Abstract
Purpose: Natamycin (NTM) ophthalmic suspension is the only FDA-approved formulation commercially available for treating ocular fungal infections. However, precorneal residence times and losses/drainage remain the foremost challenges associated with current ocular antifungal pharmacotherapy. In our previous investigations, NTM loaded polyethylene glycol nanolipid carriers (NTM-PNLCs) showed enhanced corneal permeation, both in vitro and in vivo. To further improve the corneal retention of NTM-PNLCs, this study aimed to develop a gelling system composed of carboxyvinyl polymer, guar gum, and boric acid in which the NTM-PNLCs were loaded.
Methods: A 23 factorial design was employed in formulating and optimizing the gelling system for NTM-PNLCs, where the independent factors were the gelling excipients (guar gum, boric acid, and Carbopol® 940) and dependent variables were gelling time, gel depot collapse time, rheology, firmness, and work of adhesion. Optimized gel was evaluated for transcorneal permeation using rabbit cornea, in vitro; and tear pharmacokinetics and ocular biodistribution in male New Zealand White rabbits, in vivo.
Results: Optimized NTM-PNLC-GEL was found to exhibit shear thinning rheology, adequate firmness, and spreadability, and formed a depot that did not collapse immediately. In addition, the in vitro transcorneal evaluation studies indicated that the NTM-PNLC-GEL exhibited a lower/slower flux and rate in comparison to Natacyn® suspension. NTM-PNLC-GEL (0.3%), at a 16-fold lower dose, exhibited mean residence time and elimination half-life comparable to Natacyn (5%), and provided similar in vivo concentrations in the innermost tissues of the eye.
Conclusion: The data indicate that the NTM-PNLC-GEL formulation could serve as an alternative during ophthalmic antifungal therapy.
Keywords: fungal infections, antifungal pharmacotherapy, gelling systems, pharmacokinetic evaluations, in vivo evaluations
Introduction
Currently, natamycin (NTM) ophthalmic suspension (Natacyn® topical eye drops) is the only FDA-approved, commercially available, formulation for the treatment of ocular fungal infections (OFI).1 NTM exhibits activity against filamentous fungal species, particularly Fusarium and Aspergillus species, common causes of OFI such as keratitis and endophthalmitis.2–4 NTM also exhibits better safety and tolerability profiles compared to the other polyene and azole antifungal drugs,5 and has, thus, become a favored first-line antifungal agent in treating OFI, particularly fungal keratitis.1,6
Fungal keratitis and endophthalmitis are characterized by fungal infections, primarily in the cornea and aqueous humors (AH) and vitreous humors (VH), respectively.2,7–9 Fungal keratitis severely affects the corneal integrity and could be localized initially, but if left untreated, could lead to the spread of fungal infection to the inner ocular tissues causing fungal endophthalmitis and/or deep-seated mycoses.2,7–9 Also, such cases of OFI are characterized by a loss of corneal integrity, which additionally facilitates quicker passage of antifungal drugs (such as NTM), thereby further achieving higher drug levels in the intraocular tissues. Despite this, the current therapy involves the need for frequent instillation/dosing of the marketed suspension owing to the precorneal losses/drainage5,10
Short residence times in the conjunctival cul de sac, postinstillation, and low permeability across the corneal membrane, limits ocular bioavailability of NTM (≈2%) from the Natacyn topical eye drops. This necessitates frequent instillation of the NTM eye drops, initially given every hour or 2 h and then gradually reduced to about 6–8 times a day.10,11 Also, Natacyn suspension (5%) is associated with adverse reactions/side effects such as allergic reaction, change in vision, chest pain, corneal opacity, dyspnea, eye discomfort, eye edema, eye hyperemia, eye irritation, eye pain, foreign body sensation, paresthesia, and tearing.12 In our previous studies, we developed NTM loaded polyethylene glycol nanolipid carriers (NTM-PNLCs) that showed enhanced in vitro permeation across the intact cornea and improved ocular bioavailability compared to the commercial NTM suspension (Natacyn eye drops)6 at a 16-fold lower dose (NTM-PNLC: 0.3% vs. Natacyn: 5%), thereby also potentially reducing the chances of the aforementioned adverse reactions. The goal of this research was to improve the retention of the NTM-PNLCs at a lower dose (compared to Natacyn suspension) on the ocular surface without compromising the enhanced transcorneal delivery characteristics.
Gelling systems containing nanoparticles and/or nanolipid carriers can improve precorneal and corneal residence times of therapeutic agents, reducing precorneal losses, and may improve the permeation of therapeutic agents across the corneal barriers owing to prolonged contact. In addition, the composition may provide a sustained/prolonged release profile of the therapeutic agent/s, facilitating a reduction in the frequency of application.13–17 Carboxyvinyl polymers (such as Carbopol® grades) have been evaluated as gelling agents in the ocular delivery of various therapeutic agents. They have found a favor as gelling and/or viscosity-enhancing agents owing to their ability to provide thickening and gelling effect (by cross-linking) at lower concentration, bioadhesive properties (at the corneal surface), clear gel appearance, compatibility with various therapeutic agents, and excellent patient compliance due to their ability to not interfere with the patients' vision.18–21 The borate-guar gum combination, along with carboxyvinyl polymer in the system, stabilizes the cross-linked polymer by providing additional cross-links (borate anion reacts with the galactomannan sugar contributed by guar gum to form additional cross-links).22 Therefore, this study aimed to develop and optimize a carboxyvinyl polymer-guar gum-borate gelling system containing NTM-PNLCs (NTM-PNLC-GEL) and compare its in vivo performance against NTM-PNLC and Natacyn.
Methods
Chemicals
NTM was procured from Cayman Chemicals. N-(carbonyl-methoxypolyethylenglycol-2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (mPEG-2K-DSPE sodium salt) was bought from Lipoid (Ludwigshafen, Germany). Precirol® ATO 5 (glyceryl distearate) was a gift from Gattefossé. Castor oil, surfactants (Span® 80, Tween® 80, and poloxamer 188) and glycerin were all bought from Acros Organics. The gelling components—guar gum, boric acid crystals, and Carbopol 940 and all the solvents (analytical grade) were purchased from Fisher Scientific. The excipients used in the fabrication of NTM-PNLC-GEL have generally been regarded as safe status and used in FDA-approved ophthalmic products.
Methods
Preparation of NTM-PNLCs
Homogenization method was employed in the preparation of NTM-PNLCs, which has been reported earlier.6 Briefly, an aqueous phase consisting of surfactants—Tween 80 (0.75% w/v; primary surfactant), poloxamer 188 (0.25% w/v; secondary surfactant), and glycerin (2.25% w/v) was prepared in deionized water and heated. Separately, a molten lipid phase containing NTM (0.3% w/v), castor oil (1% w/v), Precirol ATO 5 (1.5% w/v), mPEG-2K-DSPE sodium salt (1.5% w/v), and Span 80 (0.1% w/v) was prepared. The aqueous phase was gradually added to the lipid phase under continuous stirring at 2,000 rpm for 5 min. The coarse emulsion was then emulsified (Ultra-Turrax, 16,000 rpm, 5 min) to form a fine emulsion. The fine emulsion was then subjected to high-pressure homogenization (15,000 psi) using temperature-controlled Avestin® Emulsiflex C5 homogenizer that resulted in the formation of NTM-PNLCs. Temperature was maintained at a constant 80°C ± 2°C during the entire process of NTM-PNLC preparation.
Experimental design
A 23 factorial design [8 formulation experimental run; design generated using Design-Expert® software (8.0.7.1)] was employed to develop the gelling system for NTM-PNLCs. The independent factors were the gelling excipients (guar gum, boric acid, and Carbopol 940) and the dependent variables were gelling time, gel depot collapse time, rheology, firmness, and work of adhesion.
