Abstract
Coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has become a worldwide pandemic since it emerged in December 2019. Previous studies have reported rapid antibody response to SARS‐CoV‐2 in the first 2 to 3 weeks after symptom onset. Here, we retrospectively described the dynamic changes of serum immunoglobulin M (IgM) and IgG specifically against SARS‐CoV‐2 in later weeks (mainly 4‐10 weeks) in 97 hospitalized patients with COVID‐19. We observed that serum IgM and IgG, especially in patients with moderate‐to‐high levels, declined significantly between week 4 to 10 after illness onset. Notably, IgG levels in high percentage of patients (77.5%, 31 of 40) rapidly declined by half, from 212.5 (range, 163.7‐420.3) to 96.3 (range, 75.0‐133.4) AU/mL, within 1 to 2 weeks in the second month and then sustained at around 100 AU/mL until discharge from hospital. Significant reduction of IgM was also observed as SARS‐CoV‐2 nucleic acid turned negative (P = .002). In the recovery stage, serum IgG declined significantly (early vs late recovery stage, n = 16, P = .003) with a median reduction of 50.0% (range, 3.7%‐77.0%). Our results suggested that the decline of IgM may be an indicator of virus clearance and recovered patients may have a robust immunity against reinfection within at least 3 months after illness onset. Yet, the rapid reduction of IgG by half rises serious concerns on the robustness and sustainability of the humoral immune response in the period after discharge, which is crucial for immunity strategy and developing a vaccine.
Keywords: antibody response, COVID‐19, dynamic changes, recovery stage
Highlights
Serum IgM declined significantly between week 4‐10 after symptom onset in COVID‐19 patients.
Serum IgG declined remarkably by half and rapidly within 1‐2 weeks in the second month after symptom onset in COVID‐19 patients.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), an acute respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), was first identified in Wuhan, China on 12 December 2019 and became a worldwide pandemic in 2020. 1 , 2 Nearly, 10 million confirmed COVID‐19 cases and 500 thousand deaths have been reported around the world by 27 June 2020 according to the World Health Organization (WHO). Most of the patients had typically clinical symptoms including fever, cough, fatigue, and dyspnea, which appeared 2 to 14 days after exposure. 2 , 3 About 20% of patients may progress to a severe or critical disease with a higher mortality rate than mild cases. 3
Similar to patients infected by SARS‐CoV or MERS‐CoV, COVID‐19 patients also have antibody response to virus infection. 4 , 5 , 6 Serum immunoglobulin M (IgM) and IgG against SARS‐CoV‐2 were detectable within the first several weeks after symptom onset. 4 , 7 , 8 , 9 They were detectable as early as 4 days and reached a peak in the second week after onset. 7 , 8 Almost all the infected cases had seropositive antibodies in the first 3 weeks post‐illness onset. 4 Thus, the serological testing for specific antibodies against SARS‐CoV‐2 in the early stage may be helpful for the diagnosis of suspected cases in the context of high false‐negative risk of reverse‐transcription polymerase chain reaction (RT‐PCR) assay in detecting virus nucleic acid. 8 , 10 Serologic testing in conjunction with RT‐PCR have demonstrated enhanced sensitivity of detecting symptomatic or asymptomatic individuals infected with SARS‐CoV‐2. 11
However, most of the previous publications have only focused on the acute response phase within 2 to 3 weeks after onset of COVID‐19. Our study aims to describe the dynamic changes of serum antibody against SARS‐CoV‐2 in the later weeks after onset, especially when the virus nucleic acid turns negative and when patients are recovering from the disease.
2. MATERIALS AND METHODS
2.1. Patients and data collection
This was a retrospective study involving hospitalized patients with confirmed COVID‐19 from the Sino‐French New City Branch of Tongji Hospital, Wuhan, China from 26 January to 5 March 2020. The medical team from Beijing Hospital had taken full charge of the hospital since late January 2020. This study was approved by the Ethics Committee of Beijing Hospital, Beijing, China, and informed consent was obtained from the participants.
The demographic data, clinical manifestations, chest imaging, comorbid diseases, laboratory findings, treatment, and outcomes were obtained from electronic medical records. As the examination for serum antibody against SARS‐CoV‐2 were not carried out regularly until late February 2020, most of the included patients only had series data of serum IgM and IgG between 4th week after symptom onset and discharge date.
