | |||||
| A | 0 | 0 | 0 | 0 | 0 | ||
| B | 16.0 | 17.5 | 12.3 | 15.4 | 17.2 | ||
| C | 84.0 | 82.5 | 87.7 | 84.6 | 82.8 | ||
| Transplant status, % | 0.80 | 0.78 | |||||
| Not evaluated | 43.5 | 42.7 | 45.6 | 42.5 | 45.5 | ||
| Evaluated, but not listed | 53.5 | 54.5 | 50.9 | 53.7 | 53.0 | ||
| Listed | 3.0 | 2.8 | 3.5 | 3.7 | 1.5 |
In multivariable analysis, the only factor associated with PC referral in both the overall cohort and also in those who died was unmarried marital status (Table 3). In the group who died, PC referral was also associated with increased age and Child–Pugh score. Independent associations with hospice referral in those who died were similar to the overall cohort, including CCI, portal vein thrombosis, and HCC. When combining the PC and hospice referrals, only CCI was independently associated with the outcome in those who died.
Patients with late referrals to PC or hospice
Of the 57 who died and were referred to PC, the exact dates of referral and death were available in 54; of the 66 referred to hospice, dates were available in 59. The median time from PC referral to death was 2.5 days (IQR 1–11), and the median time from hospice referral to death was 5 days (IQR 2–12). Referrals to PC were within 1 week of death in 37 (68.5%); referrals to hospice were within 1 week of death in 37 (62.7%). Characteristics associated with these late referrals are shown in Table 5. Late referrals to both PC and hospice were younger, and late PC referrals were more likely to be married. Late hospice referrals were more likely to have used alcohol within six months.
Table 5.
Characteristics of Patients Who Died within One Week of Palliative Care or Hospice Referral
| Characteristic | PC referral >1 week before death (N = 17) | Late PC referral (N = 37) | p | Hospice referral >1 week before death (N = 22) | Late hospice referral (N = 37) | p |
|---|---|---|---|---|---|---|
| Age, years, mean (SD) | 64.1 (10.1) | 58.6 (9.7) | 0.06 | 64.8 (8.2) | 59.1 (8.8) | 0.02 |
| Males, % | 76.5 | 62.2 | 0.30 | 77.3 | 56.8 | 0.11 |
| Caucasian race, % | 82.4 | 91.7 | 0.37 | 85.7 | 83.8 | 0.99 |
| Married, % | 17.6 | 48.6 | 0.03 | 54.5 | 45.9 | 0.52 |
| Lives alone, % | 47.1 | 48.6 | 0.91 | 31.8 | 43.2 | 0.38 |
| Alcohol use within 6 months, % | 17.6 | 16.2 | 0.99 | 4.5 | 27.0 | 0.04 |
| Substance use within 6 months, % | 0 | 10.8 | 0.30 | 4.5 | 5.4 | 0.99 |
| Psychiatric comorbidity, % | 11.8 | 17.1 | 0.99 | 4.5 | 8.6 | 0.99 |
| Acute kidney injury, % | 70.6 | 73.0 | 0.99 | 54.5 | 59.5 | 0.71 |
| Charlson comorbidity index, mean (SD) | 4.8 (2.6) | 3.9 (2.6) | 0.27 | 5.5 (2.0) | 4.6 (3.1) | 0.25 |
| Insurance, % | 0.31 | 0.16 | ||||
| Medicare | 52.9 | 32.4 | 54.5 | 40.5 | ||
| Medicaid | 11.8 | 27.0 | 4.5 | 21.6 | ||
| Private | 23.5 | 35.1 | 40.9 | 29.7 | ||
| Uninsured | 11.8 | 5.4 | 0 | 8.1 | ||
| Body mass index, kg/m2, mean (SD) | 27.5 (6.4) | 32.1 (7.7) | 0.07 | 30.6 (9.3) | 27.7 (5.9) | 0.19 |
| Liver disease etiology, % | 0.95 | 0.71 | ||||
| Alcohol | 35.3 | 29.7 | 27.3 | 29.7 | ||
| Viral | 17.6 | 21.6 | 22.7 | 29.7 | ||
| Alcohol+Viral | 11.8 | 13.5 | 9.1 | 16.2 | ||
| NASH/cryptogenic | 17.6 | 24.3 | 27.3 | 18.9 | ||
| Other | 17.6 | 10.8 | 13.6 | 5.4 | ||
| Ascites, % | 100 | 97.3 | 0.99 | 95.5 | 94.6 | 0.99 |
| Hepatic hydrothorax, % | 17.6 | 10.8 | 0.67 | 18.2 | 13.5 | 0.72 |
| Hepatic encephalopathy, % | 70.6 | 83.8 | 0.29 | 68.2 | 73.0 | 0.69 |
