Abstract
Highlights
There are ~ 2‐fold increased odds of severe coronavirus disease 2019 (COVID‐19) and a ~ 2‐fold increased risk of odds of mortality in patients with history of diabetes mellitus compared to those without diabetes mellitus.
Patients with a history of diabetes mellitus should be closely monitored if they get infected with COVID‐19.
Results of meta‐analysis showing association of diabetes mellitus with severity (Panel A) of disease and mortality (Panel B) in coronavirus disease 2019 (COVID‐19) patients
Keywords: coronavirus, COVID‐19, diabetes mellitus
To the Editor
Coronavirus disease 2019 (COVID‐19) is a viral infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 1 Because of the huge pressure that this pandemic infectious disorder is placing on healthcare services worldwide, better knowledge of factors influencing the evolution into unfavorable outcomes is urgently needed to help in appropriate allocation of residual resources. Diabetes mellitus (DM), another current epidemic around the world, is associated with high mortality and morbidity burden. Because the prevalence of DM has been reported to be high among COVID‐19 patients, 2 we carried out a pooled analysis of current studies for evaluating potential associations between DM and infection severity outcomes in COVID‐19 patients.
1. METHODS
We searched PUBMED, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) for studies published until March 31, 2020. We also searched major infectious disease, endocrinology, and general medicine journals and then performed a hand search of the bibliography of included studies.
Studies were included if they fulfilled the following criteria: (a) report history of DM in COVID‐19 patients; (b) report outcomes of interest; and (c) sample size >10. A meta‐analysis was performed to estimate the odds ratio (OR) and 95% confidence interval (CI) of DM in COVID‐19 patients with or without severe disease and in non‐survivors vs survivors. The statistical analysis was carried out using MetaXL, software Version 5.3 (EpiGear International Pty Ltd., Sunrise Beach, Australia), with inverse variance model. Finally, we performed a random effects meta‐regression using log OR to evaluate the impact of mean age and gender on association of DM with disease severity and mortality in patients with COVID‐19.
2. RESULTS
An initial search identified 348 publications. After removing duplicated or overlapping publications, and excluding reviews and editorials, 202 documents could be initially identified. A total number of 187 studies were excluded because they did not provide the rate of DM in COVID‐19 patients with different disease severity. Fifteen articles were hence selected. During hand search of the bibliography, one additional study was identified, so that our final pooled analysis included 16 studies. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 Twelve studies reported history of DM in severe vs non‐severe cases, with a sample of 2564 confirmed COVID‐19 patients (754, 29.4% being severe cases). A total number of 265 patients (10.3%) were classified as having a history of DM. Four studies with 618 patients (307, 42.5% of non‐survivors) compared the rate of DM between survivors and non‐surviving COVID‐19 patients, 96 (15.5%) of them previously diagnosed with DM. Details of the included studies are listed in Table 1.
TABLE 1.