The severity of COVID‐19 was classified as mild, moderate, severe, and critical according to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (version 7) released by the National Health Commission of China (https://ncstatic.clewm.net/rsrc/2020/0311/22/781e459d414bf3f1579bcafef0d80f12.pdf). Briefly, confirmed patients meeting any of the following criteria were defined as severe cases: (1) respiratory rate ≥30/min; (2) oxygen saturation ≤93% at rest; (3) arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤ 300 mm Hg, or critical cases: (1) respiratory failure and requiring mechanical ventilation; (2) shock; (3) with other organ failure that required ICU care. In the present study, severe and critical patients were included in the severe group while mild and moderate patients were in the nonsevere group.
Recovery was defined as SARS‐CoV‐2 nucleic acid turning negative. To avoid false negative, only patients with negative results in all of the subsequent nucleic acid tests after conversion were confirmed to be truly recovered. We defined the early recovery stage (ERS) as the first 7 days and late recovery stage (LRS) as more than 14 days after the first recovery date. 12
The serum antibodies against SARS‐CoV‐2 (IgM and IgG) was detected by enzyme linked immunosorbent assay (iFlash 2019‐nCoV IgM and IgG kits from YHLO Biotech Co, Ltd, Shenzhen, China) using an iFlash3000 Chemiluminescence Immunoassay Analyzer (YHLO Biotech Co, Ltd, Shenzhen, China).
2.2. Statistical analysis
Continuous variables were present as median and interquartile range (IQR). Independent continuous variables were compared using the Mann‐Whitney U test and paired variables with the Wilcoxon signed‐rank sum test. Categorical variables were present as number and percentage, and compared using χ 2 test or Fisher's exact test as appropriate. P < .05 was considered statistically significant. All statistical analyses and scientific graphics were made by using GraphPad Prism 8.0 (GraphPad Software, Inc, CA).
3. RESULTS
3.1. Baseline descriptions
After excluding 10 patients who only received antibody tests before 3 weeks after illness onset, a total of 97 COVID‐19 patients (62 severe and 35 nonsevere) were included in our study (Table 1). The median age was 65 years (IQR, 53‐72), and severe patients were older than nonsevere patients (P = .007). Male and current smokers were at higher risk of being severely ill (P = .022 and .003, respectively). Cough, fatigue, dyspnea and fever were the most common symptoms that were reported in 86.6, 71.1, 68.0 and 59.8% of patients, respectively. Fever, dyspnea, myalgia, and fatigue were more frequently manifested in severe patients (all P < .01). No significant differences of comorbidities except malignancy were observed between severe and nonsevere patients.
Table 1.
Baseline characteristics of COVID‐19 patients
| Variables | All patients (n = 97) | Severe patients (n = 62) | Nonsevere patients (n = 35) | P value |
|---|---|---|---|---|
| Age, y | 65.0 (53.0‐73.0) | 67.0 (60.2‐74.8) | 59.0 (49.0‐67.0) | .007 |
| Sex | .022 | |||
| Male | 51 (52.6%) | 38 (61.3%) | 13 (37.1%) | |
| Female | 46 (47.4%) | 24 (38.7%) | 22 (62.9%) | |
| Current smokers | 18 (18.6%) | 17 (27.4%) | 1 (2.8%) | .003 |
| Days from symptom onset to: | ||||
| Admission | 12.0 (5.0‐15.0) | 9.0 (5.0‐15.0) | 14.0 (10.0‐20.5) | .001 |
| Viral nucleic acid turning negative | 34.0 (23.8‐44.2) | 32.0 (23.2‐42.8) | 39.0 (27.7‐46.5) | .140 |
| Dyspneaa | 6.0 (3.0‐10.0) | 5.0 (3.0‐10.0) | 9.0 (3.5‐13.8) | .402 |
| Days from admission to discharge | 40.0 (26.0‐54.0) | 45.0 (35.0‐56.0) | 32.0 (19.0‐41.0) | <.001 |
| Comorbidities | ||||
| Hypertension | 49 (50.5%) | 35 (56.4%) | 14 (40.0%) | .120 |
| Coronary heart disease | 15 (15.5%) | 10 (16.2%) | 5 (14.3%) | .810 |
| Diabetes | 23 (23.7%) | 15 (24.2%) | 8 (22.8%) | .882 |
| Malignancy | 10 (10.3%) | 10 (16.1%) | 0 (0%) | .012 |
| COPD | 6 (6.2%) | 6 (9.7%) | 0 (0%) | .084 |
| Chronic kidney disease | 2 (2.1%) | 1 (1.6%) | 1 (2.8%) | .678 |
| Symptoms | ||||
| Fever | 58 (59.8%) | 45 (72.6%) | 13 (37.1%) | <.001 |
| Cough | 84 (86.6%) | 56 (90.3%) | 28 (80.0%) | .152 |
| Sputum production | 49 (50.5%) | 35 (56.4%) | 14 (40.0%) | .120 |
| Headache | 11 (11.3%) | 7 (11.3%) | 4 (11.4%) | >.999 |
| Dyspnea | 66 (68.0%) | 59 (95.2%) | 7 (20.0%) | <.001 |
| Pharyngalgia | 13 (13.4%) | 8 (12.9%) | 5 (14.3%) | .847 |
| Diarrhea | 20 (20.6%) | 13 (21.0%) | 7 (20.0%) | .909 |
| Nausea | 14 (14.4%) | 10 (16.1%) | 4 (11.4%) | .527 |
| Myalgia | 36 (37.1%) | 29 (46.8%) | 7 (20.0%) | .009 |
| Fatigue | 69 (71.1%) | 53 (85.5%) | 16 (45.7%) | <.001 |
| Heart rate, bpm | 94.0 (86.0‐101.0) | 98.0 (87.2‐104.0) | 89.0 (83.0‐100.0) | .091 |
| Systolic pressure, mm Hg | 135.0 (122.0‐148.0) | 135.0 (127.2‐148.8) | 130.0 (115.0‐145.0) | .222 |
Note: Data were presented as n (%) or median (IQR). P values were determined with χ 2 test, Fisher's exact test, or Mann‐Whitney U test.