| Portal vein thrombosis, % | 17.6 | 5.6 | 0.31 | 36.4 | 13.9 | 0.06 |
| Hepatocellular carcinoma, % | 23.5 | 21.6 | 0.99 | 40.9 | 29.7 | 0.38 |
| Outside Milan criteria | 100 | 25.0 | 0.06 | 44.4 | 36.4 | 0.99 |
| MELD-Na, mean (SD) | 27.4 (8.1) | 26.7 (6.4) | 0.74 | 24.7 (7.9) | 25.4 (7.4) | 0.74 |
| Child–Pugh score, mean (SD) | 11.4 (2.0) | 11.7 (2.0) | 0.56 | 11.4 (1.8) | 11.1 (2.2) | 0.67 |
| Child–Pugh class, % | 0.99 | 0.30 | ||||
| A | 0 | 0 | 0 | 0 | ||
| B | 11.8 | 13.5 | 9.5 | 22.2 | ||
| C | 88.2 | 86.5 | 90.5 | 77.8 | ||
| Transplant status, % | 0.60 | 0.74 | ||||
| Not evaluated | 52.9 | 40.5 | 40.9 | 48.6 | ||
| Evaluated, but not listed | 47.1 | 54.1 | 59.1 | 48.6 | ||
| Listed | 0 | 5.4 | 0 | 2.7 |
Patient outcomes
Outcomes, including health care utilization and life-prolonging measures, are shown in Table 6. During follow-up, patients referred to PC had greater use of intensive care, mechanical ventilation, and vasopressors, but were also more likely to place limits on their code status. Limitations on code status were “do not resuscitate or intubate” in 30.5%, and “do not resuscitate only” in 65.2%, and the distribution of these limitations were similar in patients with and without PC referrals. Patients referred to hospice had similar use of life-prolonging measures compared with those not referred to hospice. Of those who placed limits on code status, those referred to hospice were more likely to choose both “DNR/DNI” than those not referred to hospice (44.6% vs. 23.1%; p = 0.02). In the overall cohort, 13.9% underwent liver transplant, none of whom was referred to PC or hospice.
Table 6.
Patient Outcomes
| Outcome | Overall (N = 397) | No PC (N = 336) | PC (N = 61) | p | No hospice (N = 326) | Hospice (N = 71) | p |
|---|---|---|---|---|---|---|---|
| Hospital admissions, median (IQR) | 1 (1–3) | 1 (1–3) | 1 (1–2) | 0.64 | 1 (1–3) | 1 (1–3) | 0.92 |
| Days in the hospital, median (IQR) | 15 (7–28) | 14 (7–28) | 17 (10–28.5) | 0.15 | 14.5 (7–28) | 17 (7–29) | 0.48 |
| Intensive care admission, % | 47.7 | 44.3 | 66.7 | 0.001 | 48.6 | 43.7 | 0.45 |
| Renal replacement therapy, % | 22.2 | 21.5 | 26.2 | 0.41 | 22.8 | 19.7 | 0.58 |
| Mechanical ventilation, % | 40.0 | 37.6 | 53.3 | 0.02 | 40.6 | 37.1 | 0.59 |
| Vasopressor use, % | 33.2 | 31.1 | 45.0 | 0.04 | 34.6 | 27.1 | 0.23 |
| Cardiopulmonary resuscitation, % | 7.1 | 6.9 | 8.2 | 0.79 | 7.7 | 4.2 | 0.44 |
| Limited code status, % | 43.0 | 33.8 | 96.4 | <0.001 | 33.9 | 87.7 | <0.001 |
| Liver transplant, % | 13.9 | 16.4 | 0 | 0.001 | 16.9 | 0 | <0.001 |
| Mortality, % | 50.4 | 42.6 | 93.4 | <0.001 | 41.1 | 93.0 | <0.001 |
Discussion
DC causes significant morbidity and mortality. In this study of patients requiring hospitalization and followed for 1 year, patients spent a median of 15 days in the hospital; nearly half of patients received intensive care; and half died. These poor outcomes and high utilization, coupled with the symptom burden of end-stage liver disease,7,13 underscore the potential value of PC for this population. Despite this potential benefit, we found that only 15% of patients were referred to PC, and only 33% of patients who died were referred to hospice. Importantly, most of the PC/hospice referrals came only days before death and after many had received aggressive treatments, including intensive care, mechanical ventilation, and renal replacement therapy.