Preliminary studies were performed to determine the individual levels of the gelling excipients. Factorial design was then utilized to select the optimum levels of all the 3 gelling excipients to obtain a suitable optimized formulation with desired properties. Tables 1 and 2 present the details on the experimental 23 factorial design.
Table 1.
Independent Factors (at Their 2 Levels) and Dependent Variables in the Experimental 23 Factorial Design
| Factors |
Levels |
|
|---|---|---|
| Independent factors | Level 1 | Level 2 |
| Guar gum (% w/v) | 0.1 | 0.2 |
| Boric acid (% w/v) | 0.4 | 0.5 |
| Carbopol® 940 (% w/v) | 0.1 | 0.4 |
| Dependent variables | Constraints | |
| Gelling time | Minimum | |
| Gel depot collapse time | Maximum | |
| Rheology | Optimum (shear-thinning rheology) | |
| Firmness | Maximum | |
| Work of adhesion | Maximum | |
Table 2.
23 Factorial Design for the Gelling System Containing Natamycin Loaded Polyethylene Glycol Nanolipid Carriers
| Code | Guar gum (% w/v) | Boric acid (% w/v) | Carbopol 940 (% w/v) | |
|---|---|---|---|---|
| All the codes contain NTM-PNLC (0.3% w/v) | B1 | 0.2 | 0.4 | 0.1 |
| B2 | 0.2 | 0.4 | 0.4 | |
| B3 | 0.1 | 0.4 | 0.1 | |
| B4 | 0.1 | 0.4 | 0.4 | |
| B5 | 0.1 | 0.5 | 0.1 | |
| B6 | 0.1 | 0.5 | 0.4 | |
| B7 | 0.2 | 0.5 | 0.1 | |
| B8 | 0.2 | 0.5 | 0.4 |
NTM-PNLC, Natamycin loaded polyethylene glycol nanolipid carriers.
Fabrication of NTM-PNLC-GEL
Weighed quantities of guar gum, boric acid, and Carbopol 940, according to the experimental runs (Table 1), were added into the NTM-PNLCs, prepared as described above. Stirring was continued for an additional 15 min. The NTM-PNLC-GELs thus obtained (compositions as described in Table 2) were then evaluated with respect to the dependent variables as specified in Table 1.
Characterization of NTM-PNLC-GEL
Gelling time and gel depot collapse time for NTM-PNLC-GELS
Simulated tear fluid [STF; 10 mL; composition: NaCl (0.68% w/v), NaHCO3 (0.22% w/v), CaCl2 (0.008% w/v), and KCl (0.140% w/v) in deionized water], pH = 7.4, was taken in multiple scintillation vials. Five hundred microliters of the 8 different NTM-PNLC-GEL in situ gelling samples (existing as liquids) was added and observed for their in situ gelling in STF. Time required for the gel to form and the duration of time the depot were maintained was determined.
Rheological evaluations of NTM-PNLC-GELS
Rheological evaluations were carried out using Bohlin Visco 88 viscometer (Malvern Panalytical). A cup and bob attachment with a C14 probe was used to determine the rheological behavior of the NTM-PNLC-GEL. Six hundred microliters of the gel was placed in the cup and the rheology analyses were carried out in the “up & down” ramp mode, at predefined multiple shear rates (66.5–506.5 1/s), at room temperature. The rheology data analysis was accomplished using Bohlin Software (version 6.32.1.2).
Texture analyses (firmness and work of adhesion) of NTM-PNLC-GEL
Texture analyses for NTM-PNLC-GEL was performed using Texture Analyzer model TA.XT2i (Texture Technologies Corp.) along with a 1″ diameter (TA-3), cylindrical, acrylic probe, and a soft matter kit (TA-275).23 NTM-PNLC-GELs were placed in the soft matter fixture and placed below the TA-3 probe, and the texture analyses were performed according to the parameters specified in Table 3.
Table 3.
Parameters for Texture Analyses of Gelling System Containing Natamycin Loaded Polyethylene Glycol Nanolipid Carriers
| Parameter | Set value |
|---|---|
| Test mode | Compression |
| Pretest speed | 0.5 mm/s |
| Test speed | 0.5 mm/s |
| Posttest speed | 0.5 mm/s |
| Target mode | Distance |
| Distance | 1.0 mm |
| Trigger type | Auto (force) |
| Trigger force | 2.0 g |
| Temperature | Room temperature |
In vitro transcorneal permeation of NTM
Transcorneal flux, permeability, and permeation rate of NTM from NTM-PNLC-GEL formulation were assessed across rabbit cornea, procured from Pel-Freez Biologicals, using vertical Franz diffusion cells (PermeGear, Inc.).6,24 The underlying aim of the transcorneal permeation study was to select the most optimum guar gum: boric acid ratio and compare the gelling system to Natacyn (0.3% w/v; dose: 150 mg).
Cornea was placed in between the donor and receiver compartments, with the corneal epithelial surface facing the donor cell. Five hundred microliters of the test formulations, diluted Natacyn (dose normalized; 0.3% w/v), NTM-PNLCs (0.3% w/v), or NTM-PNLC-GEL (0.3% w/v), was placed in the donor compartment. Dulbecco's phosphate-buffered saline (DPBS) containing 2.5% w/v randomly methylated-β-cyclodextrin (RMBCD) was used as the receiver medium (5 mL).25,26 Aliquots (600 μL) were withdrawn at predetermined time intervals over a duration of 3 h and immediately replenished with an equal volume of RMBCD in DPBS. NTM concentration in the in vitro aliquots/samples was quantified using an high-performance liquid chromatography (HPLC) method as described in the subsequent Analytical Methods section.
NTM content in optimized NTM-PNLC-GEL
Predetermined amount of NTM-PNLC-GEL was taken from 3 different regions (bottom, middle, and top) of the gel formulation and dissolved in methanol (extracting solvent). The gel samples were centrifuged at 13,000 rpm for 10 min and the supernatant analyzed for NTM.
pH of the optimized NTM-PNLC-GEL
pH of the optimized NTM-PNLC-GEL formulation was determined using a SevenMulti™ Mettler Toledo pH meter.
In vitro release and release kinetics of optimized NTM-PNLC-GEL
Twenty milliliters of STF, pH = 7.4, was taken in 6 scintillation vials, to evaluate the release of NTM from NTM-PNLC-GEL system and NTM suspension under stink condition. STF was selected as a biorelevant medium to study the drug release at/in the precorneal region, since the gelling system was intended to be applied/instilled in the precorneal region.27 The study was carried out under continuous magnetic stirring of the STF at 32°C ± 1°C. Briefly, 200 μL of the samples was loaded on to the dialysis cassettes (Slide-A-Lyzer® Mini Dialysis Devices, 10K molecular weight cut-off), which contained a cellulose membrane with diffusion area of 0.64 cm2. At predetermined time points (t = 0, 0.5, 1, 1.5, 2, 4, 6, 12, 24, and 48 h), 600 μL of the aliquot sample of STF was withdrawn from the scintillation vial and an equal amount of STF was replaced in the vial after every withdrawal. The aliquot sample of STF was then analyzed using HPLC to quantify the amount of NTM released across the active diffusion membrane. The release data were analyzed to model the release kinetics using different release models—Higuchi square root, first order, zero order, and Korsmeyer-Peppas models.