Abbreviations: COPD, chronic obstructive pulmonary disease; IQR, interquartile range.
Data from 66 patients (59 severe and 7 nonsevere) manifesting dyspnea.
All patients were discharged from hospital before 8 April 2020, except one critical patient who died from acute respiratory distress syndrome and multiple organ dysfunction syndrome 33 days after symptom onset. Severe patients had shorter duration from symptom onset to admission to hospital (median 9.0 vs 14.0 days, P = .001) but a longer duration of hospitalization (median 45.0 vs 32.0 days, P < .001). The median time for SARS‐CoV‐2 nucleic acid turning negative was 34.0 days with no significant difference between severe and nonsevere groups.
3.2. Levels of serum antibodies at different weeks
We illustrate the overall profile of serum IgM and IgG against SARS‐CoV‐2 from week 4 to 10 after illness onset in Figure 1. The median serum IgM levels from week 4 to 6 were 59.9 (IQR, 27.1‐92.2), 37.1 (IQR, 21.7‐98.8), and 36.2 (IQR, 12.3‐65.3) AU/mL, respectively, which were, however, not significantly different between weeks. The levels in week 7 to 9 reduced to 22.7 (IQR, 7.4‐49.7), 14.6 (IQR, 4.8‐37.1), and 8.5 (IQR, 3.8‐20.3) AU/mL, respectively, which were significantly lower than those in week 4 to 6 (all P < .05). Median serum IgG was detected as high as 150 AU/mL or more in week 4 to 6 but markedly reduced to nearly 100 AU/mL, which plateaued during week 7 to 10 (Figure 1B).
Figure 1.
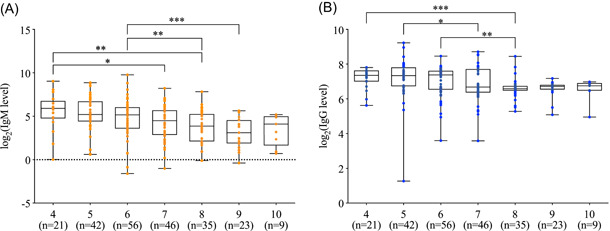
The levels of antibodies against SARS‐CoV‐2 in patients at 4 to 10 weeks after symptom onset. A, Serum IgM levels. B, Serum IgG levels. The boxplots show medians and 1st and 3rd quartiles, while the whisker shows the range. The antibody levels are compared by the Mann‐Whitney U test. *P < .05; **P < .01; ***P < .001
When stratifying patients according to severity, we found the antibody profile of serum IgM and IgG in severe and nonsevere patients were similar to that of the total sample. In addition, the difference of antibody levels between severe and nonsevere groups was not statistically significant, although nonsevere patients had slightly lower median antibody levels in some weeks (Table 2), which may be due to the small sample size. No difference was observed between male and female.
Table 2.
