Abstract
The global pandemic of coronavirus disease‐2019 (COVID‐19) has led to significant disruptions in healthcare delivery. Patients with chronic liver diseases require a high level of care and are therefore particularly vulnerable to disruptions in medical services during COVID‐19. Recent data have also identified chronic liver disease as an independent risk factor for COVID‐19 related hospital mortality. In response to the pandemic, national and international societies have recommended interim changes to the management of patients with liver diseases. These modifications included the implementation of telehealth, postponement or cancelation of elective procedures, and other non‐urgent patient care‐related activities. There is concern that reduced access to diagnosis and treatment can also lead to increased morbidity in patients with liver diseases and we may witness a delayed surge of hospitalizations related to decompensated liver disease after the COVID‐19 pandemic has receded. Therefore, it is paramount that liver practices craft a comprehensive plan for safe resumption of clinical operations while minimizing the risk of exposure to patients and health‐care professionals. Here, we provide a broad roadmap for how to safely resume care for patients with chronic liver disease according to various phases of the pandemic with particular emphasis on outpatient care, liver transplantation, liver cancer care, and endoscopy.
Keywords: Chronic liver disease, COVID‐19, Endoscopy, Hepatocellular carcinoma, Hepatology, Liver transplantation, Outpatient, Post‐pandemic, SARS‐CoV‐2
Introduction
The global pandemic of coronavirus disease‐2019 (COVID‐19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), has overwhelmed health‐care systems in many countries and led to significant disruptions in care delivery. The burden of chronic liver diseases including hepatitis B, hepatitis C, alcoholic liver disease, and non‐alcoholic fatty liver disease has been increasing around the world. 1 Patients with chronic liver diseases need a high level of care and are therefore particularly vulnerable to disruptions in medical services during COVID‐19, either directly or indirectly. 1 For instance, a recent analysis of national transplant databases from France and the USA showed a striking reduction in liver transplantation during the COVID‐19 era. 2 Furthermore, another study conducted across the Veterans Health Administration showed a significant decrease in hospital admissions related to cirrhosis during this pandemic. 3 Chronic liver disease has also been identified as an independent risk factor for COVID‐19‐related hospital mortality in recent studies, including data from the COVID‐Hep.net and SECURE‐Cirrhosis registries. 4 , 5 , 6
In response to the pandemic, national and international societies have recommended interim changes to the management of patients with liver diseases, including the American Association for the Study of Liver Diseases (AASLD), 7 , 8 the British Society of Gastroenterology, 9 the European Association for the Study of the Liver (EASL), 10 and the Gastroenterological Society of Australia. 11 These modifications included the transition of a large number of outpatient clinic visits to telemedicine, postponement or cancelation of elective procedures, and other non‐urgent patient care‐related activities. There is significant concern that even restrictions on routine medical care can be detrimental, as reduced access to diagnosis and treatment can lead to increased morbidity in patients with liver diseases. 12 Multiple experts have warned that health‐care facilities could witness a delayed surge of hospitalizations related to decompensated liver disease, after COVID‐19 has receded. Hence, liver practices must craft a comprehensive plan for safe resumption of clinical operations while minimizing the risk of exposure to patients as well as medical staff.
Phases of the pandemic
COVID‐19 has had a worldwide impact, but there are significant geographical differences in the burden and temporal trends of the pandemic. Broadly, the World Health Organization categorizes the COVID‐19 pandemic into five phases, namely, acceleration, peak, deceleration, post‐peak, and post‐pandemic phases. 13 Countries such as China, South Korea, New Zealand, Australia, Spain, and Italy are currently in the post‐peak phase, whereas large swathes of the USA remain either in the acceleration or deceleration phase of the pandemic. Most recommendations from international societies have discussed specific strategies for care delivery in the context of the “acceleration” or “peak” phase. However, as regions move across the pandemic phases, we need a plan to reintroduce routine healthcare in a safe and timely manner during the post‐peak phases. Additionally, we need to lay the groundwork to ramp up preparedness for future pandemics during the post‐pandemic phase, while simultaneously remaining vigilant for further surges of cases of COVID‐19 cases that may emerge. In this review, we discuss broad principles applicable to reopening care for patients with liver diseases with a specific reference to the various phases of the pandemic (Figure 1).
FIGURE 1.