These low utilization rates are consistent with prior observations. Several small studies of patients with DC have demonstrated PC referral rates of 10% to 35%.13,14,18 However, these studies largely excluded patients undergoing transplant evaluation; we included patients regardless of their transplant eligibility and found that those listed for transplant were less likely to see PC (even after controlling for disease severity). Notably, none of the patients who saw PC received a transplant during follow-up. This failure to refer patients seeking transplant is a missed opportunity to improve the lives of patients with advanced liver disease, and likely reflects clinicians' view that both PC and hospice are only for the sickest of patients who are imminently dying.31 This view contradicts the modern notion of PC as a service for patients with serious illness, which can be provided at any time during disease trajectory, and which can be provided concurrently with curative treatments.32,33 In patients awaiting transplant, Baumann et al. showed that PC improved symptoms and mood.11 Further work is needed to examine whether these benefits in patient-reported outcomes translate to other outcomes (e.g., health care utilization).
Larger studies based on national data have also shown low PC rates. Rush et al. found that 4.5% of patients with DC saw PC in the hospital, and Patel et al. found a rate of 30% in terminal hospitalizations.12,20 In both of these studies, PC was strongly associated with HCC. In our study, HCC was not associated with PC referral, but it was associated with hospice. Others have shown higher rates of hospice use for patients with HCC15–17 as compared with cirrhosis.19 Considering this association in our study, hospice utilization in DC is particularly poor, as many patients are likely referred to hospice because of their cancer, as opposed to the underlying liver disease. Indeed, of those who died, 58% of those with HCC were referred to hospice, as opposed to only 27% of those without HCC. These findings are consistent with studies demonstrating strong associations between hospice and concomitant cancer diagnoses.19 In one study of decedents with HCC, patients under the care of an oncologist were more likely to receive hospice.15 Given the central role of gastroenterologists and hepatologists in caring for patients with DC (including those with HCC), expanding awareness of PC and hospice among these providers has the potential to create a positive impact on the cirrhosis population.
In addition to HCC and transplant listing status, several other patient-level factors were associated with PC and hospice referrals. Notably, unmarried patients were more likely to have a PC referral compared with married patients. This relationship remained strong after multivariable adjustment and when examined in just those patients who died. Reasons for this association are unclear, but it may indicate referring providers' heightened awareness of unmet psychosocial needs for patients without significant social support. In this way, providers may be attempting to “substitute” PC social resources for inadequate existing social support. Alternatively, providers may be underestimating the burden that caregivers face, or they may be unaware of the support that PC can provide to caregivers. Regardless of the reasons, reduced referrals for married patients represents another missed opportunity for PC to improve the lives of patients and caregivers. Other findings of note include reduced hospice utilization in patients with psychiatric disease. Disparities in end-of-life care for patients with mental illness have been documented,34 and are likely exacerbated in patients with chronic liver disease, who are affected disproportionately by mental illness.35
In our cohort, referrals to both PC and hospice were often late. Late referrals in this population have been seen previously,19 and likely have multifactorial causes, including uncertainty surrounding prognosis.7,10 The number of patients with referrals was relatively small, limiting power to detect patient-level factors associated with late referral. However, those with late referrals tended to be younger and married, with recent alcohol use associated with late hospice referral. Focusing on these groups to increase timely referrals has the potential to improve quality of life and reduce health care utilization. This increased utilization in patients referred to PC (e.g., intensive care, mechanical ventilation, vasopressors) likely reflects the lateness of these referrals.