Profile of IgM and IgG antibodies against SARS‐CoV‐2 in COVID‐19 patients
| W4 | W5 | W6 | W7 | W8 | W9 | W10 | |
|---|---|---|---|---|---|---|---|
| N | 21 (6/15) | 42 (13/29) | 56 (15/41) | 46 (18/28) | 35 (14/21) | 23 (6/17) | 9 (2/7) |
| IgM | |||||||
| Total | 59.9 (27.1‐92.2) | 37.1 (21.7‐98.8) | 36.2 (12.3‐65.3) | 22.7 (7.4‐49.7) | 14.6 (4.8‐37.1) | 8.5 (3.8‐20.3) | 17.2 (5.1‐29.5) |
| Nonsevere | 35.5 (27.7‐105.5) | 22.3 (14.0‐34.8) | 37.9 (14.5‐133.2) | 14.9 (4.6‐76.5) | 19.6 (4.2‐59.2) | 7.2 (5.4‐19.7) | a |
| Severe | 60.4 (35.0‐90.3) | 53.2 (29.2‐120.0) | 34.6 (12.5‐55.3) | 31.0 (9.0‐47.3) | 14.6 (5.3‐32.8) | 8.5 (3.5‐17.1) | 17.2 (9.6‐31.9) |
| P | .850 | .052 | .388 | .692 | .449 | .507 | .444 |
| IgG | |||||||
| Total | 163.7 (128.8‐197.6) | 162.4 (107.4‐219.1) | 166.3 (93.0‐194.8) | 102.4 (82.9‐205.8) | 96.2 (90.4‐105.7) | 105.1 (96.9‐111.1) | 107.9 (89.2‐119.7) |
| Nonsevere | 149.0 (110.8‐192.5) | 138.6 (104.3‐188.2) | 166.8 (94.8‐186.2) | 87.6 (72.8‐188.1) | 89.6 (74.1‐102.8) | 103.6 (101.9‐112.8) | a |
| Severe | 163.7 (139.3‐192.6) | 169.3 (136.1‐225.3) | 165.8 (93.1‐201.1) | 108.3 (89.1‐198.5) | 96.6 (93.5‐109.4) | 106.3 (93.2‐110.2) | 90.2 (89.2‐116.6) |
| P | .622 | .316 | .640 | .160 | .142 | .962 | .472 |
Only two patients. N, number of cases (nonsevere/severe group); unit, AU/ml. All data were presented as median (interquartile range). P values were determined by the Mann‐Whitney U test comparing the antibody level of nonsevere and severe groups.
3.3. Changes of serum antibodies using paired data
As the immune response may differ greatly among individuals, the change pattern of serum antibodies revealed by analyzing unpaired date may be biased. To investigate the change pattern more precisely, we extracted paired data from patients who had antibody tests in at least two consecutive weeks and compared the serum antibody levels between consecutive weeks using the Wilcoxon signed‐rank sum test (Figure 2).
Figure 2.
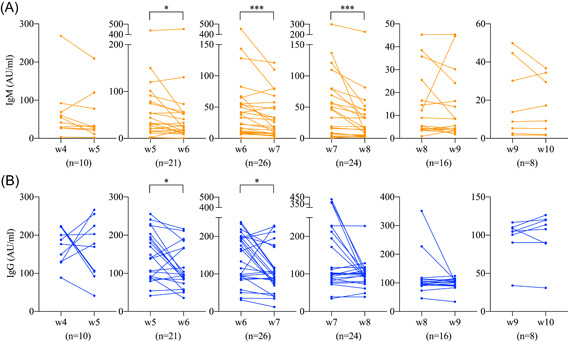
The weekly changes of antibodies against SARS‐CoV‐2 using individual data of two consecutive weeks. A, Serum IgM levels. B, Serum IgG levels. The antibody levels are compared by Wilcoxon signed‐rank sum test. *P < .05; **P < 0.01; ***P < .001
In week 5 to 6 (n = 21), the IgM level reduced from 32.9 (IQR, 18.3‐85.0) to 26.8 (IQR, 13.8‐54.4) AU/mL (P = .032) and IgG from 140.4 (IQR, 89.0‐198.7) to 94.6 (IQR, 78.8‐166.3) AU/mL (P = .029). In week 6 to 7 (n = 26), the IgM level reduced from 35.5 (IQR, 10.5‐58.5) to 17.6 (IQR, 7.3‐52.2) AU/mL (P < .001) and IgG from 147.2 (IQR, 92.9‐196) to 95.9 (IQR, 73.6‐130.7) AU/mL (P = .012). In week 7 to 8 (n = 24), the IgM level reduced from 39.7 (IQR, 7.4‐76.5) to 15.6 (IQR, 4.6‐42.6) AU/mL (P < .001) while IgG was not obviously altered. In the other week pairs, the antibody levels had no obvious change.