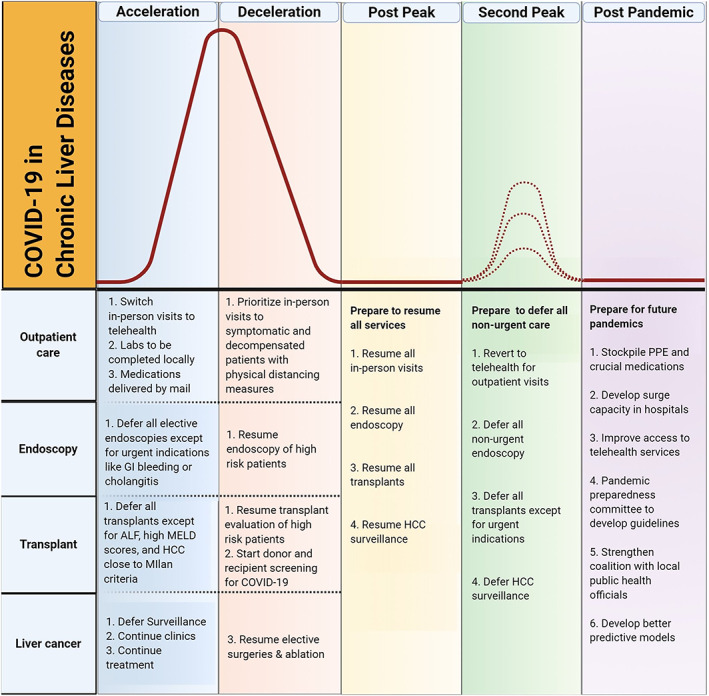
Proposed care model during different phases of the pandemic. ALF, acute liver failure; COVID‐19, coronavirus disease‐2019; HCC, hepatocellular carcinoma; MELD, model for end‐stage liver disease. [Color figure can be viewed at wileyonlinelibrary.com]
General considerations in the care of patients with chronic liver disease
As an international group of liver physicians, our collective experience makes it clear that a one‐size‐fits‐all approach will not work given the tremendous variation in patterns of care delivery around the world. We discuss here some general recommendations which can hopefully be adopted and modified to suit local practice preferences. Before any consideration to restarting in‐person care, practices need to effectively communicate and coordinate with local public health officials to gather information on the local status of the pandemic. Once they have confirmed sustained regional decelerations or entry into the post‐peak phase, health‐care facilities can plan to safely resume non‐COVID‐19 care. Moreover, health‐care systems have to be agile and be prepared to transition back to mitigation efforts if public health officials report a local surge in cases.
During the acceleration and peak phases of the pandemic, most practices have switched in‐person care to telehealth. Telehealth is both patient‐centered and conducive to social isolation practices with provider–patient communication methods ranging from phone calls to smartphone apps or webcam enabled computers. 14 Telehealth will likely continue to play a prominent role in the care of patients with liver diseases during the deceleration phase. We suggest a stepwise approach using telehealth as a buffer while we increase the capacity for face‐to‐face visits. which are clinically indicated. During the initial step, patients with a need for in‐person visits should be scheduled in the clinic, which may comprise 25–50% of clinic sessions, and telehealth continued for all other patients. In the subsequent step, most patients can come back for in‐person visits in the clinic, but telehealth should continue to be offered for those with stable disease and those who prefer to continue to use this modality. In the end, the proportion of sessions that can be indefinitely repurposed as telehealth visits should be decided by individual clinics. All clinical practices need to develop their own best practice guidelines outlining how physical distancing will be maintained and how clinical staff will use personal protective equipment (PPE) during in‐person visits. These guidelines will likely need to be refined throughout the pandemic. It is also important to develop local policies for how patients who are undergoing high‐risk procedures, like endoscopy, will be screened for COVID‐19 before arrival either via point of care polymerase chain reaction (PCR) testing or symptom‐based questionnaires. To facilitate this, we propose a system that implements a triage for symptoms 24–48 h prior to visit, the continued surveillance for COVID‐19 among symptomatic patients and mandatory temperature checks for both staff and patients. Inpatient settings have to be prepared for a possible surge of non‐COVID‐19‐related admissions, and they need to isolate the COVID‐19 patients from the other patients. Designating hospital areas into either COVID‐19 zones or non‐COVID‐19 zones has been suggested as one of the measures that can minimize exposure for both patients and staff. 15 In addition, the following steps can be adopted to maintain social distancing in the clinic such as electronic check‐in and check‐out processes, staggered staff schedules, universal masking for everyone inside health‐care facilities, and limiting family members and patient visitors. These infection prevention and control policies should be continued regardless of the pandemic phase.
