Abstract
Liver impairment is frequent in patients with novel coronavirus disease (COVID‐19) and direct viral tropism for the liver has been proven. Since several of the currently administered drugs against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) are possibly hepatotoxic, the management of patients with COVID‐19 and liver failure is still an almost unexplored field. Taking this challenging case of acute HBV with persistent hyperbilirubinemia and SARS‐COV‐2 infection with respiratory distress as a starting point, we here loop through this condition. Where the available therapeutic options are scarce, we here propose hemoperfusion (HP) as an attractive alternative to both delay any late‐stage progression of hyper inflammation process in COVID‐19 and remove the toxins involved in acute liver failure.
Keywords: COVID‐19, HBV, hemoperfusion
1. INTRODUCTION
Beyond the well‐known catastrophic pulmonary effects of coronavirus disease 2019 (COVID‐19), the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has also been associated with a significant damage to other organ systems, including kidney, heart, vessels and liver. 1 , 2 , 3 , 4 , 5 Specifically, the increase in liver enzymes in COVID‐19 patients has been reported worldwide 6 , 7 but the prevalence of liver injury and the associated clinical features of these patients are currently scarce. 8
Xu et al recently presented a case report of acute on chronic hepatic failure, 9 but no other cases of acute hepatitis occurring during the course of COVID‐19 are reported and treatment strategies are currently not well‐defined.
Viral hepatitis and COVID‐19 is still an almost unexplored field. It is indeed controversial whether patients with chronic Hepatitis B virus (HBV) are likely to have a worse outcome of COVID‐19. 10 , 11 According to Zou et al, 12 patients with SARS‐CoV‐2 and chronic HBV co‐infection with liver injury and coagulation dysfunction were more likely to develop severe illness and had higher mortality.
This selected case of acute HBV hepatitis and COVID‐19 is a cue to loop through the challenging coinfection.
We selected the patient case from the SMatteo COvid19 Registry (SMACORE), which is the cohort of patients with a confirmed diagnosis of COVID‐19 disease referred to the IRCCS Policlinico San Matteo Hospital of Pavia, Italy, from February 2020.
The SMACORE database includes demographic, clinical laboratory tests, treatment and outcome data. Ethics approval for observational research using SMACORE data was obtained from the local ethics committee.
2. CASE REPORT
A previously healthy 64 years old man presented with a 2 days history of generalized jaundice, scleral icterus, dark urine and malaise in the Hospital of Vigevano, province of Pavia, Lombardy, northern Italy on March 2020.
He was not on any medications or supplements and denied any alcohol consumption, recent transfusions of blood products and high‐risk sexual behaviour.
Admission laboratory study revealed aspartate aminotransferase (AST) 1670 U/L, alanine aminotransferase (ALT) 1540 U/L and significant levels of direct hyperbilirubinaemia (Total bilirubin 37 mg/dL, Direct bilirubin 30 mg/dL) supporting the clinical hypothesis of acute hepatitis.
Additional testing indicated a positive hepatitis B surface antigen (HBsAg), positive hepatitis B core IgM (HBcAb IgM), negative hepatitis B surface antibody (HBsAb), positive hepatitis Be antigen (HBeAg) and a negative hepatitis Be antibody (HBeAb) consistent with acute hepatitis B infection. HBV‐DNA PCR, indeed, returned with >170 million IU/mL.
Serologic and PCR tests for hepatitis A virus, hepatitis C virus, hepatitis D virus, hepatitis E virus, Epstein‐Barr virus, cytomegalovirus and human immunodeficiency virus were negative.
A liver ultrasound did not point out any anomalies, showing an echogenic liver without cirrhosis, common bile duct obstruction or gallstones.
At hospital admission, the transcriptase‐polymerase chain reaction (RT‐PCR) of the patient's nasal swab was negative for acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection.
By the third day of hospitalization, the patient's symptoms had partially resolved: his transaminitis had significantly dropped (Alanine aminotransferase ALT 120 U/L) though direct hyperbilirubinemia persisted (Total bilirubin 30 mg/dL, Direct bilirubin 20 mg/dL) and a progressive impairment of the renal function had occurred (creatinine 1.7 mg/dL; eGFR 52 mL/min/1.73 m2).
Despite further investigations were still ongoing, the patient chose to leave the hospital after 7 days of hospitalization.
Within one week after hospital self‐discharge, the patient developed fever (body temperature of 38.3°C), cough, dyspnoea and chest distress. He was then admitted to the Infectious Diseases department of our Hospital, IRCCS Policlinico San Matteo of Pavia, Northern Italy. This time, the RT‐PCR of his nasal swab confirmed SARS‐CoV‐2 infection. In this respect, we are unable to rule out either in‐hospital or community transmission of the infection.
