Abbreviations
- COPD
chronic obstructive pulmonary disease
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- ICU
intensive care unit
- IQR
interquartile range
- LDH
lactate dehydrogenase
- MDT
multidisciplinary team
- SpO2:FiO2
ratio of peripheral capillary oxygen saturation compared to fraction of inspired oxygen
To the Editors:
Coronavirus disease 2019 (COVID‐19) infection, an illness caused by severe acute respiratory coronavirus 2 (SARS‐CoV‐2), has spread rapidly worldwide resulting in a global pandemic. A subset of individuals with COVID‐19 present with severe pneumonia, evolving in some cases to acute respiratory distress syndrome (ARDS), coupled with clinical and biochemical features of hyperinflammatory syndrome, and characterized by increased levels of ferritin, C‐reactive protein (CRP), interleukin (IL)‐6 and d‐dimer. 1 , 2 , 3 These acute phase proteins may reflect circulating IL‐6, possibly a key driver of a dysregulated inflammatory response in COVID‐19. 2
Tocilizumab is a humanized monoclonal antibody targeting the IL‐6 receptor. Used to treat inflammatory arthritis, tocilizumab has been effectively used to treat both secondary hemophagocytic lymphohistiocytosis, an autoimmune‐based hyperinflammatory syndrome, as well as cytokine release syndrome, a side effect of chimeric antigen receptor T (CAR‐T) cell immunotherapy. 4 , 5 Currently, published data on the use of tocilizumab in the setting of COVID‐19 infection is scarce. 6 , 7 We report data on six individuals with severe COVID‐19 pneumonia and hyperinflammatory state treated with tocilizumab, with outcomes suggesting a potential role for tocilizumab in specific clinical circumstances in COVID‐19 infection that could inform future clinical trial design.
Between 7 March and 7 April 2020, 193 patients were admitted with confirmed COVID‐19 infection to St. Vincent's University Hospital, an 836‐bed tertiary referral centre in Dublin, Ireland. All patients enrolled into the All‐Ireland Infectious Diseases Cohort Study, a multicentre, prospective cohort study. This study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practices, and was approved by the St. Vincent's Healthcare Group Research Ethics Committee. All patients who agreed to participate provided written informed consent.
Patients were considered for tocilizumab based on the presence of severe COVID‐19 pneumonia and evidence of a hyperinflammatory response. Cases were discussed at multidisciplinary team (MDT) meetings involving infectious disease, pulmonary, critical care and rheumatology physicians. Individuals with evidence of disease progression manifested by moderate to severe respiratory failure as defined by a ratio of peripheral capillary oxygen saturation compared to fraction of inspired oxygen (SpO2:FiO2) of ≤315 mm Hg, 8 progression of or new pulmonary infiltrates on chest imaging and evidence of hyperinflammation (including three of temperature >38°C in the past 48 h, d‐dimer >1.5 μg/mL and elevated levels of CRP, ferritin, lactate dehydrogenase (LDH) or fibrinogen) were considered for MDT discussion. Use of tocilizumab was avoided in those with imminent requirement for intubation/mechanical ventilation, known immunosuppression, active malignancy, uncontrolled bacterial infection, liver transaminases 10 times the normal values or history of significant gastrointestinal ulcerative disease.
Of the 193 cases, 8 (4.1%) were considered for tocilizumab therapy of whom 6 patients were treated with a single dose of intravenous tocilizumab at 8 mg/kg (maximum dose: 800 mg). The reasons that two patients did not receive tocilizumab after multidisciplinary discussion were severe frailty with multiple comorbidities in one patient and rapid progression of respiratory failure requiring imminent intubation in the other patient. Baseline demographic/clinical characteristics of the six treated patients are outlined in Table 1. On admission, four had pulmonary infiltrates on imaging, all had systemic inflammatory response with increased CRP (median: 72.3 mg/L, interquartile range (IQR): 40.1–127.8 mg/L) and ferritin levels (median: 1803 mg/L, IQR: 1071–3163 mg/L) and the median SpO2:FiO2 ratio was 322 mm Hg (IQR: 291–421 mm Hg) (Table 1,Fig. 1,Table S1 (Supplementary Information)).
Table 1.