Resuming in‐person outpatient clinical care
Routine outpatient care was significantly impacted during the onset of COVID‐19. When practices resume in‐person clinic visits, they need to ensure they are following appropriate screening practices for COVID‐19 and also enforce adequate social distancing in the liver clinic. Given these new requirements, it is likely that the number of patients who can be seen in‐person will not immediately reach pre‐pandemic levels, once outpatient clinical care is reopened. Clinical practices need to develop a strategic framework to identify and prioritize patients who need in‐person visits earlier.
During the deceleration phase, and to an even greater extent during the post‐peak period, we urge physicians to work closely with the liver clinic administrative staff to identify patients with advanced chronic liver diseases whose visits were either canceled or postponed during the pandemic and to ensure these patients are not lost to follow up. Additionally, a comprehensive review of the clinic backlog should be performed, with earlier scheduling of patients based on disease severity and clinical need. Symptomatic patients, patients with hepatic decompensation, those whose liver tests are worsening, or patients with liver cancer should be prioritized to be brought back to the clinic sooner, while routine follow up of patients with stable liver disease from hepatitis B or non‐alcoholic steatohepatitis can be slightly delayed (Table 1). Special attention needs to be given for liver cancer patients who have received locoregional therapy, which represents a heterogenous group, and optimal follow up and rescheduling should be considered in the context of a multidisciplinary team. This review should include new patient consults and also secure early outpatient follow up of patients recently discharged from the hospital. A proposed general schema is as follows:
Priority 1—needs to be seen right away; examples include patients who are decompensated that have not been stabilized, listed for liver transplant, newly diagnosed with liver cancer, and under consideration of resection or recent locoregional therapy.
Priority 2—can be delayed by 2–3 months; examples of which include patients who are decompensated but on stable doses of medication, treatment‐naïve HCV patients.
Priority 3—can be delayed by 3–6 months; examples of which include patients with compensated cirrhosis without ongoing liver injury, patients with hepatitis C cirrhosis who have achieved sustained virologic response, chronic hepatitis B on treatment, newly diagnosed non‐alcoholic fatty liver disease or non‐alcoholic steatohepatitis patients.
Priority 4—can be delayed by 6–9 months; examples of which include patients with or without cirrhosis seen in the last 6 months, patients with hepatitis C who have achieved sustained virologic response, stable non‐alcoholic fatty liver disease, early stage primary biliary cholangitis, or primary sclerosing cholangitis with stable liver chemistries.
TABLE 1.
A suggested framework for prioritizing visits
| In‐person visits |
| General hepatology clinic |
| Patients with decompensated cirrhosis |
| Patients requiring active adjustment of medications (diuretics, encephalopathy medications) |
| Pre‐transplant evaluations |
| Patients with alcohol‐related liver disease who restart drinking |
| Evaluation for elevated liver enzymes >3 times upper limit of normal |
| Hepatitis C treatment |
| Consider for patients with advanced fibrosis/cirrhosis |
| Hepatocellular carcinoma |
| Initial diagnostic evaluation |
| Case‐by‐case consideration for patients with autoimmune hepatitis, Wilson's disease, etc. requiring more urgent clinic evaluation |
| Continue telehealth |
| Routine visits for compensated cirrhosis |
| Hepatitis C treatment for patients without advanced fibrosis/cirrhosis |
| Evaluation for elevated liver enzymes <3 times upper limit of normal |
| Initial evaluation of non‐alcoholic fatty liver disease |
Another issue that needs to be addressed as part of the reopening is maintenance of continuity of care. During the pandemic, many liver physicians were deployed to other areas of need in the health‐care system, such as emergency rooms and COVID‐19 dedicated wards, while selected colleagues saw their patients in the liver clinic. Ideally, patients should be reassigned to their pre‐pandemic care providers to maintain continuity in the patient–provider relationship.
Assessing the degree of fibrosis is an important aspect in the care of patients with chronic liver disease and practices need to judiciously resume the use of ancillary testing such as transient elastography (TE) to determine fibrosis score. TE such as FIbroScan® is typically performed in the office, by the consulting hepatologist or an advanced provider and involves close contact with patients. While it is considered to be safe to resume in‐clinic TE in the deceleration or post‐peak phases of the pandemic, providers need to wear appropriate PPE throughout the procedure. 16 This should be according to local infection prevention and control policies and at a minimum should include surgical face masks for both the patient and care provider and disposable gloves. Even with appropriate precautions, TE is still associated with risk for exposure to SARS‐Co‐V2, given the close proximity to the patient, duration of contact with patient, and unknown adequacy of current disinfection protocols for transducer probes. Hence, it is important to continue to limit the use of TE where alternative non‐invasive robust markers for fibrosis such as FIB‐4, AST to platelet ratio index, or non‐alcoholic fatty liver disease fibrosis score are available. 12
With appropriate planning and implementation, we can hopefully restore adequate levels of outpatient care for patients with chronic liver disease in a timely manner. We propose a general strategy for in‐person visit prioritization in Table 1, which can be adopted and modified to fit institutional and provider preferences (Figure 1).