His physical exam revealed generalized jaundice on the one hand, and on the other hand a respiratory rate (RR) of 42 and an oxygen saturation of 89% on room air, improving to 95% on a 15 L/min non‐rebreather mask.
Admission laboratory results (Table 1) showed elevated levels of C reactive protein and several inflammatory cytokines together with neutrophilic leukocytosis, which are consistent with the hyper‐inflammation phase of SARS‐CoV‐2 infection, as already described in the literature. 1 , 2 , 3 In addition, renal failure persisted.
TABLE 1.
Biochemical and Cytokine serum level
| Laboratory | Value | Measures | Reference range |
|---|---|---|---|
| Interferon gamma (Ig γ) | 0.1 | pg/mL | <15.6 |
| Interleuchina‐1 (IL‐1) | 40.8 | pg/mL | 0‐3.9 |
| Interleuchina‐10 (IL‐10) | 45.1 | pg/mL | 0‐7.8 |
| Interleuchina‐2 (IL‐2) | 14.40 | pg/mL | 0‐31.2 |
| Interleuchina‐6 (IL‐6) | 69.9 | pg/mL | 0‐3.1 |
| Interleuchina‐8 (IL‐8) | 68.5 | pg/mL | 0‐31.2 |
| Tumor necrosis factor alpha | 0.12 | pg/mL | 0‐15.6 |
| Leukocytes | 11.40 | ×103/µL | 4.00‐10.00 |
| Neutrophils | 10.45 | ×103/µL | 2.0‐8.0 |
| Lymphocytes | 2.06 | ×103/µL | 1.5‐4.0 |
| LDH | 453 | mU/mL | 125‐220 |
| CRP | 8.6 | mg/dL | <0.5 |
| PCTI | 1.1 | ng/ml | 0.0‐0.5 |
| AST | 81 | U/L | <37 |
| ALT | 70 | U/L | <41 |
| Total bilirubin | 33.4 | U/L | <1.0 |
| Direct bilirubin | 28.4 | U/L | <0.2 |
| GGT | 177 | U/L | 5‐61 |
| Creatinine | 2 | U/L | 0.80‐1.20 |
| eGFR | 33 | mL/min/1.73m2 | |
| BUN | 95 | mg/dL | 10‐50 |
| Albumin | 1.3 | g/dL | 3.5‐5.0 |
| INR | 1.18 | ||
| PT | 76 | % | 70‐120 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C reactive protein; eGFR, estimated Glomerular Filtration Rate; GGT, γ‐glutamyl transferase; IgY, interferon gamma; IL‐1, interleuchina‐1; IL‐10, interleuchina‐10; IL‐2, interleuchina‐2; IL‐6, interleuchina‐6; IL‐8, interleuchina‐8; LDH, lactate dehydrogenasis; PCTI, procalcitonin; PT, prothrombin time ratio.
Regarding liver function tests, there was a still significant direct hyperbilirubinemia.
The chest radiograph showed an interstitial pneumonia with bilateral infiltrates.
The patient was treated with enoxaparin 6000 IU, hydroxychloroquine 400 mg per day and empiric antibiotic coverage with ceftriaxone 2 g once a day for the first 5 days of hospitalization. Although no evidence of fulminant hepatitis, the persistent increase in bilirubin levels was concerning and Entecavir 1 mg once a day was started. 13
As a result of the acute viral hepatitis and the reports of sudden acute decompensation in patients with COVID‐19, 4 despite being hemodynamically stable, the patient was moved to the preintensive care unit in our hospital.
As a result of the kidney failure and oliguria, two cycles of continuous renal replacement therapy (CRRT) were performed on day 6 and 7 obtaining a progressive clinical improvement though the creatinine levels stocked on higher levels than patient's basal (Figure 1).
FIGURE 1.