3.4. Rapid reduction of serum IgG
Dynamics of serum antibody levels of 40 patients who received antibody tests in at least 3 weeks among week 4 to 9 after illness onset is shown in Figure 3. In addition, the changes of antibody levels of each patients are shown in Figure 4. We stratified patients according to the antibody level of the first antibody test.
Figure 3.
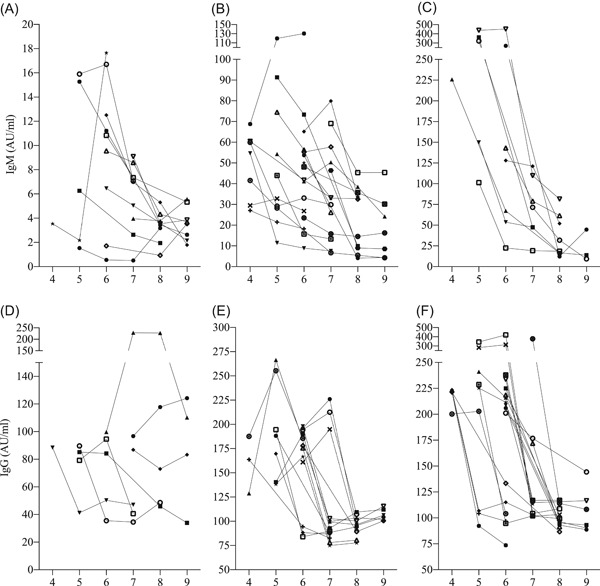
Dynamics of antibody against severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in 4 to 9 weeks after symptom onset stratified by antibody level in the first antibody test in 40 patients who received antibody tests in at least 3 weeks. A‐C, Low, moderate, and high IgM group (<20 AU/mL, n = 12; 20‐100 AU/mL, n = 19; >100 AU/mL, n = 9, respectively). D‐F, Low, moderate, and high IgG group (<100 AU/mL, n = 7; 100‐200 AU/mL, n = 15; > 200 AU/mL, n = 18, respectively)
Figure 4.

Changes of serum immunoglobulin M (IgM) and IgG in 4 to 9 weeks after symptom onset stratified by antibody level in the first antibody test in 40 patients who received antibody tests in at least 3 weeks. A‐C, Low, moderate, and high IgM group (<20 AU/mL, n = 12; 20‐100 AU/mL, n = 19; >100 AU/mL, n = 9, respectively). D‐F, Low, moderate, and high IgG group (<100 AU/mL, n = 7; 100‐200 AU/mL, n = 15; >200 AU/mL, n = 18, respectively)
Patients in the low‐IgM group (<20 AU/mL, n = 12) had persistently low IgM levels at week 4 to 9 without obvious changes (Figures 3A and 4A). Meanwhile, the serum IgM of patients in moderate‐IgM group (20‐100 AU/mL, n = 19) gradually declined (Figures 3B and 4B) and IgM of patients in high‐IgM group (>100 AU/mL, n = 9) had a significant reduction of more than 50 AU/mL in week 4 to 9 (Figures 3C and 4C).
The serum IgG of most patients in low‐IgG group (<100 AU/mL, n = 7) also sustained at a low level (Figures 3D and 4D). In the moderate‐IgG group (100‐200 AU/mL, n = 15), serum IgG of seven patients increased first to a peak and then rapidly declined, while the others declined directly (Figures 3E and 4E). In the high‐IgG group (>200 AU/mL, n = 18), most patients had great reduction of >100 AU/mL in IgG (Figures 3F and 4F). However, serum IgG in moderate and high groups sustained at around 100 AU/mL after significant reduction until discharge from hospital.
Notably, serum IgG in 60.0% (24 of 40) of patients rapidly declined within 1 week and 17.5% (7 of 40) within 2 weeks (no antibody tests in the intermediate week) and then sustained until discharge. The median levels of serum IgG before and after rapid decline were 212.5 (range, 163.7‐420.3) and 96.3 (range, 75.0‐133.4) AU/mL, respectively, and the median reduction was 52.7% (range, 39.8%‐75.2%). Therefore, it was reasonable to infer that most patients had a sudden reduction of IgG by half within 1 week in the second month after illness onset.
In the rest of the patients, we found 4 patients with low IgG levels (<100 AU/mL) also had sharp reduction of IgG by half (range, 45.5%‐60.4%) within 1 to 2 weeks.