Despite this study's novel findings, it has several limitations. As a retrospective study, the design does not allow for assessment of patient-reported outcomes or individual goals and treatment preferences that are important in understanding both the utilization and impact of PC and hospice. We also limited the sample to a single center, which limits generalizability. However, as a tertiary transplant center, Indiana University Hospital has similar characteristics to many U.S. transplant centers. In addition, limiting to a single center allows for uniform data collection that enhances internal validity. Confirmation of these findings elsewhere should enhance external validity. Lastly, the data are from 2012 and the field of PC has since grown considerably. However, others have previously shown significant growth in PC before 2012, and more recent studies have shown similar PC rates.12,20,21 Furthermore, we anticipate that these findings can be used as a baseline for comparison in future studies, as the knowledge base for PC in cirrhosis continues to grow. Indeed, we believe this study can serve as a benchmark for future studies that can compare differences with concurrent care with transplant, similarities and differences at other tertiary centers, and differences depending on the culture or structure of PC services at other institutions. In contrast to these weaknesses, the study benefits from having a large, well-characterized cohort. Other larger studies on this topic have often relied on administrative data, which do not provide comparable granularity. The sample size and granularity also allowed us to examine novel variables, including the separate examination of PC and hospice, as well as late referrals. Additionally, our broad inclusion criteria allowed for a detailed examination of HCC and transplant listing status in relation to outcomes. Lastly, the one-year follow-up period provided adequate time to examine a host of clinical outcomes.
In conclusion, we found that despite high mortality and health care utilization, patients with DC had low PC/hospice referral rates, with the majority of referrals occurring shortly before death. We identified novel factors associated with these referrals, which can be targeted in future efforts to expand the appropriate use of these services to better meet the needs of this population.
Acknowledgments
The sponsors played no role in the study design, collection, analysis, or interpretation of the data, or in the writing of the report.
Funding Information
This work was supported, in part, by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K23DK109202. The work was independent of the funding.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. D'Amico G, Garcia-Tsao G, Pagliaro L: Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J Hepatol 2006;44:217–231 [DOI] [PubMed] [Google Scholar]
- 2. Marchesini G, Bianchi G, Amodio P, et al. : Factors associated with poor health-related quality of life of patients with cirrhosis. Gastroenterology 2001;120:170–178 [DOI] [PubMed] [Google Scholar]
- 3. Ghabril M, Jackson M, Gotur R, et al. : Most individuals with advanced cirrhosis have sleep disturbances, which are associated with poor quality of life. Clin Gastroenterol Hepatology 2017;15:1271–1278.e1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanwal F, Hays RD, Kilbourne AM, et al. : Are physician-derived disease severity indices associated with health-related quality of life in patients with end-stage liver disease? Am J Gastroenterol 2004;99:1726. [DOI] [PubMed] [Google Scholar]
- 5. Orman ES, Ghabril M, Emmett TW, et al. : Hospital Readmissions in Patients with Cirrhosis: A Systematic Review. J Hosp Med 2018. [Epub ahead of print]; DOI: 10.12788/jhm.2967 [DOI] [PMC free article] [PubMed]
- 6. Desai AP, Reau N: The burden of rehospitalization for patients with liver cirrhosis. Hosp Pract (1995) 2016;44:60–69 [DOI] [PubMed] [Google Scholar]
- 7. Low J, Davis S, Vickerstaff V, et al. : Advanced chronic liver disease in the last year of life: A mixed methods study to understand how care in a specialist liver unit could be improved. BMJ Open 2017;7:e016887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roth K, Lynn J, Zhong Z, et al. : Dying with end stage liver disease with cirrhosis: Insights from support. J Am Geriatr Soc 2000;48(Suppl 1):S122–S130 [PubMed] [Google Scholar]
- 9. Low JTS, Rohde G, Pittordou K, et al. : Supportive and palliative care in people with cirrhosis: International systematic review of the perspective of patients, family members and health professionals. J Hepatol 2018;69:1260–1273 [DOI] [PubMed] [Google Scholar]
- 10. Kimbell B, Boyd K, Kendall M, et al. : Managing uncertainty in advanced liver disease: A qualitative, multiperspective, serial interview study. BMJ Open 2015;5:e009241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baumann AJ, Wheeler DS, James M, et al. : Benefit of early palliative care intervention in end-stage liver disease patients awaiting liver transplantation. J Pain Symptom Manage 2015;50:882–886.e882. [DOI] [PubMed] [Google Scholar]