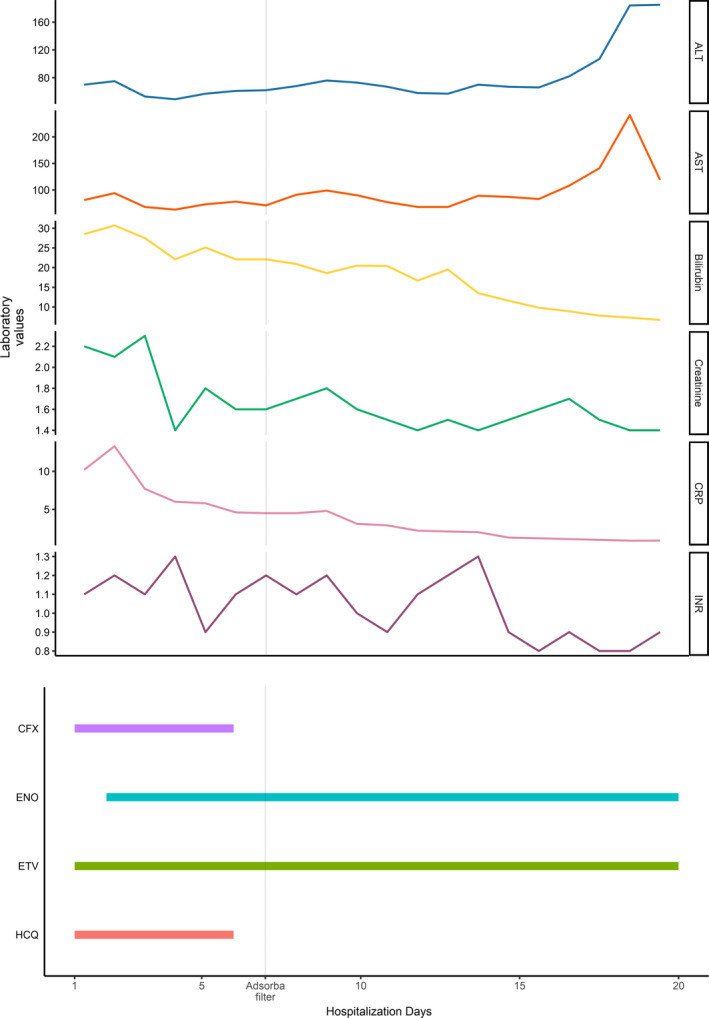
Trend of the laboratory values according to the days of hospitalization and Adsorba filter (on day 7) and other treatments administered during the hospitalization. ALT, alanine aminotransferase; AST, aspartate aminotransferase; CFX, ceftriaxone; CRP, C reactive protein; ENO, enoxaparin; ETV, entecavir; HCQ, hydroxychloroquine; INR, international normalized ratio
Furthermore, since cytokines and bilirubin tend to be difficult to remove with conventional haemodialysis or CCRT, two cycles of hemoperfusion (HP) treatment (Adsorba filter) were performed on day 7 and 8. As shown in Figure 1, the levels of bilirubin had slowly dropped within the next few days as well as those of inflammatory markers. The respiratory function had also improved significantly, and the patient was finally discharged on day 20. The patient also becomes negative for SARS‐CoV‐2 after 5 days from hospitalization and then persisted negative over time.
At the time of discharge, an assessment of hepatitis B serology was repeated and showed seroconversion, consisting of loss of HBeAg and appearance of HBeAb. In addition, hepatitis B virus (HBV) DNA had dropped to 748 IU/mL.
3. DISCUSSION
Alterations of LFT are frequent in COVID‐19 patients but their prognostic value is controversial (18, 19). Direct SARS‐COV‐2 infection of the liver has been demonstrated, and elevated levels of cholestatic indexes in COVID‐19 patients are possibly because of the viral tropism for ACE2 receptors expressed in cholangiocytes. 14 Moreover, a study led by Cai et al on 417 COVID‐19 patients highlighted the role played by hepatotoxic drugs in the onset of liver injury. 15
To our knowledge, this was the first reported case of acute HBV and SARS‐COV‐2 co‐infection that forced us to face the absence of data about managing COVID‐19 patients whit acute liver damage because of a different cause.
Wafa A. Aldhaleei et al recently reported a clinical case of HBV reactivation caused by SARS‐CoV‐2 in a young man with confusion and severe transaminitis. 16 However, since the patient was completely asymptomatic for COVID‐19, no specific treatment was deemed necessary.
To date, evidence on safety and efficacy of drugs against SARS‐CoV‐2 are missing and all approaches are empirical.
Even though there is no substantiated proofs about the COVID‐19 pathogenesis, it has been assumed that the disease presents with two distinct phases: an earlier viral phase, targeted by antiviral agents like Remdesivir, followed by a dysregulated immune response, known as cytokine storm, (with higher levels of cytokines as IL‐1, IL‐10, IL‐6 and TNF) which leads to clinical deterioration and acute respiratory distress syndrome (ARDS). Several treatments, including corticosteroids and IL‐6 blockage with Tocilizumab, have been used to control and mitigate the cytokine storm. 13 Most of these drugs are contraindicated in patients with liver damage because of their hepatotoxicity.