Characteristics of the studies included
| Study | Total sample size | Severe patients/non‐survivors | Non‐severe patients/survivors | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n (%) | Age (yrs) a | Women (%) | Diabetes n (%) | n (%) | Age (yrs) a | Women (%) | Diabetes n (%) | ||
| Chen et al 2020 a 18 | 150 | 24 (16%) | 68.5 | 6 (25%) | 5 (20.8%) | 126 (84%) | 57.1 | 60 (47.6%) | 15 (11.9%) |
| Deng et al 2020 3 | 225 | 109 (48.5%) | 69 (62‐74) | 36 (33%) | 17 (15.6%) | 116 (51.5%) | 40 (33‐57) | 65 (56%) | 9 (7.8%) |
| Guan et al 2020 4 | 1099 | 173 (15.7%) | 52 (40‐65) | 73 (42%) | 28 (16.2%) | 926 (84.3%) | 45 (34‐57) | 386 (42%) | 53 (5.7%) |
| Huang et al 2020 10 | 41 | 13 (31.7%) | 49 (41‐61) | 2 (15%) | 1 (8%) | 28 (68.3%) | 49 (41‐57.5) | 9 (32%) | 7 (25%) |
| Liu et al 2020 6 | 78 | 11 (14.1%) | 66 (51‐70) | 4 (52.2%) | 2 (18.2%) | 67 (85.9%) | 37 (32‐41) | 35 (36.4%) | 6 (9.0%) |
| Liu et al 2020 a 7 | 12 | 6 (50%) | 64 | 3 (50%) | 1 (16%) | 6 (50%) | 43.3 | 1 (16%) | 1 (16%) |
| Qin et al 2020 8 | 452 | 286 (63.3%) | 61 (51‐69) | 131 (45.8%) | 53 (18.5%) | 166 (36.7%) | 53 (41.25‐62) | 86 (51.8%) | 22 (13.3%) |
| Ruan et al 2020 9 | 150 | 68 (45.3%) | 67 (15‐81) | 19 (28%) | 12 (18%) | 82 (54.6%) | 50 (44‐81) | 29 (35%) | 13 (16%) |
| Tianxin et al a 14 | 49 | 9 (18.3%) | 53 | 1 (11.1%) | 2 (2.2%) | 40 (81.7%) | 40.6 | 15 (37.5%) | 0 (0%) |
| Wan et al 2020 10 | 135 | 40 (29.6%) | 56 (52‐73) | 19 (47.5%) | 9 (22.5%) | 95 (70.4%) | 44 (33‐49) | 43 (45.3%) | 3 (3.1%) |
| Wang et al 2020 11 | 138 | 36 (26.1%) | 66 (57‐78) | 14 (39%) | 8 (22.2%) | 102 (73.9%) | 51 (37‐62) | 49 (48%) | 6 (5.9%) |
| Wang et al 2020 12 | 69 | 14 (20.3%) | 70.5 (62‐77) | 7 (50%) | 6 (43%) | 55 (79.7%) | 37 (32‐51) | 30 (55%) | 1 (2%) |
| Wu et al 2020 13 | 201 | 84 (41.7%) | 58.5 (50‐69) | 24 (28.6%) | 16 (19%) | 117 (58.3%) | 48 (40‐54) | 49 (41.9%) | 6 (5.1%) |
| Yang et al 2020 15 | 52 | 32 (61.5%) | 64.6 (11.2) | 11 (34%) | 7 (22%) | 20 (38.5%) | 51.9 (12.9) | 6(30%) | 2 (10%) |
| Zhang et al 2020 16 | 140 | 58 (41.4%) | 64 (25‐87) | 25 (43%) | 8 (13.8%) | 82 (58.6%) | 52 (26‐78) | 44 (54%) | 9 (11%) |
| Zhou et al 2020 17 | 191 | 54 | 69 (63‐76) | 16 (30%) | 17 (31%) | 137 | 52 (45‐58) | 56 (41%) | 19 (14%) |
Abbreviation: ICU, intensive care unit; MV, mechanical ventilation; NR, not reported.
Age data presented as median (interquartile range [IQR]) or mean (SD). Studies marked with (a) report age as mean (yrs).
The results of the pooled analysis are presented in Figure 1. COVID‐19 patients previously diagnosed with DM were found to be associated with a statistically significant increased risk of worse COVID‐19 infection (OR: 2.60 [95% CI: 1.96 to 3.45], I2 = 56%, Cochran's Q = 24.9, P = 0.01). In the pooled analysis of the four studies reporting mortality data, significant association was found with increased risk of mortality in COVID‐19 patients previously diagnosed with DM (OR: 2.03 [95% CI: 1.29‐3.20] I2 = 0%, Cochran's Q = 2.63, P = 0.45).
FIGURE 1.
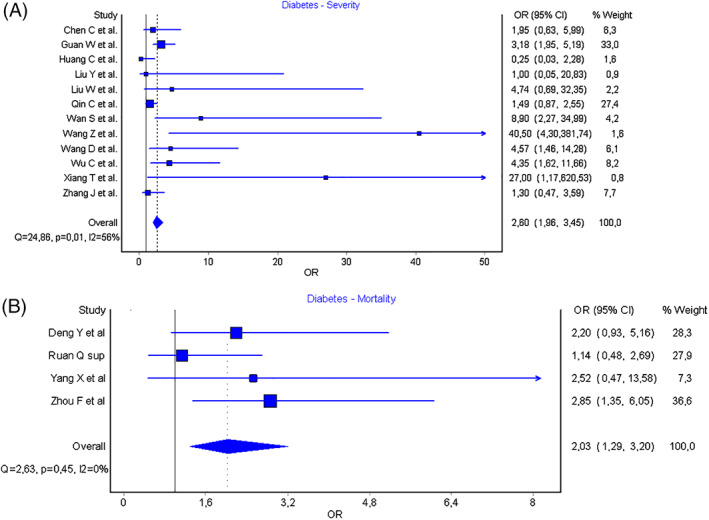
Results of meta‐analysis showing association of diabetes mellitus with severity (Panel A) of disease and mortality (Panel B) in coronavirus disease 2019 (COVID‐19) patients
Meta‐regression analysis showed no effect of age (Figure S1) or gender (Figure S2) on the association of DM with COVID‐19 infection severity or mortality.