Demographics, clinical characteristics and radiological findings
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | n/Median (IQR) |
|---|---|---|---|---|---|---|---|
| Demographics | |||||||
| Age (years) | 63 | 61 | 63 | 55 | 60 | 48 | 60.5 (56–62.5) |
| Sex | Male | Male | Female | Male | Male | Male | 5M:1F |
| Smoking history | Non‐smoker | Non‐smoker | Non‐smoker | Ex‐smoker | Ex‐smoker | Non‐smoker | n = 2/6 |
| Body mass index (kg/m2) | 33.45 | 31.08 | 46.16 | 28.32 | 25.59 | 27.05 | 29.7 (27.36–32.85) |
| Comorbidities | |||||||
| Cardiovascular disease | Cardiomyopathy, atrial fibrillation | Hypertension | — | Hypertension | Hypertension | — | n = 4/6 |
| Respiratory disease | Asthma | — | — | — | Emphysema | — | n = 2/6 |
| Metabolic disease | Dyslipidaemia | — | — | Dyslipidaemia | — | — | n = 2/6 |
| Autoimmune disease | — | — | Psoriatic arthritis | — | — | — | n = 1/6 |
| Findings on admission to hospital | |||||||
| SpO2:FiO2 ratio (mm Hg) | 452 | 447 | 182 | 303 | 342 | 287 | 322 (291–421) |
| Chest radiograph features | No focal infiltrate | No focal infiltrate | Multifocal areas of consolidation in the mid and lower zones bilaterally | Linear atelectasis in the right perihilar region and right lower zone | Bilateral peripheral midzone consolidation | Focal consolidation in the periphery of the right mid and left lower zones | n = 4/6 had abnormal chest radiograph on admission |
| Findings at the time of tocilizumab administration | |||||||
| Number of days after symptom onset | 10 | 9 | 13 | 8 | 8 | 11 | 9.5 (8–11) |
| SpO2:FiO2 ratio (mm Hg) | 224 | 325 | 98.8 | 232 | 249 | 240 | 236 (226–247) |
| Chest radiograph features | New patchy airspace opacities in the right mid and bilateral lower zones | New patchy hazy airspace opacities throughout both mid and lower zones | Progression in the multifocal consolidation, particularly in the periphery of the left mid and lower zones | New patchy peripheral hazy opacities in the right mid and bilateral lower zones | Significant progression in the midzone consolidation, particularly on the left side | Progression of existing consolidation with new right basal consolidation | n = 6/6 had progressive radiographic changes |
| Number of days of hydroxychloroquine azithromycin therapy prior to tocilizumab | 4 | 3 | 3 | 3 | 1 | 5 | 3 (3–4) |
| Required non‐invasive positive pressure ventilation | Yes | No | Yes | No | No | No | n = 2/6 |
| Admitted to intensive care unit | Yes | No | Yes | No | No | No | n = 2/6 |
| Number of days after tocilizumab discharged home | 8 | 6 | 7 | 7 | 7 | 7 | 7 (7–7) |
| Required hospital readmission | No | No | No | No | Yes | No | n = 1/6 |
IQR, interquartile range; SpO2:FiO2, ratio of peripheral capillary oxygen saturation compared to fraction of inspired oxygen.
Figure 1.
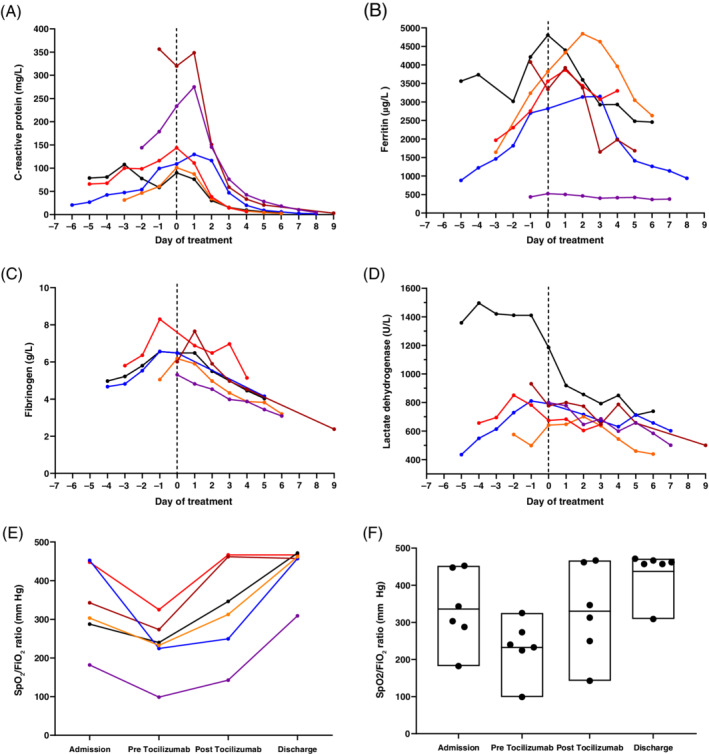
Laboratory data from six coronavirus disease 2019 (COVID‐19) patients treated with tocilizumab. Day 0 (dashed line) is the day on which tocilizumab treatment was administered and data are presented prior to this and following drug administration for C‐reactive protein (mg/L) (A), ferritin (μg/L) (B), fibrinogen (g/L) (C) and lactate dehydrogenase (U/L) (D). All patients had evidence of a systemic hyperinflammatory state with resolution of inflammation following therapy. (E) SpO2:FiO2 (ratio of peripheral capillary oxygen saturation (SpO2) compared to fraction of inspired oxygen (FiO2)) per patient at four time points. Data are displayed for oxygenation at admission, immediately before tocilizumab administration (pre tocilizumab), 3 days after administration (post tocilizumab) and at the time of discharge from hospital (range: 5–9 days). (F) SpO2:FiO2 ratio range for all six patients on admission, immediately before tocilizumab administration (pre tocilizumab), 3–4 days after administration (post tocilizumab) and at the time of discharge from hospital (range: 6–8 days).