In vivo precorneal tear pharmacokinetics
In vivo precorneal tear kinetics of the various NTM formulations was determined in triplicates (n = 3) in male New Zealand White (NZW) rabbits (2–2.5 kg), purchased from Charles River Labs. All the animal studies conformed to the tenets of the Association for Research in Vision and Ophthalmology statement on the use of animals in ophthalmic vision and research and the University of Mississippi Institutional Animal Care and Use Committee-approved protocols. The rabbits were dosed (100 μL administered as 2 drops of 50 μL each) with NTM-PNLC-GEL, NTM-PNLC, and Natacyn (5%) formulations topically. Therefore, the amount of NTM dose received by the rabbits was 0.3 mg (for NTM-PNLC-GEL and NTM-PNLC) and 5 mg (for Natacyn). The tear samples were collected using a preweighed piece of filter paper by gently touching the filter paper at the corneal surface at every time point (t = 0, 0.5, 1, 2, 3, 4, 5, and 6 h). The wet weight of the filter paper was then recorded and the difference in their dry and wet weights was used in the determination of the amount of tear fluid that was collected, which was used in the estimation of NTM from the tear biosamples.
NTM from the tear biosamples collected on filter papers was extracted with 600 μL of ice-cold methanol, mixed thoroughly using a vortex mixer, and centrifuged (13,000 rpm, 15 min) using a table-top centrifuge. The supernatant was then collected and analyzed for NTM content using a validated HPLC method that is outlined below (Analytical Methods section). The data were then analyzed using PKSolver software 2.0 to calculate the various pharmacokinetics (PK) parameters.28
In vivo ocular biodistribution of NTM
NTM-PNLC-GEL, NTM-PNLC, and Natacyn (5%) were dosed in vivo, in conscious NZW rabbits (n = 3; 12 rabbits). The dosing regimen of rabbits is detailed in Table 4. At the sacrificial time points, rabbits were anesthetized using a combination of ketamine (35 mg/kg) and xylazine (3.5 mg/kg) injected intramuscularly, and subsequently euthanized using an overdose of pentobarbital. The eyes were enucleated and washed using BSS and the intraocular tissues, AH and VH, iris-ciliary bodies (ICB), and cornea, were separated and processed to extract the NTM. Briefly, ICB and cornea were cut into pieces, and AH and VH were taken in centrifuge tubes and ice-cold methanol (600 μL) was added for protein precipitation. The biosamples were sonicated for 10 min in an ultrasonic bath and centrifuged (13,000 rpm).
Table 4.
Dosing Regimen of Rabbits (n = 3) for Ocular Biodistribution Studies
| Set | No. of animals (n) | Formulations | Volume | NTM dose, mg | Dosing frequency (t), h | Sacrificial time point (t), h |
|---|---|---|---|---|---|---|
| 1 | 3 | NTM-PNLC-GEL | 100 μL as 2 drops 50 μL each | 0.3 | 0, 3, 6 | 9 |
| 3 | NTM-PNLC | 0.3 | ||||
| 3 | Natacyn® | 5 | ||||
| 2 | 3 | NTM-PNLC-GEL | 0.3 | 0, 4 | 8 |
NTM-PNLC-GEL, gelling system containing NTM-PNLC.
Analytical methods
Quantification of NTM in in vitro samples
NTM was quantified using a, previously reported, validated HPLC method.6,29 The HPLC system consisted of a Waters 717 plus autosampler coupled with a Waters 2487 Dual λ Absorbance UV (ultraviolet) detector, a Waters 600 controller pump, and an Agilent 3395 Integrator. The mobile phase consisted of a mixture of phosphate buffer (0.2 M, pH 5.5) and acetonitrile (70:30) with flow rate of 1 mL/min. A C18 Phenomenex Luna® (5 μm, 250 × 4.6 mm) column was used. The injection volume was 20 μL, and the UV detection wavelength was set to 304 nm at absorbance units full scale 1.00.
Quantification of NTM in ocular tissues
A Waters Xevo TQ-S triple quadrupole tandem mass spectrometer with an electrospray ionization (ESI) source, equipped with Acquity UPLC® I-Class System, was used (Waters Corporation, Milford, MA) in NTM quantification from biosamples. TargetLynx and MassLynx were utilized for data acquisition and data processing, respectively. Separation was achieved using a C18 column (Acquity UPLC BEH C18 100 mm × 2.1 m, 1.7 μm particle size). Mobile phase consisted of water (A) and acetonitrile (B), both containing 0.1% formic acid with a gradient flow rate of 1.0 mL/min (0 min, 98% A/2% B held for 0.2 min and in next 2.3 min to 100% B). Each run was followed by 1-min wash with 100% B and equilibration period of 2 min with 98% A/2% B. Column and sample temperature were maintained at 50°C and 10°C, respectively. Column effluent was directed into the ESI probe. The ESI conditions were as follows: desolvation temperature 600°C, source temperature 150°C, capillary voltage 3.0 kV, cone voltage 40 V, nebulizer gas 1,100 L·h−1 N2, and nebulizer pressure 7 bar. The collision gas utilized was Argon and collision energies ranged from 10 to 15 eV. Mass spectra were acquired in positive mode and multiple reaction monitoring (MRM) mode. The MRM mode was applied to monitor the transitions of quantifier ion to qualifier ions of m/z 666.2 → m/z 467.2, 485.2, and 503.2 for NTM and m/z 924.4 → m/z 107.5, 743.2, and 761.4 for amphotericin B (internal standard).
Using standard calibration curves for AH (0.4–100 ng/mL), VH (2–200 ng/mL), cornea (1.31–65.57 ng/mL), and ICB (1.31–65.57 ng/mL), NTM quantification was accomplished using the LC-MS/MS method. A coefficient of determination r2 ≥0.95 was obtained for all the standard curves. NTM extraction efficiency was >95% for cornea, ICB, and AH, whereas it was about 82% for VH; and process efficiency was >90% for all the tissues.6 The limit of detection for all ocular tissues corresponded to 0.13 ng/mL for all 4 intraocular tissues analyzed.6
Statistical analyses
For each study, a minimum of 3 independent experimental trials have been performed and the data values have been represented as mean ± standard deviation and/or mean ± standard error. Statistical comparison was performed using Student's t-test and/or 1-way analysis of variance (ANOVA). Statistically significant differences were considered at P value <0.05.
Results
Characterization of NTM-PNLC-GEL
Gelling time and gel depot collapse time for NTM-PNLC-GELS
The results observed from gelling time and gel depot collapse time are presented in Table 5. Out of 8 (B1–B8) factorial runs, B1, B3, B5, and B7 did not undergo gelation to form a gel depot. Therefore, these formulations from the experimental design could not be considered for statistical analyses (ANOVA). On the basis of the results, NTM-PNLC-GEL formulation codes (B2, B4, B6, and B8) were selected for further evaluation with respect to the other aforementioned dependent variables with the intent to optimize and select the most suitable NTM-PNLC-GEL formulation.
Table 5.
Results from Gelling Time and Gel Depot Collapse Time Study
| Code | Gelling components in NTM-PNLC-GEL (%) |
Gelling time | Depot collapse time | Firmness (g) | Work of adhesion (g-s) | ||
|---|---|---|---|---|---|---|---|
| Guar gum | Boric acid | Carbopol 940 | |||||
| B1 | 0.2 | 0.4 | 0.1 | (−) | NA | NA | NA |
| B2 | 0.2 | 0.4 | 0.4 | (+) | Depot until >12 h | 7.36 ± 2.86 | 21.54 ± 1.52 |
| B3 | 0.1 | 0.4 | 0.1 | (−) | NA | NA | NA |
| B4 | 0.1 | 0.4 | 0.4 | (+) | Depot until >12 h | 10.32 ± 0.28 | 23.59 ± 3.04 |
| B5 | 0.1 | 0.5 | 0.1 | (−) | NA | NA | NA |
| B6 | 0.1 | 0.5 | 0.4 | (+) | Depot until >12 h | 11.30 ± 1.37 | 23.41 ± 4.31 |
| B7 | 0.2 | 0.5 | 0.1 | (−) | NA | NA | NA |
| B8 | 0.2 | 0.5 | 0.4 | (+) | Depot until >12 h | 8.42 ± 3.79 | 20.87 ± 2.18 |
(−), Does not form a gel depot and instantaneously collapses in STF. (+), Forms a gel depot immediately (<1 min).