3.5. Antibody changes as the nucleic acid turns negative
We analyzed paired data from 18 patients who had antibody tests in the acute stage (AS, the period when the SARS‐CoV‐2 nucleic acid can be found in the respiratory specimen) and ERS to investigate the changes of serum antibodies as the nucleic acid turns negative (ie, recovery). The median days from illness onset to recovery in these patients were 46.0 (IQR, 36.8‐50.8). If a patient had multiple antibody data in AS, only the last one was used. The IgM level significantly decreased (AS: 28.2 [IQR, 8.7‐230]; ERS: 16.0 [IQR, 6.5‐32.6]; P = .002) as the viral RNA turned negative (Figure 5A). The IgG level decreased from 133.0 (IQR, 81.9‐209.9) to 96.4 (IQR, 87.1‐118.4) AU/mL but the difference did not reach statistical significance (P = .108; Figure 5B).
Figure 5.
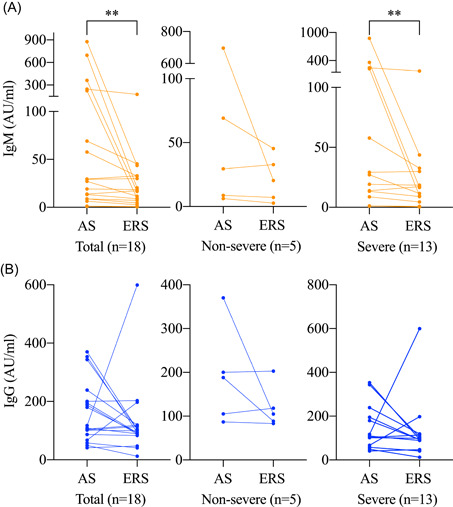
Dynamic changes of antibody against SARS‐CoV‐2 as the nucleic acid turns negative. A, Serum IgM levels. B, Serum IgG levels. AS, acute stage; ERS, early recovery stage; IgM, immunoglobulin M; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2. The antibody levels are compared by Wilcoxon signed‐rank sum test. *P < .05; **P < .01; ***P < .001
3.6. Change pattern in recovery stage
Paired data of antibody levels in ERS and LRS from 16 patients were included to investigate the change pattern in the recovery stage of COVID‐19 (Figure 6). The median recovery date of these patients was 34.0 (range, 21‐44) days after symptom onset. Significant reductions were observed in the IgM level (median, 23.6 vs 10.6; P = .006) and IgG level (median, 193.7 vs 101.0; P = .003) from ERS to LRS. The IgG levels in 87.5% (14 of 16) of patients decreased in the recovery stage, and the median percentage of reduction was 50.0% (range, 3.7%‐77.0%).
Figure 6.
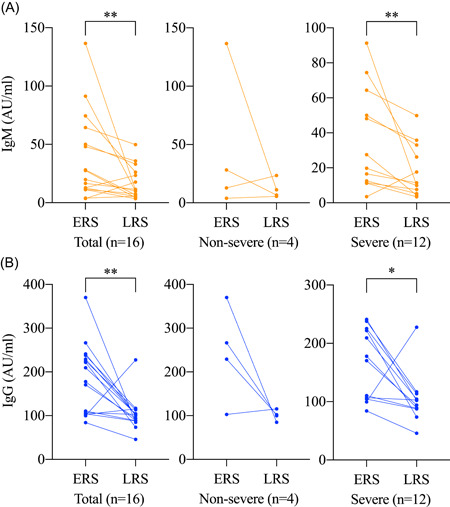
Dynamic changes of antibody against SARS‐CoV‐2 in the recovery stage. A, Serum IgM levels. B, Serum IgG levels. ERS, early recovery stage; IgM, immunoglobulin M; LRS, late recovery stage; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2. The antibody levels are compared by Wilcoxon signed‐rank sum test. *P < .05; **P < .01; *** P < .001
4. DISCUSSION
The current study described the dynamic changes of serum IgM and IgG against SARS‐CoV‐2 after 3 weeks following illness onset in hospitalized patients with COVID‐19. Overall, IgM and IgG declined in the second month after illness onset, especially in patients with moderate‐to‐high levels of serum antibodies that represented a stronger antibody response to SARS‐CoV‐2.
Antibody response is critical for virus clearance and preventing reinfection. Previous studies have reported rapid production of specific IgM and IgG to SARS‐CoV‐2 within the first week and a peak level of antibodies by 2 to 3 weeks after disease onset. 4 , 10 , 13 The peak of antibodies may not persist for long durations. We found the decline of serum IgM after peak mainly occurred in the second month (week 5‐8) after onset. This was parallel to the virological assessment results that SARS‐CoV‐2 nucleic acid of most patients turned negative in week 4 to 7 (median 34 days; IQR, 23.8‐44.2) since illness onset. Subsequent paired‐data analysis revealed that the IgM level significantly declined as the virus was completely cleared. Thus, it seems to be conclusive that IgM titer increases rapidly within the first 2 weeks, persists in the following 1 to 2 weeks as the virus is being cleared, and declines by the 4th week after illness onset as the virus nucleic acid turns negative. 14 Therefore, the decline of IgM may be an indicator of virus clearance, which may help determine the true nucleic acid conversion negative in conjunction with RT‐PCR testing. It could serve as a criterion for discharge and in ending quarantine, especially for mildly symptomatic or asymptomatic cases.