Liver transplantation: Considerations during the coronavirus disease‐2019 era
Patients requiring liver transplantation have been disproportionately affected by the pandemic as living donor programs have come to a halt in many countries and rates of deceased donor liver transplantation have plummeted. 2 During the acceleration and peak phases of the pandemic, liver transplantation evaluations were paused for non‐urgent indications. Several recommendations have been made for screening recipients and donors for COVID‐19 by the various international transplant societies to ensure the safety of patients and medical staff. Furthermore, the role of immunosuppression in disease severity remains controversial, 17 , 18 leading to wide variations in how hepatologists manage post‐transplant patients. We will discuss the care of patients with chronic liver disease with relation to liver transplantation in three separate parts—pre‐transplant evaluation, transplant surgery, and post‐transplant management.
Pre‐transplant evaluation
During the deceleration and the post‐peak phases of the pandemic, transplant centers will look to ramp up transplant evaluation and listing. Although some aspects of transplant evaluation are feasible via telehealth and using local laboratories, the face‐to‐face interaction with the surgery team, social worker, and hepatologist is of utmost value. It is important for centers to decide which aspects of transplant evaluation could continue via telehealth in the post‐peak phase. For example, psychosocial evaluation, nutritional assessment, social work evaluation, or visit with pharmacists can all be achieved via telehealth, even more efficiently, as multiple providers do not have to be present in a single space during the evaluation. For now, it is still best to take a stepwise approach and continue to do as much telehealth as possible. One of the most important steps before fully opening up in‐person clinics is to work with local officials to understand the COVID‐19 local incidence, capacity for testing, and local mandates for isolation.
It has been recommended that transplant offers should be limited to patients with a high model for end‐stage liver disease score, patients with hepatocellular carcinoma (HCC) close to Milan criteria limit, and those with acute liver failure during the acceleration and peak phases of the pandemic. 7 , 11 , 19 This approach should be continued until there is a sustained local decrease in the number of COVID‐19 cases for at least 14 days. At that point, centers should consider broadening the transplantation criteria. However, we have to remain vigilant and anticipate further surges of COVID‐19 cases, with a plan to reenter mitigation efforts and scale back transplantation as needed.
Rapid testing for COVID‐19 by real‐time PCR should be continued for all patients who are high on the waiting list. There is a lack of understanding as to whether transmissibility decreases after resolution of COVID‐19 and if viral shedding directly implies transmissibility. This will make it challenging to triage patients who test positive for SARS‐CoV‐2 but are otherwise suitable candidates for transplant. In one study, patients were retested in 8 days, and if negative, the transplant team proceeded with reactivation of the transplantation waiting list. 20 Because patients can shed virus for many more days without symptoms, negative PCR seems a reasonable approach prior to activation on the list.
Transplant surgery
Most elective surgeries including liver transplantation and hepatic resections for cancer have seen a decline in numbers during the COVID‐19 pandemic, as shown by an Italian survey. 21 In the USA, the Center for Medicare and Medicaid Services designated deceased donor liver transplantation as a tier 3b procedure, putting it in the “do not postpone” category. Despite this designation, there was a significant drop in both deceased donor and living donor liver transplantation in the USA and also in most parts of the world. 2 The main aim of developing reopening strategies is to safely restore the numbers of liver transplantation back to pre‐COVID‐19 levels. These efforts largely depend on the stage of the pandemic, which in turn determines availability of resources and feasibility to safely proceed with transplantation.