STF, simulated tear fluid.
Rheological evaluations of NTM-PNLC-GELS
The rheological data for the selected NTM-PNLC-GEL formulations (B2, B4, B6, and B8) are depicted in Fig. 1. All 4 selected gel formulations exhibited shear thinning rheology with a viscosity of 394.64 ± 18.12 cP for B2, 287.30 ± 4.23 cP for B4, 243.17 ± 2.03 cP for B6, and 428.12 ± 8.25 cP for B8 at the highest shear rate (≈500 1/s).
FIG. 1.
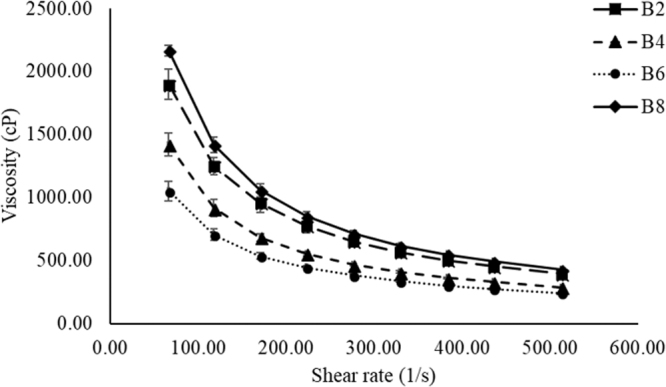
Plot of viscosity versus shear rate for selected NTM-PNLC-GEL formulations (B2, B4, B6, and B8) exhibiting shear-thinning rheology. NTM-PNLC-GEL, gelling system containing Natamycin loaded polyethylene glycol nanolipid carriers.
Texture analyses (firmness and work of adhesion) of NTM-PNLC-GELS
The results obtained for firmness and work of adhesion are presented in Table 5 for the selected formulation codes—B2, B4, B6, and B8.
In vitro transcorneal permeation of NTM
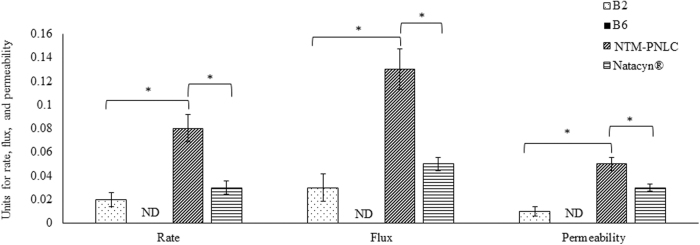
From the in vitro transcorneal permeation study, it was observed that (guar gum:boric acid) should be used in their lowest ratio (1:2) for effective transcorneal NTM permeation (higher amounts of boric acid in NTM-PNLC-GEL, in contrast, did not show any NTM permeation at the end of 3 h). Hence, gel formulation B2 with a ratio of guar gum to boric acid (1:2) was chosen to be the most suitable gel formulation in comparison to the gel formulation B6, which had a higher amount of boric acid (1:5). Transcorneal permeability (cm/s) of NTM-PNLC-GEL-B2 [(0.010 ± 0.007) × 10−5] was significantly lower than NTM-PNLCs [(0.050 ± 0.010) × 10−5], and Natacyn [(0.030 ± 0.005) × 10−5]) (N = 3). The trend for transcorneal flux and rate was NTM-PNLC-GEL < Natacyn < NTM-PNLCs (Fig. 2).
FIG. 2.
Plot of rate (μg/min), flux (μg/min/cm2), and permeability ( × 10−5 cm/s) for NTM permeation across the isolated cornea from NTM-PNLC-GEL-B2, NTM-PNLC-GEL-B6, NTM-PNLC, and Natacyn® (dose normalized: diluted to 0.3% w/v) over 3 h, (n = 3); data represented as mean ± standard error of mean. *Denotes statistically significant difference at P < 0.05.
NTM content in optimized NTM-PNLC-GEL
The mean NTM content in the optimized and most suitable NTM-PNLC-GEL (formulation B2) from the 3 different regions was found to be 95.53% ± 0.61%.
pH of the optimized NTM-PNLC-GEL
The pH of the selected optimized NTM-PNLC-GEL formulation (B2) was 5.5 ± 0.1.
In vitro release and release kinetics of optimized NTM-PNLC-GEL
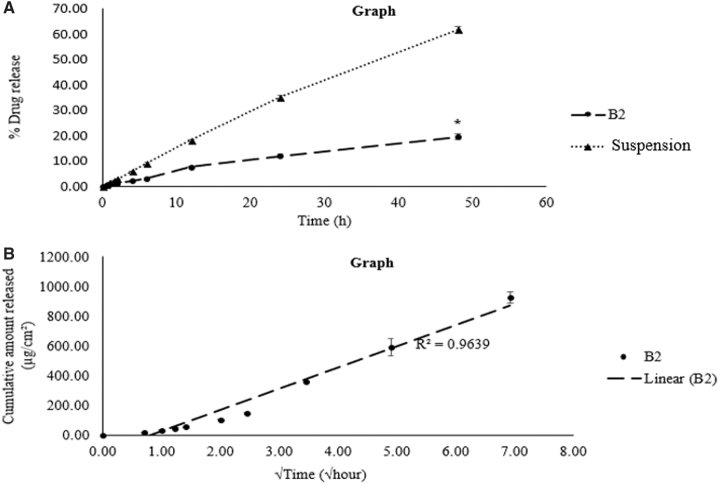
From Fig. 3, it is evident that NTM-PNLC-GEL system provided a significantly slower/sustained release in comparison to the control—NTM suspension (≈3-fold lower)—over a period of 48 h and exhibited a better fit for Higuchi model for drug release kinetics (r2 > 0.96) compared to the other release models (first order, zero order, and Korsmeyer-Peppas; r2 < 0.94).
FIG. 3.
(A) Comparison of NTM release from NTM-PNLC-GEL (formulation B2) and NTM solution; (B) fit to Higuchi model for drug release kinetics for NTM-PNLC-GEL (formulation B2).
In vivo precorneal tear PK
Instantaneous gelation was observed upon the instillation of NTM-PNLC-GEL and results obtained from the precorneal tear PK parameters [area under curve from time t = 0 to t = 6 h (AUC0–t), half-life (T0.5), maximum concentration (Cmax), and mean residence time (MRT0–∞)] are summarized in Table 6 and represented in Fig. 4.
Table 6.
Precorneal Tear Pharmacokinetics Parameters Obtained for Gelling System Containing Natamycin Loaded Polyethylene Glycol Nanolipid Carriers and Natacyn (5%)
| Test formulations | NTM-PNLC-GEL | NTM-PNLC | Natacyn | |
|---|---|---|---|---|
| Dose | 0.3 mg | 0.3 mg | 5 mg | |
| PK parameter | Units | |||
| AUC0–t | μg/μL × h | 1.15 ± 0.02 | 0.58 ± 0.01a | 2.72 ± 0.17b |
| Dose normalized AUC0–t | (μg/μL × h)/mg | 3.83 ± 0.06 | 1.93 ± 0.03a | 0.54 ± 0.03b |
| T0.5 | h | 2.82 ± 0.23 | 0.93 ± 0.14a | 2.78 ± 0.85 |
| Cmax | μg/μL | 2.57 ± 0.09 | 1.37 ± 0.06a | 3.00 ± 0.18b |
| Dose normalized Cmax | (μg/μL)/mg | 8.57 ± 0.3 | 4.56 ± 0.2a | 0.6 ± 0.03b |
| MRT0–∞ | h | 2.16 ± 0.26 | 1.09 ± 0.21a | 1.56 ± 0.32 |
Statistically significant difference between NTM-PNLC-GEL and NTM-PNLC at P < 0.05.