Sustained IgG level is crucial for shaping memory immune to prevent reinfection. Previous studies revealed that detectable antibody levels persisted for more than 2 years in SARS‐CoV‐ and MERS‐CoV‐infected patients. 15 , 16 It is unclear how long specific IgG against SARS‐CoV‐2 will last. The present study showed a significant reduction of IgG in week 5‐7 that happened within 1‐2 weeks, which sustained since 7th week after illness onset until discharge. The level of IgG decreased from over 150 AU/mL and then plateaued around 100 AU/mL. Serum IgG was still detectable in two patients as long as 11 weeks since illness onset. Analysis using paired data showed that IgG may occur in the process of recovery. Our findings were consistent with previous studies. 17 , 18 Long et al 17 observed that IgG levels in recovered patients decreased in 2 to 3 months upon infection, while Wang et al 18 reported that neutralizing antibodies in half of 8 convalescent patients declined 6 to 7 weeks after symptom onset. Our study, by using longitudinal data, suggested that the IgG level may sustain after the decline for several weeks until at least 3 months after illness onset. This was also supported by Long et al's 17 findings that 87.1% of symptomatic patients still had seropositive IgG 8 weeks after they were discharged from hospital. Notably, the decline of IgG mainly occurred in patients with moderate‐to‐high IgG levels, indicating that stronger antibody response did not always necessarily convert to more robust long‐term humoral immunity against reinfection.
Additionally, dynamic analysis in the current study showed that the IgG levels in most patients rapidly declined by half within 1 to 2 weeks in the second month after illness onset. Several patients with IgG levels <100 AU/mL also had a rapid reduction by half. This may arise serious concerns that IgG would have logarithmically declined at certain time intervals in the future and recovered patients may become vulnerable to SARS‐CoV‐2 again. More serological studies investigating the dynamics of antibodies in recovery patients for a longer time period (3 months or more after discharge) are urgently need to determine the duration of humoral immunity, which is pivotal for immunity strategy and developing a vaccine.
The immune response to SARS‐CoV‐2 may differ by disease severity. Immunological survey demonstrated that critical ill cases had dramatically higher levels of interleukin‐2R (IL‐2R), IL‐6, IL‐10, and tumor necrosis factor‐α but lower counts of T lymphocytes, CD4+ T cells, and CD8+ T cells as compared with moderate cases, 19 so that the IL‐2R/lymphocyte may be a useful marker for monitoring the progression and predicting the progression. 20 Serological assays also revealed higher titers of IgG 4 and total antibody 10 in severe patients than in nonsevere patients, which was associated with clinical outcomes. This may suggest an effect of antibody‐dependent enhancement (ADE). ADE, characterized by enhanced virus entry mediated by antibodies and induction of severe inflammatory activation, may contribute to lung injury. 21 This was previously observed in SARS‐CoV‐1‐infected rhesus macaques. 22 However, it is still unclear whether higher IgG titers in critical ill patients contributed to lung pathology in SARS‐CoV‐2 infection. The divergence of IgG titers between different severity groups was mainly observed in the first 2 to 3 weeks. 4 On the contrary, Zhao et al 10 reported no significant difference of IgM or IgG levels between critical and noncritical patients in about 2‐week after onset. Our study revealed that serum IgM and IgG levels in 4 or more weeks after symptom onset were comparable between severe and nonsevere patients. Notably, nonsevere patients had lower median IgM levels than severe patients in week 4 and 5, but the difference did not reach statistical significance (P = .850 and .052, respectively), which may be due to the small sample size. Therefore, more studies are needed to clarify the precise association between antibody‐mediated immune response and disease severity.
Some limitations in our study should be noted. First, this is a retrospective study and serological testing had not become regular until late February 2020. Thus, most patients had incomplete weekly data of antibody testing from the 4th week to discharge, and only a small proportion of patients were included in paired analysis. Second, we did not discriminate binding antibodies specific to SARS‐CoV‐2 N and S protein (ie N‐IgM, N‐IgG, S‐IgM, S‐IgG). Third, the sample size was relatively small with inherently reduced statistical power. Furthermore, subgroup analysis stratified by age and comorbidities were not available in our study.