3. COMMENT
The results of our pooled analysis demonstrate that the presence of DM may significantly worsen the clinical course of COVID‐19. Overall, we found a ~ 2‐fold increased odds of severe COVID‐19 and a ~ 2‐fold increased odds of mortality in DM patients with this infection compared to non‐DM patients.
There are several possible mechanisms explaining these findings. Patients with DM have been inherently known to have higher cumulative mortality, mostly owing to cardiovascular and renal disease. 19 DM has also been previously associated with worse outcomes in patients with SARS infection. 20 The circulating levels of some cytokines such as interleukin‐6 (IL‐6) were found to be higher in COVID‐19 patients with DM, which suggests the presence of an underlying proinflammatory milieu as one mechanism linking DM to worse severity outcomes in COVID‐19 patients. 21 It is also noteworthy that DM patients are more frequently overweight or have a higher prevalence of obesity, which could also contribute to worsen the prognosis of restrictive lung diseases.
A limitation of our analysis is in the fact that we could not use exclusion criteria to obtain data from the largest possible number of studies. We did perform sensitivity analysis and analysis for publication bias to assess for heterogeneity. To assess the effect of age and gender as confounding variables in our analysis, we also performed a meta‐regression that showed no impact on association of DM with disease severity or mortality in COVID‐19 patients. Because the included studies were observational, we cannot rule out possibility of confounding and reverse causation. We did not have data on use of antihyperglycemic agents, duration of diabetes, and associated diabetic micro‐ and macrovascular complications. Owing to the limited number of studies and small sample size, large prospective studies would be advisable to confirm our findings, data regarding COVID‐19 are still in nascent stage and our findings may help clinicians and policymakers implement risk stratification models and put the limited healthcare resources to judicious use.
DISCLOSURE
None declared.
2019年冠状病毒病(COVID‐19)是由严重急性呼吸综合征冠状病毒2型引起的一种病毒性传染病。由于这种大流行传染病给全球医疗服务带来了巨大压力, 需要更好地了解相关影响因素, 以帮助合理分配医疗资源。糖尿病是目前在世界范围内流行的另一种疾病, 与高死亡率和高发病率负担有关。因为有报道新冠肺炎患者中糖尿病的患病率很高, 我们对目前的研究进行了综合分析, 以评估糖尿病与COVID‐19患者感染严重程度之间的潜在相关性。
通过在PubMed、EMBASE和Cochrane Central Register of Control Trials(CENTAL)上检索发表在2020年3月31日前的研究以及主要传染病、内分泌学和普通医学期刊的文章, 最终筛选出16项研究进行分析。其中12项研究包括2 564例新冠感染病例(29.4%为重症患者)报告了重症和非重症患者的糖尿病病史, 其中265名患者(10.3%)有糖尿病病史。另外四项研究共有618例患者, 比较了幸存者和新冠肺炎死亡患者之间的DM发生率, 其中96例(15.5%)曾被诊断为糖尿病。
我们的综合分析结果表明, 糖尿病的存在可能会明显恶化新冠肺炎的临床病程。与非糖尿病患者相比, 糖尿病患者严重新冠肺炎的几率增加约2倍, 死亡的几率增加约2倍。有几种可能的机制可以解释这些发现。由于更多的心血管和肾脏疾病, 糖尿病患者的累积死亡率较高。此外, 糖尿病也与严重急性呼吸系统综合征感染患者的不良预后有关。在新冠肺炎糖尿病患者中, 发现一些细胞因子, 如白细胞介素‐6(IL‐6)的循环水平更高, 这表明潜在的促炎环境的存在是将糖尿病与新冠肺炎患者更严重的预后联系起来的机制之一。还值得注意的是, 糖尿病患者更容易超重或肥胖, 这也可能加重限制性肺病的预后。为了评估年龄和性别作为混杂变量的效果, 我们还进行了一项meta回归分析, 结果显示在新冠肺炎患者中, 糖尿病与疾病严重程度或死亡率的相关性没有影响。由于研究数量有限, 样本量小, 建议进行大型前瞻性研究来证实我们的发现, 有关新冠肺炎的数据仍处于初级阶段, 我们的发现可能有助于临床医生和政策制定者实施风险分层模型, 并合理利用有限的医疗资源。
Supporting information
Figure S1 Results of meta‐regression showing no impact of age on association of diabetes mellitus with severity of disease (Panel A) or mortality (Panel B) in coronavirus disease 2019 (COVID‐19) patients.