Statistically significant difference between NTM-PNLC-GEL and Natacyn (5%) at P < 0.05.
AUC, area under curve; Cmax, maximum concentration; MRT, mean residence time; PK, pharmacokinetics; T0.5, half-life.
FIG. 4.
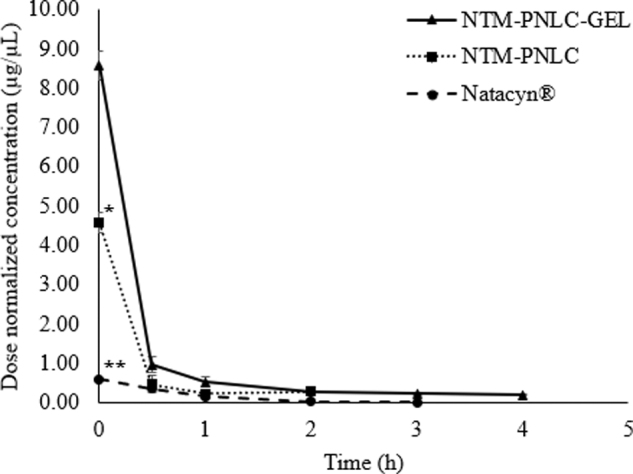
Plot of dose-normalized NTM concentrations (μg/μL) versus time (hours) profile for NTM-PNLC-GEL, NTM-PNLC, and Natacyn. *,**Denotes statistically significant difference between (NTM-PNLC-GEL and NTM-PNLC) and (NTM-PNLC-GEL and Natacyn) at P < 0.05, respectively; (n = 3, data represented as mean ± standard error).
In vivo ocular biodistribution of NTM
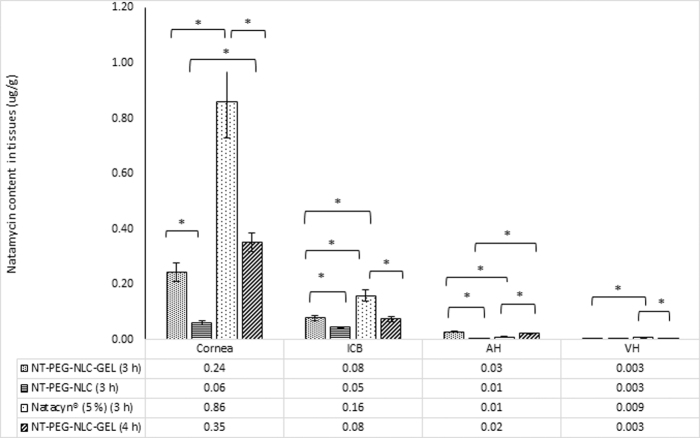
Figure 5 illustrates that all the NTM formulations [NTM-PNLC-GEL, NTM-PNLC, and Natacyn (5%)] could deliver NTM to AH, VH, ICB, and cornea in both study sets.
FIG. 5.
NTM concentrations (μg/g) in cornea, ICB, AH, and VH from NTM-PNLC-GEL (dose: 0.3 mg; instillation volume: 100 μL), NTM-PNLC (dose: 0.3 mg; instillation volume: 100 μL), and Natacyn (dose: 5 mg; instillation volume: 100 μL) obtained after instillation every 3 and 4 h for a 9- and an 8-h study, respectively. *Denotes statistically significant difference at P < 0.05 (n = 3, data represented as mean ± standard error). AH, aqueous humors; ICB, iris-ciliary bodies; VH, vitreous humors.
Moreover, it is evident from Fig. 5 that, there is no statistical difference observed between NTM levels obtained from NTM-PNLC-GEL following 2 different dosing regimens (Table 4) in AH, VH, ICB, and cornea (P > 0.05).
In the cornea (Fig. 5), NTM concentration from NTM-PNLC-GEL (for both dosing regimens detailed in Table 4) was significantly higher than NTM concentration obtained from NTM-PNLC formulation (P < 0.05). The NTM concentration from Natacyn (5%), however, was significantly higher than that obtained from NTM-PNLC-GEL (in both study sets) (P < 0.05).
NTM concentration from NTM-PNLC-GEL (for both dosing regimens) (Table 4) was found to be significantly lower than Natacyn (5%) in ICB (P < 0.05). In ICB, a statistical difference was found for NTM concentrations obtained from NTM-PNLC-GEL (for both dosing regimens) (Table 4) and NTM-PNLC (P < 0.05) (Fig. 5).
In AH (Fig. 5), concentration of NTM from NTM-PNLC-GEL (for both dosing regimens) (Table 4) was significantly higher than the NTM concentrations obtained from the NTM-PNLC and Natacyn (5%) formulations (P < 0.05).
In case of VH (Fig. 5), significantly lower concentration was observed in NTM concentrations obtained from the NTM-PNLC-GEL (for both dosing regimens) (Table 4) compared to Natacyn (5%) (P < 0.05). However, a nonsignificant difference was observed between NTM levels obtained from NTM-PNLC-GEL and NTM-PNLC (P > 0.05).
Discussion
NTM has been a mainstay in the pharmacotherapy of fungal keratitis and endophthalmitis.1,3,5,10 The current therapeutic regimen comprises an ophthalmic suspension of NTM—Natacyn (5%); however, “lower retention” at precorneal/corneal sites has been one of the foremost challenges that has been associated with the current Natacyn therapy.1,5,10,30 In our previous work,6 NTM-PNLCs showed better permeation across the intact corneal barrier owing to PEGylation. However, the initial formulations were simple eye drops, without any viscosity/mucoadhesive modifiers, and thus had lower retention at the ocular surface. Hence, to enhance the residence at the precorneal/corneal sites, we sought to develop and optimize a suitable gelling system to incorporate the NTM carriers—NTM-PNLCs (developed and optimized in our previous study6)—for topical ocular administration.
A couple of studies have reported on the design and performance of NTM gelling systems for ocular application.31,32 The study by Janga et al. reports on the design and evaluation of NTM loaded bilosome hydrogel system intended for the back-of-the-eye delivery. In their study, the developed and optimized system showed enhancement in transcorneal flux in vitro and in vivo. In another study by Paradkar and Parmar, NTM loaded niosomal gel also exhibited improved transcorneal permeation, in vitro. The fabrication processes for the formulations in both the publications involves the use of organic solvents [dimethyl sulfoxide (DMSO), chloroform, ethanol, and methanol], which may be toxic even in residual amounts. So also, use of organic solvents makes the scale-up of the process challenging. The amount of surfactant (Span 60) used in the formulation of NTM loaded niosomes reported by Janga et al. and Paradkar and Parmar, is more than ∼5 times the amount of Span used in the fabrication of NTM-PNLC-GEL investigated in this study. Use of such high amount of surfactant could also lead to potential irritations/toxicities. The formulation investigated in this study does not use any organic solvent (potentially scalable formulation) and has a significantly lower amount of Span (∼5 times lower), thereby circumventing any potential toxicities/irritations in the aggravated diseased conditions. Also, to the best of our knowledge, ours is the first study that assesses the effectiveness of a carboxyvinyl polymer-based gelling system for ocular applications by evaluating, both, precorneal tear PK (front-of-the-eye delivery) and ocular biodistribution (back-of-the-eye delivery), in vivo.