The median duration from onset of symptoms to clinical deterioration warranting MDT discussion was 9.5 days (IQR: 8–11.5 days). At the time of MDT discussion, all patients had progression of pulmonary infiltrates on chest radiograph from the time of admission and SpO2:FiO2 ratio had deteriorated (median: 236 mm Hg, IQR: 226–247 mm Hg) (Fig.1D,E). All patients met the criteria for hyperinflammatory state, evident by increased CRP (median: 126.6 mg/L, IQR: 103.2–242.2 mg//L), ferritin (median: 3451.5 mg/L, IQR: 2950–4138.2 mg/L) and fibrinogen (median: 6.33 g/L, IQR: 5.96–6.93 g/L) (Fig. 1A–D,Table S1 (Supplementary Information)).
Following tocilizumab, we observed a rapid decline in inflammatory markers and decreased oxygen requirements in all patients. Two patients initially deteriorated following tocilizumab. Both were admitted to the intensive care unit (ICU) and maintained on continuous positive airway pressure ventilatory support, being discharged from the ICU after 2 and 3 days, respectively, without the need for mechanical ventilation. All patients were discharged home at a median of 7 days (IQR: 7–8 days) post tocilizumab. One patient was readmitted to the hospital 2 days after discharge, 9 days after tocilizumab administration, due to an exacerbation of COPD complicated by bacterial pneumonia. This individual received antibiotics in hospital and was discharged to go home 7 days thereafter.
This report describes the clinical outcomes of six patients with COVID‐19 pneumonia and hyperinflammatory response treated with tocilizumab in the pre‐ICU setting and suggests favourable outcomes in this setting. Our data stand in contrast to a previous case series of use of tocilizumab in 15 patients with varying clinical presentations, from moderate severity to critically ill, in which the authors failed to identify a consistent improvement with use of both single‐dose and multiple‐dose tocilizumab. 6 However, this case series included a heterogeneous population, including a number of patients already under critical care, and doses of tocilizumab varied considerably (80–600 mg). Of the 15 subjects, 8 were treated concurrently with repeated doses of methylprednisolone. Similarly, another case series cautioning the use of tocilizumab reported that two patients were intubated and mechanically ventilated when they received tocilizumab. 9 Further reports on the use of tocilizumab have provided varying results, with some studies administering tocilizumab to more severe respiratory failure and using multiple does of tocilizumab within 24 h. 10 , 11 A study of 21 patients from China reported positive outcomes with tocilizumab. However, this cohort also received lopinavir/ritonavir, corticosteroids and interferon, so it is difficult to determine the true efficacy. 12 Notably, another study demonstrated worse outcomes in patients who were intubated compared to those who were not. 13 Our pre‐intensive care approach was more standardized in terms of patient profile, MDT approach and the dose used. None of our patients received other concurrent immunosuppressive therapy, providing a clearer indication of the effect of a single dose of tocilizumab in this setting. Although immunomodulatory therapy carries concerns relating to unwanted effects of immunosuppression, apart from one patient suffering an exacerbation of COPD, we observed no other safety signals.
Our data support opinions that therapeutic approaches directly targeting key cytokines to halt the innate immune response may be an important adjunct in moderate to severe cases of COVID‐19. 14 We demonstrate a marked reduction in the levels of CRP, ferritin and fibrinogen following tocilizumab therapy. While the reduction in CRP levels is likely a direct effect of tocilizumab, the other markers may be more representative of a change in inflammatory state. Interestingly, changes in serum LDH in our series did not track with reductions observed in other inflammatory markers and it is therefore unclear if LDH accurately reflects IL‐6‐driven inflammation in this case series. Of note, in this cohort, all patients had elevated body mass index (BMI). It is known that IL‐6 levels correlate with BMI and that enhanced IL‐6 signalling drives inflammation in obesity. 15 A limitation of this report is that serum IL‐6 levels were not measured, as this was not a routinely available clinical biomarker. However, CRP and ferritin are both acute phase proteins that are released in response to IL‐6 stimulation, and can be used as surrogates.