In conclusion, our study described the dynamic changes of serum antibodies of patients with COVID‐19 and provided deep insight into the clinical course and humoral immune response to SARS‐CoV‐2 during month 2 to 3 after illness onset.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
YL conceptualized the study. WZ, XX, ZC, HW, XZ, XT, and TL collected and analyzed the data. YL and WZ interpreted the data. WZ drafted the manuscript. All authors critically revised the manuscript and approved the final version for publication.
ACKNOWLEDGMENTS
This study was supported by the Special Foundation for National Science and Technology Basic Research Program of China (2017FY101200).
Zhou W, Xu X, Chang Z, et al. The dynamic changes of serum IgM and IgG against SARS‐CoV‐2 in patients with COVID‐19. J Med Virol. 2021;93:924–933. 10.1002/jmv.26353
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270‐273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Long QX, Liu BZ, Deng HJ, et al. Antibody responses to SARS‐CoV‐2 in patients with COVID‐19. Nat Med. 2020;26(6):845‐848. 10.1038/s41591-020-0897-1 [DOI] [PubMed] [Google Scholar]
- 5. Serologic responses of 42 MERS‐coronavirus‐infected patients according to the disease severity. Diagn Microbiol Infect Dis. 2017;89(2):106‐111. 10.1016/j.diagmicrobio.2017.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi Y, Wan Z, Li L, et al. Antibody responses against SARS‐coronavirus and its nucleocaspid in SARS patients. J Clin Virol. 2004;31(1):66‐68. 10.1016/j.jcv.2004.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun B, Feng Y, Mo X, et al. Kinetics of SARS‐CoV‐2 specific IgM and IgG responses in COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):940‐948. 10.1080/22221751.2020.1762515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiang F, Wang X, He X, et al. Antibody detection and dynamic characteristics in patients with COVID‐19. Clin Infect Dis. 2020. 10.1093/cid/ciaa461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okba NMA, Müller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2‐specific antibody responses in coronavirus disease patients. Emerg Infect Dis. 2020;26(7):1478‐1488. 10.3201/eid2607.200841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Espejo AP, Akgun Y, Al Mana AF, et al. Review of current advances in serologic testing for COVID‐19. Am J Clin Pathol. 2020;154(3):293‐304. 10.1093/ajcp/aqaa112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wen W, Su W, Tang H, et al. Immune cell profiling of COVID‐19 patients in the recovery stage by single‐cell sequencing. Cell Discov. 2020;6:31. 10.1038/s41421-020-0168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lou B, Li TD, Zheng SF, et al. Serology characteristics of SARS‐CoV‐2 infection since exposure and post symptom onset. Eur Respir J. 2020:2000763. 10.1183/13993003.00763-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Y, Jie Y, Hu Q, et al. A polymeric solid‐phase microextraction fiber for the detection of pharmaceuticals in water samples. J Chromatogr A. 2020;1623:461171. 10.1016/j.chroma.2020.461171 Jul 19 Jul 19 2020;1623:461171 [DOI] [PubMed] [Google Scholar]
- 15. Payne DC, Iblan I, Rha B, et al. Persistence of antibodies against Middle East respiratory syndrome coronavirus. Emerg Infect Dis. 2016;22(10):1824‐1826. 10.3201/eid2210.160706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu LP, Wang NC, Chang YH, et al. Duration of antibody responses after severe acute respiratory syndrome. Emerg Infect Dis. 2007;13(10):1562‐1564. 10.3201/eid1310.070576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med. 2020;26(8):1200‐1204. 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 18. Wang X, Guo X, Xin Q, et al. Neutralizing antibodies responses to SARS‐CoV‐2 in COVID‐19 inpatients and convalescent patients. Clin Infect Dis. 2020. 10.1093/cid/ciaa721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620‐2629. 10.1172/JCI137244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hou H, Zhang B, Huang H, et al. Using IL‐2R/lymphocytes for predicting the clinical progression of patients with COVID‐19. Clin Exp Immunol. 2020;201(1):76‐84. 10.1111/cei.13450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor A, Foo SS, Bruzzone R, Dinh LV, King NJ, Mahalingam S. Fc receptors in antibody‐dependent enhancement of viral infections. Immunol Rev. 2015;268(1):340‐364. 10.1111/imr.12367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu L, Wei Q, Lin Q, et al. Anti‐spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS‐CoV infection. JCI Insight. 2019;4(4):e123158. 10.1172/jci.insight.123158 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