In this study, different gel formulations were evaluated for their critical quality attributes (CQAs); first, being the gelling time and gel depot collapse time. From Table 5, it is evident that the gel formulations (B1, B3, B5, and B7) having low Carbopol 940 concentration (<0.4% w/v) did not form a gel depot upon addition in STF. The formulations containing higher amounts of Carbopol 940 (0.4% w/v) formed the gel depot in STF that persisted for more than 12 h. It is evident that the presence of Carbopol 940 in higher amounts (>0.1% w/v) is essential for maintaining the gel characteristics of the NTM-PNLC-GEL formulation. Therefore, formulations B2, B4, B6, and B8, having higher concentration of Carbopol 940 (0.4% w/v), were selected for further evaluation of their CQA.
The second CQA was rheology and viscosity. It is recommended that the ocular gelling systems exhibit pseudoplastic flow patterns, which implies that the gelling systems should undergo a reduction in their viscosity upon the application of shear, that is, exhibit a shear-thinning rheology.33 The mechanism of blinking generates shear that ranges between 10,000 and 40,000 1/s.33–35 Therefore, if the gelling formulation exhibits shear-thinning rheology, the process of blinking is not affected, avoiding any discomfort to the eye, thereby enhancing patient compliance.36 Also, it is reported that ocular gelling systems having viscosity between 5 and 1,000 cP exhibit ease of application without causing any ocular discomfort or interfering with the blinking process.36,37 The NTM-PNLC-GELS exhibit a viscosity of ∼500 cP at 500 1/s shear rate (20 times lower shear than the shear generated by blinking), indicating that the viscosity would be even lower at the blinking shear rates. Therefore, the application of a gelling system with shear-thinning rheology leads to a reduction in the viscosity of the gelling formulation, owing to the shearing force associated with blinking. Accordingly, all the selected gel-forming formulations (B2, B4, B6, and B8) exhibited shear-thinning rheology and a marked decrease in viscosity with increasing shear rates (Fig. 1).
Gel texture analysis parameters such as firmness and work of adhesion (Table 5) are the CQAs that provide information on the gel characteristics. Firmness describes the ability of the gel to maintain its cohesivity and work of adhesion describes the extent of ability of spreading of the gel as a thin film upon the application of shear.38 The firmness and work of adhesion values for gel formulations B2, B4, B6, and B8, indicate that they would maintain the cohesivity of the gel film and help stabilize it as a thin film over the ocular surface. In addition, a statistically nonsignificant difference was observed in the values for viscosity, rheological patterns, firmness, and work of adhesion of these formulations. The concentration of Carbopol 940 in these formulations was the same (0.4% w/v), which suggests that Carbopol 940 is primarily responsible for affecting these 2 CQA in these formulations. This is consistent with reports that Carbopol (all grades) is known to have a dominant effect on the texture properties of the formulations containing it.23,39–44 The shear-thinning rheology of the gels coupled with favorable firmness and adhesivity values would aid in the stabilization of the gel film over the ocular surface without affecting the corneal tear film.34,38,45
Since all the gelling formulations (B2, B4, B6, and B8) exhibited similar rheological and textural properties, the most optimum gelling system was selected on the basis of guar gum-to-boric acid ratio. In all the abovementioned formulations, guar gum: boric acid ratio ranged from 1:2 (B2), 1:2.5 (B8), 1:4 (B4), to 1:5 (B6). Although the texture characteristics of B2, B4, B6, and B8 were not significantly different, the unstressed gel viscosity of formulation B2 was the highest, whereas that of B6 was the lowest. Thus, the ratio of guar gum and boric acid had an effect on the viscosity of the gel formed on contact of the formulation with the STF, B2 (1:2) demonstrating higher gel viscosity than any of the others (1:2.5, 1:4, or 1:5).
Formulations B2 and B6 were further evaluated for their transcorneal permeation characteristics to understand if guar gum: boric acid ratio had any impact on the permeation of NTM-PNLCs from the gelling system. From Fig. 2, it is observed that the gelling formulation B6 (guar gum:boric acid::1:5) did not show any transcorneal NTM permeation at the end of the 3-h study, whereas gelling formulation B2 (guar gum:boric acid::1:2) showed transcorneal NTM permeation. This could be attributed to the presence of a higher amount of boric acid (borate anion), which could have led to the formation of a borate-NTM (a polyene antifungal) complex, which would have potentially reduced the solubility and dissolution rate, and reduced/controlled the transcorneal NTM permeation.46 Thus, the gelling formulation B2 was chosen as the most suitable and optimized formulation for further evaluations.
The trend for transcorneal rate of permeation, flux, and permeability followed the order—NTM-PNLC-GEL < Natacyn < NTM-PNLCs. The trend was the highest for NTM-PNLCs, which is consistent with the reported literature (PEGylation facilitates improved penetration across the corneal mucosa and epithelium).6,47–49 However, in case of NTM-PNLC-GEL, the trend was lowest, which could be ascribed to the entrapment of the NTM-PNLCs in the gelling system.32 The gelling system manifested as a primary barrier for NTM release from the embedded NTM-PNLCs.32
The low standard deviation for the mean NTM content (95.53 ± 0.61) % w/v obtained from 3 different regions of the formulation indicates a homogenous formulation. The pH of an ophthalmic formulation plays a critical role in assessing it potential utility, since extreme pH ranges could lead to ocular discomfort, irritation, and redness.36 pH of the FDA-approved marketed suspension (Natacyn) ranges between pH 5.0 and 7.5; therefore, the optimized gelling system reported in this study, having a pH value (5.5 ± 0.1), would also be well tolerated.50
The rate of release of NTM was sustained and slower from the NTM-PNLC-GEL in comparison to the NTM suspension (control) (Fig. 3A). A 3-fold decrease was found in the NTM release from the gelling system in comparison to the control, indicating that the entrapment of NTM in NLCs and further entrapment of the NTM-PNLCs in the cross-linked gelling system sustained/slowed the release of NTM from NTM-PNLC-GEL. The modeling of release data for release kinetics revealed that the NTM release from NTM-PNLC-GEL showed a better fit to Higuchi release kinetics compared to the other release models (higher r2 value) (Fig. 3B).51 This fit implies that assumptions of Higuchi kinetics hold true.36,52,53
The tear kinetics data show that the MRT0–∞ and T0.5 with the NTM-PNLC-GEL was significantly greater than NTM-PNLCs, indicating that the gelling system prolongs the MRT of NTM by decreasing its precorneal loss (Table 6). The dose-normalized Cmax and AUC0–t values associated with the NTM-PNLC-GEL was ≈2-fold higher than NTM-PNLCs. NTM was detectable in the tear until the end of 5 h from the gelling system, in contrast to NTM-PNLCs, wherein NTM was detected until the end of 3 h only. Interestingly, a 16-fold higher dose of NTM with the Natacyn suspension formulation exhibited similar T0.5 and MRT values (statistically nonsignificant). The dose-normalized Cmax (Fig. 4) and AUC0–t of NTM from Natacyn were, thus, disproportionately ≈14-fold and 7-fold lower than that from NTM-PNLC-GEL. The NTM-PNLC-GEL system at a lower dose (1/16th of Natacyn) achieves significantly better NTM PK parameters compared to Natacyn, suggesting that NTM-PNLC-GEL could be a potential alternative to Natacyn in case of superficial fungal infections.