This report adds to the need for data on the potential efficacy of tocilizumab as an approach for cytokine release associated with COVID‐19. The fact that all individuals receiving tocilizumab in this study avoided the need for mechanical ventilation, despite being critically unwell, is encouraging. Nevertheless, interpretation of the results require caution due to several other considerations, including the cohort being small, the patient group being relatively young and the absence of an appropriately matched control group. Hence, to determine the true efficacy and safety of tocilizumab in COVID‐19, randomized controlled trials are needed.
Author contributions
Conceptualization: C.M., S.S., E.R.F., M.W.B., L.O., M.P.K., P.W.M. Data curation: C.M., S.S., C.O., L.O., P.W.M. Formal analysis: C.M., S.S., M.W.B., L.O., D.J.M., P.W.M. Investigation: C.M., S.S., C.O., S.R., L.O., C.G.G., E.F.M., S.W., P.W.M. Methodology: C.M., E.R.F., M.W.B., L.O., D.J.M., C.G.G., E.F.M., S.W., A.C., P.D., M.P.K., P.W.M. Project administration: C.M., P.W.M. Supervision: P.W.M. Writing—original draft: C.M., S.S., E.R.F., M.W.B., M.P.K., P.W.M. Writing—review and editing: C.M., S.S., E.R.F., M.W.B., C.O., S.R., L.O., D.J.M., C.G.G., E.F.M., S.W., A.C., P.D., M.P.K., P.W.M
Supporting information
Table S1. Laboratory results.
McCarthy C, Savinelli S, Feeney ER, et al. Tocilizumab therapy in individuals with COVID‐19 infection and hyperinflammatory state. Respirology. 2020;25:1090–1094. 10.1111/resp.13912
Received 4 June 2020; invited to revise 23 June 2020; revised 24 June 2020; accepted 30 June 2020
Peer review handled by Editors‐in‐Chief: Phil Bardin and Paul Reynolds
REFERENCES
- 1. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to covid‐19 in italy. JAMA. 2020; 323(8): 1775–1776. [DOI] [PubMed] [Google Scholar]
- 2. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen H, Wang F, Zhang P, Zhang Y, Chen Y, Fan X, Cao X, Liu J, Yang Y, Wang B et al. Management of cytokine release syndrome related to CAR‐T cell therapy. Front. Med. 2019; 13: 610–7. [DOI] [PubMed] [Google Scholar]
- 5. Watanabe E, Sugawara H, Yamashita T, Ishii A, Oda A, Terai C. Successful tocilizumab therapy for macrophage activation syndrome associated with adult‐onset Still's disease: a case‐based review. Case Rep. Med. 2016; 2016: 5656320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID‐19: a single center experience. J. Med. Virol. 2020; 92: 814–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michot JM, Albiges L, Chaput N, Saada V, Pommeret F, Griscelli F, Balleyguier C, Besse B, Marabelle A, Netzer F et al. Tocilizumab, an anti‐IL6 receptor antibody, to treat Covid‐19‐related respiratory failure: a case report. Ann. Oncol. 2020; 31: 961–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest 2007; 132: 410–7. [DOI] [PubMed] [Google Scholar]
- 9. Radbel J, Narayanan N, Bhatt PJ. Use of tocilizumab for COVID‐19‐induced cytokine release syndrome: a cautionary case report. Chest 2020; 158: e15–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, Franceschini F, Airò P, Bazzani C, Beindorf EA et al. Tocilizumab for the treatment of severe COVID‐19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020; 19: 102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Campochiaro C, Della‐Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, Tomelleri A, Baldissera E, Rovere‐Querini P, Ruggeri A et al. Efficacy and safety of tocilizumab in severe COVID‐19 patients: a single‐centre retrospective cohort study. Eur. J. Intern. Med. 2020; 76: 43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X et al. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc. Natl. Acad. Sci. U. S. A. 2020; 117: 10970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morena V, Milazzo L, Oreni L, Bestetti G, Fossali T, Bassoli C, Torre A, Cossu MV, Minari C, Ballone E et al. Off‐label use of tocilizumab for the treatment of SARS‐CoV‐2 pneumonia in Milan, Italy. Eur. J. Intern. Med. 2020; 76: 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu E, Pereira MMA, Karakasilioti I, Theurich S, Al‐Maarri M, Rappl G, Waisman A, Wunderlich FT, Brüning JC. Temporal and tissue‐specific requirements for T‐lymphocyte IL‐6 signalling in obesity‐associated inflammation and insulin resistance. Nat. Commun. 2017; 8: 14803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Laboratory results.


