A 72‐year‐old female was admitted because of a 5‐day history of fever and shortness of breath. Her medical history was remarkable for hypertension and asthma. On admission, she had tachypnea, and her basal oxygen saturation was 87%. A chest X‐ray revealed bilateral diffuse patchy interstitial infiltrates. Nasopharyngeal swab test for SARS‐CoV‐2 by qualitative real‐time reverse transcription polymerase chain reaction (rRT‐PCR) assay was positive. Despite high flow oxygen therapy and prone position, her oxygen saturation remained 87%–89%. Two days after admission, she was intubated because of hypoxemia without cardiorespiratory arrest. She received standard doses of lopinavir/ritonavir, hydroxychloroquine, ceftriaxone, azithromycin, corticosteroids, meropenem, and prophylactic doses of low‐molecular‐weight heparin. After respiratory improvement, the patient was discharged from the intensive care unit. A month after admission, she presented progressively disabling myoclonus in upper limbs and negative myoclonus in lower limbs leading to falls (Supporting Information Video S1). Dysarthria, dysphagia, cognitive deficits, or ataxia were not observed.
In patients with COVID‐19, myoclonus may be caused by metabolic disturbances (liver failure, renal failure, or hypercapnia), medications (cephalosporines, quinolones, or imipenem), or hypoxia. In this patient, laboratory tests (including renal function, liver function, ammonium, urea, and CO2) were normal at the moment of evaluation. Antibiotics, antivirals, and corticosteroids had been withdrawn 2 weeks before the onset of the myoclonus. Magnetic resonance imaging (MRI) showed cortical and brainstem ischemic lesions (Fig. 1). After 2 days of treatment with low doses of clonazepam, the myoclonus almost disappeared (Supporting Information S2). Lumbar puncture, electroencephalogram (EEG), and electromyography (EMG) were not performed due to logistic limitations related to the pandemic and the patient's clinical improvement. Taking into account all the findings, myoclonus was attributed to central nervous system (CNS) hypoxia.
FIG. 1.
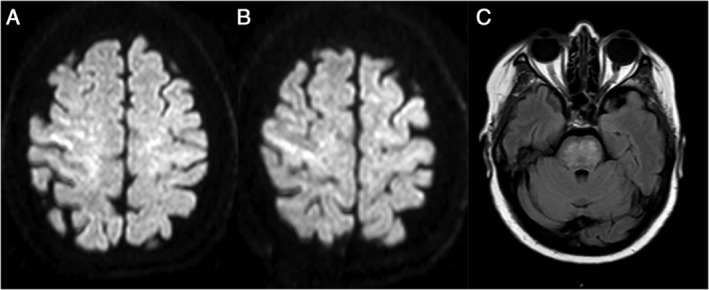
(A and B) Cortical hyperintensities in axial diffusion weighted imaging sequences. (C) Pontine hyperintense lesions in axial fluid attenuation inversion recovery imaging sequences.
Chronic post‐hypoxic myoclonus may appear days or weeks after respiratory or cardiac arrest. 1 Characteristically, it is exacerbated by muscle activation (action myoclonus) or intention (intention myoclonus). 1 Negative myoclonus may be present in lower limbs and myoclonus may be triggered by sensory stimuli such as touch or sound. 1 Neuronal loss has been described in the thalamus, striatum, mammillary bodies, or the brainstem raphe nuclei, but it is not known which injured neurons generate the myoclonus. 1 Characteristic neuroimaging findings have not been defined. 1 Neurophysiological studies may help distinguish myoclonus from other movement disorders. 2 Clonazepam, valproic acid, and levetiracetam have shown to be effective. 1 In some refractory cases, deep brain stimulation has been used with different results. 1
COVID‐19 pandemic is caused by severe acute respiratory syndrome coronavirus (SARS‐CoV‐2) that has a high infectivity. 4 The virus binds to ACE2 (angiotensin‐converting enzyme 2) receptors that are expressed in the lung but also in the nervous system and skeletal muscles. 3 COVID‐19 infection can progress to acute respiratory distress syndrome (ARDS) in adults and 5% of infected patients may need intensive care. 4 Involvement of the brainstem region described in previous reports may suggest that the cardiorespiratory center contributes to the severe respiratory distress, although these suggestions need further investigation. 4 In addition, severe COVID‐19 infection is associated with a robust systemic inflammatory response and may be accompanied by vascular endothelium dysfunction that may lead to neuroinflammation. 5 We hypothesize that these factors may decrease the hypoxia threshold required for the development of post‐hypoxic myoclonus, because the patient did not suffer prolonged or severe hypoxia normally involved in Lance‐Adams syndrome.
SARS‐CoV‐2 may cause some neurologic manifestations through direct or indirect mechanisms. 3 Long‐term neurological sequelae have not been attributed to COVID‐19 infection yet. 4 Post‐hypoxic myoclonus may be a long‐term movement disorder complication of this devastating pandemic. This disabling syndrome should be suspected in survivors of COVID‐19 infection presenting with myoclonus in order to start the best treatment.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the First Draft, B. Review and Critique.
V.R.C.: 1A, 1B, 1C, 3A, 3B
C.Q.: 1A, 3B
J.L.S.: 1C, 3B
I.C.: 1A, 1B, 1C, 3B
Disclosures
Ethical Compliance Statement
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. The authors confirm that the approval of an institutional review board was not required for this work. Oral and written informed consent was obtained from the patient.
Funding Sources and Conflicts of Interest
The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for the Previous 12 Months
The authors declare that there are no disclosures to report.
Supporting information
Video S1. Segment 1. Sudden, brief, jerks (positive myoclonus) in the upper limbs present at rest, while maintaining posturing and during action.
Video S2. Segment 2. Brief cessation of muscular activity while posturing (negative myoclonus) in the lower limbs.
References
- 1. Gupta VH, Caviness JN. Post‐hypoxic myoclonus: Current concepts, neurophysiology, and treatment. Tremor Other Hyperkinet Mov 2016;6:409 10.7916/D89C6XM4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Merchant SHI, Vial‐Undurraga F, Leodori G, Gerpen JA, Hallett M. Myoclonus: An electrophysiological diagnosis. Mov Disord Clin Pract 2020;7:489–499. 10.1002/mdc3.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77(6):683–690. 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Papa SM, Brundin P, Fung VSC, et al. Impact of the COVID‐19 pandemic on Parkinson's disease and movement disorders. Mov Disord Clin Pract 2020;7:357–360. 10.1002/mds.28067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coolen T, Lolli V, Sadeghi N, et al. Early postmortem brain MRI findings in COVID‐19 non‐survivors. Neurology 2020. 10.1212/WNL.0000000000010116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Segment 1. Sudden, brief, jerks (positive myoclonus) in the upper limbs present at rest, while maintaining posturing and during action.
Video S2. Segment 2. Brief cessation of muscular activity while posturing (negative myoclonus) in the lower limbs.