Moreover, since the use of corticosteroids has been an object of debate since the early days of COVID‐19 pandemic and immunosuppressive drugs are not indicated during HBV active replication, 13 steroids were out of our case, despite an antiviral treatment had been started.
Hemoperfusion is indicated in hepatic failure and hepatic encephalopathy to remove several toxins involved in liver damage, such as inflammatory cytokines (IL‐1, IL‐2, IL‐6, IL‐10), bile acids and bilirubin. Despite the lack of treatment guidelines and controlled human trials, HP has been used with efficacy to reduce IL‐6 levels in septic patients and also has been proposed to mitigate disease progression in COVID‐19 patients. 17
In such a complex and miscellaneous condition the conventional treatments to manage SARS‐COV‐2 infection were not indicated and, in the absence of available weapons, we decided to perform HP with Adsorba cartridge. This strategy would have “killed two birds with one stone” both lowering bilirubin levels and removing inflammatory cytokines. What would have happened without such treatment is clearly unknown to us but all we are aware of is that a significant improvement of pulmonary symptoms was obtained.
Hence, it is reasonable to assume an improvement in terms of respiratory mechanics and inflammation markers after the aforementioned treatment while an only partial benefit in reducing hyperbilirubinemia.
In addition, our case also challenged us with an acute kidney injury, which appears to be another significant issue. Renal impairment is indeed relatively frequent in COVID‐19 patients, probably because of the expression of ACE2 receptor. 15 , 16 On the other hand, our patient's kidney damage might have been related to bile cast nephropathy (BCN) because of the high levels of bilirubin and bile acids. Unfortunately, in the absence of a kidney biopsy, BCN remains a diagnosis by exclusion. 17 A liver biopsy would have been also useful to characterize the histological pattern of the disease and to evaluate the role SARS‐COV‐2 infection might have played. However, because of the slow but progressive reduction of hyperbilirubinaemia and to the improvement of the hepatic and renal functionality, not even the liver biopsy has been performed.
Colaneri M, Valsecchi P, Perotti L, et al. Running out of bullets: The challenging management of acute hepatitis and SARS-COV-2 from the SMatteo COvid19 Registry (SMACORE). Liver Int. 2020;40:2655–2659. 10.1111/liv.14609
Handling Editor: Luca Valenti
REFERENCES
- 1. Wang D, Hu BO, Hu C, et al, Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen N, Zhou M, Dong X, et al, Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al, Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou F, Yu T, Du R, et al, Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu J, Liu J, Zhao X, et al, Clinical characteristics of imported cases of COVID‐19 in Jiangsu Province: a multicenter descriptive study. Clin Infect Dis. 2020. 10.1093/cid/ciaa199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guan GW, Gao L, Wang JW, et al, Exploring the mechanism of liver enzyme abnormalities in patients with novel coronavirus‐infected pneumonia. Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):100‐106. [DOI] [PubMed] [Google Scholar]
- 7. Liu C, Jiang ZC, Shao CX, et al, Preliminary study of the relationship between novel coronavirus pneumonia and liver function damage: a multicenter study. Zhonghua Gan Zang Bing Za Zhi. 2020;28(2):107‐111. [DOI] [PubMed] [Google Scholar]
- 8. Xie H, Zhao J, Lian N, Lin S, Xie Q, Zhuo H. Clinical characteristics of non‐ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40(6):1321‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen X, Jiang Q, Ma Z, et al, Clinical characteristics of hospitalized patients with SARS‐CoV‐2 and hepatitis B virus co‐infection. BMJ. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guan W, Ni Z, Hu Y, et al, Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zou X, Fang M, Li S, et al, Characteristics of liver function in patients with SARS‐CoV‐2 and chronic HBV co‐infection. Clin Gastroenterol Hepatol. 2020. 10.1016/j.cgh.2020.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lampertico P, Agarwal K, Berg T, et al, EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370‐398. [DOI] [PubMed] [Google Scholar]
- 14. Chai X, Hu L, Zhang Y, et al, Specific ACE2 Expression in Cholangiocytes May Cause Liver Damage After 2019‐nCoV Infection. bioRxiv. 2020. 10.1101/2020.02.03.931766 [DOI] [Google Scholar]
- 15. Cai Q, Huang D, Yu H, et al, COVID‐19: abnormal liver function tests. J Hepatol. 2020; 1–9. 10.1016/j.jhep.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aldhaleei WA, Alnuaimi A, Bhagavathula AS. COVID‐19 induced hepatitis B virus reactivation: a novel case from the United Arab Emirates. Cureus. 2020;12(6):e8645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coutts ER. Evaluation of adsorbent haemoperfusion in interventional therapy of sepsis. PQDT ‐ UK & Ireland. 2016.