In our previous investigations, NTM-PNLCs (0.3%) showed enhanced NTM permeation across the intact corneal barrier, and the NTM levels from NTM-PNLCs were similar to the marketed suspension (Natacyn, 5%) in inner ocular tissues (AH, VH, and ICB), in vivo.6 Therefore, to assess the effectiveness of the gelling formulation in NTM delivery in the current ocular biodistribution study, dosing specifications (amount and volume of dose) were kept similar to the previously published study. NTM formulations containing 0.3 mg NTM were chosen, since they were highest NTM-loaded formulations that were optimized and stabilized, which provided enhanced transcorneal permeation.6 One hundred microliters of the formulations was instilled to provide the highest NTM dose (0.3 mg) from the formulations and was instilled as 2 drops of 50 μL to prevent spillage from the cul de sac. A high dose of NTM was chosen, since the in vivo evaluations were to be conducted across intact and uninflamed cornea, which would pose a significant barrier for NTM permeation than a fungal infected and inflamed corneal barrier. Since Natacyn is clinically administered at 5% dose, in these in vivo investigations, its clinical dose was followed to compare its profile to the NTM formulations at lower dose. The current therapeutic regimen calls for the administration of Natacyn (5%) every 1 or 2 h.1,6,10 Therefore, to evaluate if the optimized gelling system could potentially reduce the dosing frequency, every 3- and 4-h instillation was investigated.
In cornea, AH, and ICB (Fig. 5), NTM concentration from NTM-PNLC-GEL was significantly higher than NTM concentration obtained from NTM-PNLC, consistent with the higher MRT and T0.5 values in the tear fluid. Higher MRT and T0.5 values suggested a higher retention and lower elimination of NTM from the gelling system compared to NTM-PNLCs. The NTM concentration in cornea from Natacyn (5%) was ≈2.5- and 3.5-fold higher (every 3- and 4-h dosing, respectively), ≈2-fold higher in ICB and ≈3-fold higher in VH compared to NTM levels obtained from NTM-PNLC-GEL. However, in case of all the abovementioned observations, it should be noted that Natacyn dose, which was 16-fold higher, caused only a disproportionate increase in the corneal, ICB, and VH concentrations compared to NTM-PNLC-GEL. In contrast to the other tissues, AH NTM concentrations from the NTM-PNLC-GEL formulation were 3- and 2-fold higher (for every 3- and 4-h instillation, respectively) than Natacyn (5%). High MRT and T0.5 for NTM-PNLC-GEL coupled with the quicker diffusion of NTM-PNLCs from the gelling system across the corneal and ocular barriers, owing to PEGylation, could have led to higher NTM concentrations in AH from NTM-PNLC-GEL in comparison to Natacyn (5%).
Conclusion
The NTM-PNLC-GEL—composed of carboxyvinyl polymer guar gum-borate system reported in this study—could serve as a potential substitute to the Natacyn suspension in cases of OFI. The optimized NTM-PNLC-GEL system exhibited the formation of a gel depot that did not collapse immediately (>12 h), shear-thinning rheology, adequate firmness, and spreadability (evaluated as work of adhesion). In addition, the transcorneal permeation and release studies indicated that the NTM-PNLC-GEL exhibited a lower/slower flux, rate, and sustained release of NTM in comparison to the control/s. The NTM-PNLC-GEL demonstrated superior precorneal PK parameters and ocular biodistribution in comparison to NTM-PNLCs (without the gelling components), which indicated that incorporation of the gelling agents improves the residence and lowers the precorneal losses/drainage, thereby improving its effectiveness. The NTM-PNLC-GEL (0.3%) attained comparable MRT and T0.5 and higher dose-normalized Cmax and AUC0–t at the precorneal/corneal sites, despite a 16-fold lower dose for NTM-PNLC-GEL compared to Natacyn (5%). Thus, NTM-PNLC-GEL could be a potential alternative platform to the conventional marketed suspension during the ocular antifungal regimen.
Acknowledgment
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
The authors do not declare any conflict of interests regarding this article.
Funding Information
This research work was funded and supported by National Institute of General Medical Sciences, National Institutes of Health (P30GM122733-01A1).
References
- 1. Patil A., Lakhani P., and Majumdar S.. Current perspectives on Natamycin in ocular fungal infections. J. Drug Deliv. Sci. Technol. 41:206–212, 2017 [Google Scholar]
- 2. Centers for Disease Control and Prevention (CDC). Fungal eye infections. 2017. www.cdc.gov/fungal/diseases/fungal-eye-infections/definition.html Accessed February1, 2020
- 3. Lakhani P., Patil A., and Majumdar S.. Challenges in the polyene- and azole-based pharmacotherapy of ocular fungal infections. J. Ocul. Pharmacol. Ther. 35:6–22, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thakkar R., Patil A., Mehraj T., Dudhipala N., and Majumdar S.. Updates in ocular antifungal pharmacotherapy: formulation and clinical perspectives. Curr. Fungal Infect. Rep. 13:45–58, 2019 [Google Scholar]
- 5. Müller G., Kara-José N., and de Castro R.. Antifungals in eye infections: drugs and routes of administration. Rev. Bras. Oftalmol. 72:132–141, 2013 [Google Scholar]
- 6. Patil A., Lakhani P., Taskar P., et al. Formulation development, optimization, and in vitro-in vivo characterization of Natamycin-loaded pegylated nano-lipid carriers for ocular applications. J. Pharm. Sci. 107:2160–2171, 2018 [DOI] [PubMed] [Google Scholar]
- 7. Thomas P. Current perspectives on ophthalmic mycoses. Clin. Microbiol. Rev. 16:730–797, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas P.A. Fungal infections of the cornea. Eye (Lond). 17:852–862, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Thomas P., and Kaliamurthy J.. Mycotic keratitis: epidemiology, diagnosis and management. Clin. Microbiol. Infect. 19:210–220, 2013 [DOI] [PubMed] [Google Scholar]
- 10. Patil A., and Majumdar S.. Echinocandins in ocular therapeutics. J. Ocul. Pharmacol. Ther. 33:340–352, 2017 [DOI] [PubMed] [Google Scholar]
- 11. O'Day D., Head W., Robinson R., and Clanton J.. Corneal penetration of topical amphotericin B and Natamycin. Curr. Eye Res. 5:877–882, 1986 [DOI] [PubMed] [Google Scholar]
- 12. Natacyn product insert. www.accessdata.fda.gov/drugsatfda_docs/label/2008/050514s009lbl.pdf Accessed February3, 2020
- 13. Kumar D., Jain N., Gulati N., and Nagaich U.. Nanoparticles laden in situ gelling system for ocular drug targeting. J. Adv. Pharm. Technol. Res. 4:9–17, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Iqbal M.A., Shadab M., Sahni J.K., Baboota S., Dang S., and Ali J.. Nanostructured lipid carriers system: recent advances in drug delivery. J. Drug Target. 20:813–830, 2012 [DOI] [PubMed] [Google Scholar]
- 15. Singh V., Bushetti S.S., Raju S.A., Ahmad R., Singh M., and Ajmal M.. Polymeric ocular hydrogels and ophthalmic inserts for controlled release of timolol maleate. J. Pharm. Bioallied Sci. 3:280–285, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Patel A., Cholkar K., Agrahari V., and Mitra A.. Ocular drug delivery systems: an overview. World J. Pharmacol. 2:47–64, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhagurkar A., Repka M., and Murthy S.. A novel approach for the development of a nanostructured lipid carrier formulation by hot-melt extrusion technology. J. Pharm. Sci. 106:1085–1091, 2017 [DOI] [PubMed] [Google Scholar]