A recent autopsy study showed high SARS‐CoV‐2 viremia in 60%, and very high viral titers in the liver in 41% of the patients who died of severe COVID‐19. 22 Hence, the recommendations to avoid donors diagnosed with COVID‐19 are prudent. 23 To ensure that organs from asymptomatic donors who are SARS‐CoV‐2 positive do not inadvertently get accepted, it is important to screen donors for COVID‐19. Donors should be screened by epidemiological and clinical history for suspected COVID‐19 as well as PCR within 3 days of procurement, when feasible. Currently, the American Society of Transplantation advises against using chest computed tomography (CT) to screen asymptomatic living donors for COVID‐19 although several centers around the world include CT chest in the preoperative evaluation because living donors undergo CT of the liver as a part of surgical planning anyway. Preventative strategies and social distancing measures should be reinforced in living donors, especially within 14 days prior to planned organ donation. If a living donor is considered high risk either because of COVID‐19 symptoms or exposure, organ donation should be postponed by at least 28 days. In case of donors at intermediate risk for COVID‐10 like those with exposure but no symptoms, American Society of Transplantation recommends we consider delaying the transplant for at least 14 days. 24 If the donor risk for COVID‐19 is confirmed to be low based on clinical and epidemiological criteria, one can consider proceeding with transplantation even without PCR testing. Moreover, if the donor has had resolution of symptoms more than 28 days prior to organ donation and has negative testing repeated at least 24 h apart, organs are considered safe to use. 24
Current recommendations do not endorse SARS‐Co‐V2 antibody testing for donor screening, given the unreliable results. 24 Rapid antigen testing approved by the Food and Drug Administration in early May is also not recommended for donor screening. A case report published recently discussed the clinical course of a living liver donor who was mildly symptomatic prior to donor hepatectomy tested positive on postoperative day 3 and subsequently improved without much trouble. We do need more data about the safety of hepatectomy in asymptomatic COVID‐19 patients. 25
There are limited published data on the early experience with liver transplantation during the COVID‐19 era. A report from the hyperendemic region in Italy noted that 2 of the 17 patients who underwent liver transplantation developed COVID‐19 on postoperative days 9 and 22. 26 Whether the infections were nosocomial, donor‐derived or just delayed diagnosis of asymptomatic recipients could not be ascertained conclusively. On the other hand, a center in Sichuan, China, reported no complications in the six liver transplants performed during COVID‐19. 27 They also reported using standard immunosuppression without dose reduction. The only caveat was the level of spread in that province was not very high. Most of the centers have already adopted real‐time‐PCR and CT scan screening along with serology to rule out asymptomatic infection in donors, but unless we completely isolate the transplant process from cross‐contamination and are able to identify all asymptomatic COVID‐19 patients, the levels of transplantation may not reach the pre‐COVID19 era any time soon.
Post‐transplant care
Post‐transplant patients represent a high‐risk group in the COVID‐19 era because there is significant concern that the ongoing immunosuppression makes them more vulnerable to acquire SARS‐CoV‐2; hence, efforts to isolate them from exposure to the virus must continue. Ideally, the location of the clinic and the medical team taking care of pre‐transplant and post‐transplant patients should be segregated from one another. Measures that can be taken to minimize exposure risk are similar to pre‐transplant care and include offering telemedicine for follow up, using local laboratories for blood tests, and providing medication refills through the mail. Patients in the immediate post‐transplant phase need close follow up and frequent blood tests. One approach is to continue frequent blood work for up to 1‐month post‐transplant, and then to space out blood tests to every 2 weeks followed by every 4 weeks and eventually transition to every 3–6 months post‐transplant. This has to be individualized on a case‐by‐case basis depending on the patient's overall clinical condition.
Most expert guidelines have recommended that patients continue their stable doses of immunosuppression and warn against reductions in immunosuppression during COVID‐19 pandemic. In patients with severe COVID‐19, steroids need to be avoided if possible and immunosuppression minimized to lowest safe doses. In the post‐peak phase, transplant hepatologists need to make sure that all post‐transplant patients are on adequate doses of immunosuppression in order to avoid unwanted complications like acute rejection.
Care of patients with hepatocellular carcinoma
Patients with liver cancer face the dilemma of either being exposed to a higher risk of COVID‐19‐related mortality if they continue medical care versus a higher risk of cancer‐related mortality if care is disrupted. Patients with an underlying diagnosis of cancer are increasingly being reported to experience more severe COVID‐19 infections and higher mortality if infected. 28 , 29 , 30 Most clinical practices have tried to continue to provide uninterrupted care for patients with liver cancer during COVID‐19 by embracing telemedicine. The uniform recommendations from AASLD, EASL, and the International Liver Cancer Association have been to continue imaging surveillance, to maintain virtual multidisciplinary tumor boards, and to sustain transarterial therapies and systemic therapies as indicated. 7 , 31
In reality, despite our best efforts, several aspects of clinical care will undoubtedly be interrupted for patients with liver cancer. For instance, elective surgical procedures like liver resection and thermal ablation have been canceled or rescheduled. Although only a select group of patients who present with single, small tumors are generally eligible for these, there is significant concern that postponing these procedures could have led to progression of cancer and these patients subsequently could become ineligible for curative procedures. When reopening care, every effort must be made to quickly reschedule these elective procedures for eligible patients with HCC. Liver transplant evaluation or listing might have been delayed for patients with liver cancer who are candidates for transplant. In the post‐peak phase, these patients should be prioritized to undergo expedited transplant listing because they already have extended wait times before they can avail exception points. It is likely that transarterial therapies like chemoembolization or radioembolization were continually offered during the COVID‐19 peak in most practices. If there were any interruptions either because of patient cancelations or limitations in access, these therapies have to be expeditiously resumed to avoid cancer progression.