- 12. Rush B, Walley KR, Celi LA, et al. : Palliative care access for hospitalized patients with end-stage liver disease across the United States. Hepatology 2017;66:1585–1591 [DOI] [PubMed] [Google Scholar]
- 13. Poonja Z, Brisebois A, van Zanten SV, et al. : Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin Gastroenterol Hepatol 2014;12:692–698 [DOI] [PubMed] [Google Scholar]
- 14. Kelly SG, Campbell TC, Hillman L, et al. : The utilization of palliative care services in patients with cirrhosis who have been denied liver transplantation: A single center retrospective review. Ann Hepatol 2017;16:395–401 [DOI] [PubMed] [Google Scholar]
- 15. Sanoff HK, Chang Y, Reimers M, et al. : Hospice utilization and its effect on acute care needs at the end of life in medicare beneficiaries with hepatocellular carcinoma. J Oncol Pract 2017;13:e197–e206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fukui N, Golabi P, Otgonsuren M, et al. : Hospice care in Medicare patients with primary liver cancer: The impact on resource utilisation and mortality. Aliment Pharmacol Ther 2018;47:680–688 [DOI] [PubMed] [Google Scholar]
- 17. Zou WY, El-Serag HB, Sada YH, et al. : Determinants and outcomes of hospice utilization among patients with advance-staged hepatocellular carcinoma in a veteran affairs population. Dig Dis Sci 2018;63:1173–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kathpalia P, Smith A, Lai JC: Underutilization of palliative care services in the liver transplant population. World J Transplant 2016;6:594–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown CL, Hammill BG, Qualls LG, et al. : Significant morbidity and mortality among hospitalized end-stage liver disease patients in Medicare. J Pain Symptom Manage 2016;52:412–419.e411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel AA, Walling AM, Ricks-Oddie J, et al. : Palliative care and health care utilization for patients with end-stage liver disease at the end of life. Clin Gastroenterol Hepatol 2017;15:1612–1619.e1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Plunkett A, Mortimore M, Good P: Palliative care in cirrhosis with decompensation. Intern Med J 2019;49:904–908 [DOI] [PubMed] [Google Scholar]
- 22. Nehra MS, Ma Y, Clark C, et al. : Use of administrative claims data for identifying patients with cirrhosis. J Clin Gastroenterol 2013;47:e50–e54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Humphreys J, Harman S: Late referral to palliative care consultation service: Length of stay and in-hospital mortality outcomes. J Community Support Oncol 2014;12:129–136 [DOI] [PubMed] [Google Scholar]
- 24. Diamond EL, Russell D, Kryza-Lacombe M, et al. : Rates and risks for late referral to hospice in patients with primary malignant brain tumors. Neuro Oncol 2016;18:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Angeli P, Gines P, Wong F, et al. : Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J Hepatol 2015;62:968–974 [DOI] [PubMed] [Google Scholar]
- 26. Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 27. Myers RP, Quan H, Hubbard JN, et al. : Predicting in-hospital mortality in patients with cirrhosis: Results differ across risk adjustment methods. Hepatology 2009;49:568–577 [DOI] [PubMed] [Google Scholar]
- 28. Pugh RN, Murray-Lyon IM, Dawson JL, et al. : Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973;60:646–649 [DOI] [PubMed] [Google Scholar]
- 29. Organ Procurement and Transplantation Network: Policy 9: Allocation of Livers and Liver-Intestines. 2019. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf (Last accessed May22, 2019)
- 30. Mazzaferro V, Regalia E, Doci R, et al. : Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693–699 [DOI] [PubMed] [Google Scholar]
- 31. Beck KR, Pantilat SZ, O'Riordan DL, et al. : Use of palliative care consultation for patients with end-stage liver disease: Survey of liver transplant service providers. J Palliat Med 2016;19:836–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferrell BR, Temel JS, Temin S, et al. : Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:96–112 [DOI] [PubMed] [Google Scholar]
- 33. Institute of Medicine: Dying in America: Improving Quality and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press, 2015 [PubMed] [Google Scholar]
- 34. Shalev D, Brewster K, Arbuckle MR, et al. : A staggered edge: End-of-life care in patients with severe mental illness. Gen Hosp Psychiatry 2017;44:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hughes E, Bassi S, Gilbody S, et al. : Prevalence of HIV, hepatitis B, and hepatitis C in people with severe mental illness: A systematic review and meta-analysis. Lancet Psychiatry 2016;3:40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]