Figure S2 Results of meta‐regression showing no impact of gender on association of diabetes mellitus with severity of disease (Panel A) or mortality (Panel B) in coronavirus disease 2019 (COVID‐19) patients
ACKNOWLEDGEMENT
Fabian Sanchis‐Gomar is supported by a postdoctoral contract granted by “Subprograma Atracció de Talent ‐ Contractes Postdoctorals de la Universitat de València.” Other authors: No funding received.
Aggarwal G, Lippi G, Lavie CJ, Henry BM, Sanchis‐Gomar F. Diabetes mellitus association with coronavirus disease 2019 (COVID‐19) severity and mortality: A pooled analysis. Journal of Diabetes. 2020;12:851–855. 10.1111/1753-0407.13091
Funding information Subprograma Atracció de Talent ‐ Contractes Postdoctorals de la Universitat de València
REFERENCES
- 1. Lippi G, Sanchis‐Gomar F, Henry BM. Coronavirus disease 2019 (COVID‐19): the portrait of a perfect storm. Ann Transl Med. 2020;8:497. 10.21037/atm.2020.03.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Emami A, Javanmardi F, Pirbonyeh N, Akbari A. Prevalence of underlying diseases in hospitalized patients with COVID‐19: a systematic review and meta‐analysis. Arch Acad Emerg Med. 2020;8(1):e35. [PMC free article] [PubMed] [Google Scholar]
- 3. Deng Y, Liu W, Liu K, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID‐19) in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133:1261‐1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu W, Tao ZW, Lei W, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J (Engl). 2020;133(9):1032‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019‐nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364‐374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID‐19 patients in Northeast Chongqing. J Med Virol. 2020;92:797‐806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71(15):769‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tianxin X, Jiaming L, Fei X, et al. Analysis of clinical characteristics of 49 patients with new type of coronavirus pneumonia in Jiangxi region. Chin J Resp Crit Car. 2020;19:154‐160. [Google Scholar]
- 15. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med. 2020;8:475‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75:1730‐1741. [DOI] [PubMed] [Google Scholar]
- 17. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen C, Chen C, Jiangtao Y, Ning Z, Jianping Z, Daowen W. Analysis of myocardial injury and cardiovascular diseases in critical patients with new coronavirus pneumonia. Chin J Cardiovasc Dis. 2020. [Google Scholar]
- 19. Rodriguez BL, Abbott RD, Fujimoto W, et al. The American Diabetes Association and World Health Organization classifications for diabetes. Their impact on diabetes prevalence and total and cardiovascular disease mortality in elderly Japanese‐American men. Diabetes Care. 2002;25(6):951‐955. [DOI] [PubMed] [Google Scholar]
- 20. Booth CM, Matukas LM, Tomlinson GA, et al. Clinical features and short‐term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801‐2809. [DOI] [PubMed] [Google Scholar]
- 21. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Results of meta‐regression showing no impact of age on association of diabetes mellitus with severity of disease (Panel A) or mortality (Panel B) in coronavirus disease 2019 (COVID‐19) patients.
Figure S2 Results of meta‐regression showing no impact of gender on association of diabetes mellitus with severity of disease (Panel A) or mortality (Panel B) in coronavirus disease 2019 (COVID‐19) patients