Radiology departments are expected to experience a surge in need for imaging services once routine care resumes. All patients with HCC whose surveillance imaging had been postponed will need to be prioritized when the radiology division opens up, to ensure we can monitor tumor response to therapy and decide upon appropriate next steps. Moreover, surveillance ultrasound scans for patients with cirrhosis have also been rescheduled based on current recommendations from international societies. Patients whose procedures were postponed need to be sent appropriate reminders to ensure they attend their new appointments and do not miss these crucial tests needed for early detection of HCC. In case of limited availability of radiologic testing for HCC surveillance, we suggest prioritizing patients with a high risk of HCC, including those with Liver Imaging Reporting and Data System 3/4 lesions on previous imaging, patients on the transplant list, and patients who have not had regular previous surveillance. Finally, clinical trial enrolment for patients with advanced HCC has been significantly impacted because most centers interrupted clinical trial enrolment during the peak of COVID‐19. 32 Strategic planning and coordination will be needed to ensure robust enrolment of patients with advanced HCC in ongoing clinical trials once we reopen routine care in the post‐peak and post‐pandemic phases of COVID‐19.
Resuming endoscopy after coronavirus disease‐2019
During the acceleration and peak phases of the COVID‐19 pandemic, most endoscopy activity has been suspended except for urgent, life threatening conditions and time‐sensitive cases. 33 , 34 , 35 , 36 Elective procedures specific to patients with liver diseases that may have been deferred include esophagogastroduodenoscopy for variceal screening and endoscopic retrograde cholangiopancreatography for dilatation or stent replacement in post‐liver transplantation or primary sclerosing cholangitis. 19 As the tide of the pandemic turns, a stepwise, 36 phased 37 resumption of elective endoscopy services will need to be implemented with strategies to ensure that deferred endoscopies are tracked.
Recently, various societies have offered guidance on resuming elective endoscopies. The joint American Gastroenterological Association/Digestive Health Physicians Association guidance 38 specifically mentions the importance of abiding by local authorities' guidance when considering the timing and approach to reopening. Scheduled endoscopies should continue to be prioritized by level of urgency, based on individual patient considerations and physician's judgment. The American Society for Gastrointestinal Endoscopy recommends prioritizing into three tiers, urgent (tier 1), semi‐urgent (tier 2), and elective (tier 3). 39 Strict screening procedures may prevent the spread of COVID‐19 via digestive endoscopy during this period. 40 , 41 The concept of a “shielded” category of patients who are essentially immunocompromised and require more protection is advocated by the British Society of Gastroenterology guidance. 37 It is suggested that all patients should receive PCR‐based testing for active COVID‐19 infection wherever possible, 37 , 38 , 39 and if not feasible, then patients should keep a daily temperature log for 10 days prior to the procedure. A symptom questionnaire and temperature check should be administered to all patients on the day of procedure. Of note, many of the protective measures with regard to infection prevention and control are kept in force with mask recommendations based on the availability of PCR‐based COVID‐19 testing and PPE supplies. 38 Although, less flexibility is allowed by the American Society for Gastrointestinal Endoscopy guidance, which suggests the use of N95 respirators or equivalent regardless of COVID‐19 test results given the moderate negative predictive value of PCR testing done on nasal swab samples and insufficient data regarding the correlation between infectivity and antibody development. 39
Specifically for variceal screening, during the peak of COVID‐19, EASL 11 recommended risk assessments by applying the Baveno VI criteria for risk stratification, where patients with a liver stiffness <20 kPa with a platelet count >150 000 have a very low risk of having clinically significant varices that require treatment and can avoid screening endoscopy altogether. 42 The AASLD 7 goes even further to suggest primary prophylaxis with non‐specific beta blockers instead of screening endoscopy in patients with clinically significant portal hypertension or high risk of decompensation. This is supported by a recent study where long‐term treatment with beta blockers could increase decompensation‐free survival in patients with compensated cirrhosis and clinically significant portal hypertension, although the benefits were mainly observed for patients with ascites. 43 A protocol has been devised to help manage these patients if there is limited access to endoscopy. In general, it is thought that the benefit of endoscopy outweighs the risk of COVID‐19 for patients with recent variceal bleeding and for patients with compensated and decompensated cirrhosis who are intolerant to non‐specific beta blockers. 44 We would like to emphasize that these recommendations are largely based on expert opinion and hence need to be modified to suit local practice patterns.