- 18. Felt O., Einmahl S., Furrer P., Baeyens V., and Gurny R.. Polymeric systems for ophthalmic drug delivery. In: Dumitriu S., ed. Polymeric Biomaterials, Revised and Expanded. New York: CRC Press; 2001; p. 377–421 [Google Scholar]
- 19. Baranowski P., Karolewicz B., Gajda M., and Pluta J.. Ophthalmic drug dosage forms: characterisation and research methods. ScientificWorldJournal 2014:14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shaikh R., Raj Singh T.R., Garland M.J., Woolfson A.D., and Donnelly R.F.. Mucoadhesive drug delivery systems. J. Pharm. Bioallied Sci. 3:89–100, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo J.-H. Carbopol polymers for pharmaceutical drug delivery applications. Drug Deliv. Technol. 3:1–3, 2003 [Google Scholar]
- 22. Chowhan M.A., Ghosh M., Asgharian B., and Han W.W. Carboxyvinyl polymer-containing nanoparticle suspensions. Patent US 8921337 B2. Fort Worth, TX: Alcon Research Ltd.; 2014
- 23. Bhagurkar A.M., Angamuthu M., Patil H., et al. Development of an ointment formulation using hot-melt extrusion technology. AAPS PharmSciTech. 17:158–166, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lakhani P., Patil A., Taskar P., Ashour E., and Majumdar S.. Curcumin-loaded nanostructured lipid carriers for ocular drug delivery: design optimization and characterization. J. Drug Deliv. Sci. Technol. 47:159–166, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Majumdar S., Hingorani T., Srirangam R., Gadepalli R.S., Rimoldi J.M., and Repka M.A.. Transcorneal permeation of l- and d-aspartate ester prodrugs of acyclovir: delineation of passive diffusion versus transporter involvement. Pharm. Res. 26:1261–1269, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Warsi M.H., Anwar M., Garg V., et al. Dorzolamide-loaded PLGA/vitamin E TPGS nanoparticles for glaucoma therapy: pharmacoscintigraphy study and evaluation of extended ocular hypotensive effect in rabbits. Colloids Surf. B Biointerfaces 122:423–431, 2014 [DOI] [PubMed] [Google Scholar]
- 27. Marques M., Löbenberg R., and Almukainzi M.. Simulated biological fluids with possible application in dissolution testing. Diss.Tech. 8:15–28, 2011 [Google Scholar]
- 28. Zhang Y., Huo M., Zhou J., and Xie S.. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput. Methods Programs Biomed. 99:306–314, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Thangabalan B., and Kumar V.. Analytical method development and validation of Natamycin in eye drop by RP-HPLC. Asian J. Pharm. Clin. Res. 6:134–135, 2013 [Google Scholar]
- 30. Lakhani P., Patil A., Wu K.-W., et al. Optimization, stabilization, and characterization of amphotericin B loaded nanostructured lipid carriers for ocular drug delivery. Int. J. Pharm. 572:118771, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Paradkar M., and Parmar M.. Formulation development and evaluation of Natamycin niosomal in-situ gel for ophthalmic drug delivery. J. Drug Deliv. Sci. Technol. 39:113–122, 2017 [Google Scholar]
- 32. Janga K.Y., Tatke A., Balguri S.P., et al. Ion-sensitive in situ hydrogels of Natamycin bilosomes for enhanced and prolonged ocular pharmacotherapy: in vitro permeability, cytotoxicity and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 46:1039–1050, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zignani M., Tabatabay C., and Gurny R.. Topical semi-solid drug delivery: kinetics and tolerance of ophthalmic hydrogels. Adv. Drug Deliv. Rev. 16:51–60, 1995 [Google Scholar]
- 34. Bother H., and Waaler T.. Rheological characterization of tear substitutes. Drug Dev. Ind. Pharm. 16:755–768, 1990 [Google Scholar]
- 35. Schoenwald R.D., Ward R.L., DeSantis L.M., and Roehrs R.E.. Inlfuence of high-viscosity vehicles on miotic effect of pilocarpine. J. Pharm. Sci. 67:1280–1283, 1978 [DOI] [PubMed] [Google Scholar]
- 36. Patil A., Singh S., Opere C., and Dash A.. Sustained-release delivery system of a slow hydrogen sulfide donor, GYY 4137, for potential application in glaucoma. AAPS PharmSciTech. 18:2291–2302, 2017 [DOI] [PubMed] [Google Scholar]
- 37. Rathore K. In situ gelling ophthalmic drug delivery system: an overview. Int. J. Pharm. Sci. 2:30–34, 2010 [Google Scholar]
- 38. Hurler J., Engesland A., Poorahmary Kermany B., and Škalko-Basnet N.. Improved texture analysis for hydrogel characterization: gel cohesiveness, adhesiveness, and hardness. J. Appl. Polym. Sci. 125:180–188, 2012 [Google Scholar]
- 39. Poorahmary Kermany B. Carbopol hydrogels for topical administration: treatment of wounds. Drug Transport and Delivery Research Group. Tromsø: University of Tromsø; 2010
- 40. A-sasutjarit R., Sirivat A., and Vayumhasuwan P.. Viscoelastic properties of Carbopol 940 gels and their relationships to piroxicam diffusion coefficients in gel bases. Pharm. Res. 22:2134–2140, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Dantas M.G.B., Reis S.A.G.B., Damasceno C.M.D., et al. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. ScientificWorldJournal 2016:7394685, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shukr M., and Metwally G.. Evaluation of topical gel bases formulated with various essential oils for antibacterial activity against methicillin resistant Staphylococcus aureus. Trop. J. Pharm. Res. 12:877–884, 2013 [Google Scholar]
- 43. Barry B.W., and Meyer M.C.. The rheological properties of carbopol gels I. Continuous shear and creep properties of carbopol gels. Int. J. Pharm. 2:1–25, 1979 [Google Scholar]
- 44. Todica M., Pop C.V., Udrescu L., and Pop M.. Rheological behavior of some aqueous gels of Carbopol with pharmaceutical applications. Chinese Phys. Lett. 27:018301, 2010 [Google Scholar]
- 45. Manjappa A.S., Nanjwade B.K., Manvi F.V., and Murthy R.S.R.. Sustained ophthalmic in situ gel of ketorolac tromethamine: rheology and in vivo studies. Drug Dev. Res. 70:417–424, 2009 [Google Scholar]
- 46. Vandeputte J. Borate complex of polyene macrolide antibiotics. In: Office USP, ed. ER New York, NY: Squibb and Sons, LLC; 1973
- 47. Mun E., Morrison P., Williams A., and Khutoryanskiy V.. On the barrier properties of the cornea: a microscopy study of the penetration of fluorescently labeled nanoparticles, polymers, and sodium fluorescein. Mol. Pharm. 11:3556–3564, 2014 [DOI] [PubMed] [Google Scholar]
- 48. Lai S., Wang Y., and Hanes J.. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 61:158–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Xu Q., Boylan N., Cai S., Miao B., Patel H., and Hanes J.. Scalable method to produce biodegradable nanoparticles that rapidly penetrate human mucus. J. Control. Release 170:279–286, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Natacyn®. United States Food and Drug Administration. www.accessdata.fda.gov/drugsatfda_docs/label/2008/050514s009lbl.pdf Accessed February1, 2020
- 51. Patil A. Preparation, characterization and toxicity evaluation of a novel sustained release delivery system for a hydrogen sulfide donor—GYY 4137. Pharmaceutical Sciences. Omaha, NE: Creighton University; 2016; p. 147
- 52. Dash S., Murthy P., Nath L., and Chowdhury P.. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 67:217–223, 2010 [PubMed] [Google Scholar]
- 53. Schwartz J., Simonelli A., and Higuchi W.. Drug release from wax matrices. I. Analysis of data with first-order kinetics and with the diffusion-controlled model. J. Pharm. Sci. 57:274–277, 1968 [DOI] [PubMed] [Google Scholar]