Preparedness for post‐pandemic phase
All the reopening measures need to be considered in the context of the pandemic where the possibility of a second peak or even further peaks is still possible. Long‐term plans need to be in place as some models project that the effects of COVID‐19 may last until 2022. 45 Any efforts for reopening clinical services will need to take into account the local situation regarding the outbreak and allow for the flexibility to recalibrate the overall strategy. Contingency plans should be established with rapid response measures in coordination with public health officials. Factors that may need to be reevaluated continuously include the availability of effective diagnostics, levels of PPE available, and future developments that include population immunity and the availability of vaccines.
The COVID‐19 outbreak has been a humbling experience for all. In the post‐pandemic phase, efforts would be shifted to prepare for future pandemics. 46 , 47 Measures would include the stockpiling of PPE, medical equipment and crucial medications, developing surge capacity within health‐care systems, improving access to telehealth services, establishing pandemic preparedness committees to develop clinical frameworks for future outbreaks, strengthening the coalition with local public health officials, and developing better predictive models.
Conclusions
The COVID‐19 pandemic has disrupted health‐care delivery for patients with chronic liver diseases all over the world. Fortunately, most regions of the world are transitioning either to the deceleration on post‐peak phase of the COVD‐19 pandemic. As we move into these later phases of this pandemic, the resumption of normal clinical services will become increasingly important to ensure patients receive adequate and timely care. Close liaison with local public health authorities for coordinated strategies and having the flexibility to adapt to the ebbs and flows of the pandemic will be essential for the safe reopening of services. In this review, we have provided a broad roadmap for how to safely resume care for patients with chronic liver diseases in various clinical settings including outpatient care, liver transplantation, and endoscopy.
We are hoping that the most grueling phases of the COVID‐19 pandemic are now behind us so we can focus on rebuilding our clinical practices. Patients with chronic liver diseases have missed clinic appointments, surveillance ultrasounds, elective endoscopies, laboratory tests, and even medication refills during this pandemic. We owe these patients a comprehensive and realistic plan to safely and expeditiously restore access to all levels of care while minimizing their exposure to SARS‐CoV‐2. Lastly, we hope that the lessons learnt from managing the current outbreak will enhance preparedness for future pandemics.
Kapuria, D. , Bollipo, S. , Rabiee, A. , Ben‐Yakov, G. , Kumar, G. , Siau, K. , Lee, H.‐W. , Congly, S. , Turnes, J. , Dhanasekaran, R. , Lui, R. N. , and the Global Online Alliance for Liver Studies (GOAL) (2021) Roadmap to resuming care for liver diseases after coronavirus disease‐2019. Journal of Gastroenterology and Hepatology, 36: 885–892. 10.1111/jgh.15178.
Declaration of conflict of interest: All authors declare no conflicts of interest
References
- 1. GBD 2017 Cirrhosis Collaborators . The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020. Mar; 5: 245–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Loupy A, Aubert O, Reese PP, Bastien O, Bayer F, Jacquelinet C. Organ procurement and transplantation during the COVID‐19 pandemic [Internet]. Lancet 2020. Available from:; 395: e95–e96. 10.1016/s0140-6736(20)31040-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mahmud N, Hubbard RA, Kaplan DE, Serper M. Declining cirrhosis hospitalizations in the wake of the COVID‐19 pandemic: a national cohort study [Internet]. Gastroenterology 2020. Available from:. 10.1053/j.gastro.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moon AM, Webb GJ, Aloman C et al. High mortality rates for SARS‐CoV‐2 infection in patients with pre‐existing chronic liver disease and cirrhosis: preliminary results from an international registry. J. Hepatol. [Internet] 2020. May. Available from:. https://linkinghub.elsevier.com/retrieve/pii/S0168827820303056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. The OpenSAFELY Collaborative , Williamson E, Walker AJ et al. OpenSAFELY: factors associated with COVID‐19‐related hospital death in the linked electronic health records of 17 million adult NHS patients. medRxiv. 2020. May 7;2020.05.06.20092999.
- 6. Singh S, Khan A. Clinical characteristics and outcomes of COVID‐19 among patients with pre‐existing liver disease in United States: a multi‐center research network study. Gastroenterol. Int. 2020. May 3. Available from:. 10.1053/j.gastro.2020.04.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fix OK, Hameed B, Fontana RJ et al. Clinical best practice advice for hepatology and liver transplant providers during the COVID‐19 pandemic: AASLD expert panel consensus statement [Internet]. Hepatology 2020. Available from:. 10.1002/hep.31281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. AASLD clinical insights for hepatology and liver transplant providers during the covid‐19 pandemic.pdf.
- 9.Service recovery documents: the what, when and how | The British Society of Gastroenterology [Internet]. The British Society of Gastroenterology. 2020 [cited 2020 May 24]. Available from: https://www.bsg.org.uk/covid-19-advice/service-recovery-documents-the-what-when-and-how/
- 10. Boettler T, Newsome PN, Mondelli MU et al. Care of patients with liver disease during the COVID‐19 pandemic: EASL‐ESCMID position paper. JHEP Rep. 2020. Jun; 2: 100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Considerations for management of liver diseases during the COVID‐19 pandemic. https://www.gesa.org.au/public/13/files/COVID-19/Considerations%20for%20management%20of%20liver%20diseases%2028May20%20final.pdf accessed on 27 June 2020
- 12. Tapper EB, Asrani SK. The COVID‐19 pandemic will have a long‐lasting impact on the quality of cirrhosis care. J. Hepatol. [Internet] 2020. Apr 13. Available from:. 10.1016/j.jhep.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. WHO Pandemic phase descriptions and main actions by phase [Internet]. [cited 2020 May 13]. Available from: https://www.who.int/influenza/resources/documents/pandemic_phase_descriptions_and_actions.pdf
- 14. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid‐19. N. Engl. J. Med. 2020. Apr 30; 382: 1679–1681. [DOI] [PubMed] [Google Scholar]
- 15. OPENING UP AMERICA AGAIN [Internet]. cms.gov. [cited 2020 May 15]. Available from: https://www.cms.gov/files/document/covid-flexibility-reopen-essential-non-covid-services.pdf
- 16. Crespo J, Andrade R, Alberca de Las Parras F et al. Resumption of activity in gastroenterology departments. Recommendations by SEPD, AEEH, GETECCU and AEG. Gastroenterol. Hepatol. [Internet] 2020. Apr 25. Available from:. 10.1016/j.gastrohep.2020.04.001 [DOI] [PubMed] [Google Scholar]
- 17. Ahn C, Amer H, Anglicheau D et al. Global transplantation COVID report March 2020. Transplantation 2020. Apr 1. Available from:. 10.1097/TP.0000000000003258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boyarsky BJ, Chiang TP‐Y, Werbel WA et al. Early impact of COVID‐19 on transplant center practices and policies in the United States. Am. J. Transplant. [Internet] 2020. Apr 13. Available from:. http://doi.wiley.com/10.1111/ajt.15915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bollipo S, Kapuria D, Rabiee A et al. One world, one pandemic, many guidelines—management of liver diseases during COVID‐19. Accepted Gut Apr 2020. [DOI] [PMC free article] [PubMed]
- 20. Tzedakis S, Jeddou H, Houssel‐Debry P, Sulpice L, Boudjema K. COVID‐19: thoughts and comments from a tertiary liver transplant center in France. Am. J. Transplant. [Internet] 2020. Apr 26. Available from:. https://onlinelibrary.wiley.com/doi/abs/10.1111/ajt.15918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Torzilli G, Belghiti J, Kokudo N et al. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations? An observational study of the HCC East‐West study group. Ann. Surg. 2013; 257: 929–937. [DOI] [PubMed] [Google Scholar]
- 22. Wichmann D, Sperhake J‐P, Lütgehetmann M et al. Autopsy findings and venous thromboembolism in patients with COVID‐19: a prospective cohort study. Ann Intern Med [Internet] 2020. May 6. Available from:. 10.7326/M20-2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah MB, Lynch RJ, El‐Haddad H, Doby B, Brockmeier D, Goldberg DS. Utilization of deceased donors during a pandemic: an argument against using SARS‐CoV‐2 positive donors [Internet]. Am. J. Transplant. 2020. Available from:. 10.1111/ajt.15969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. American Society of Transplantation . Recommendations and guidance for organ donor testing [Internet]. 2020. [cited 2020 May 23]. Available from: https://www.myast.org/sites/default/files/COVID19%20FAQ%20Donor%20Testing%2005.19.2020_0.pdf
- 25. Hong H, Kim S, Choi DL, Kwon HH. A case of coronavirus disease 2019–infected liver transplant donor. Am. J. Transplant. [Internet] 2020. May 12. Available from:. https://onlinelibrary.wiley.com/doi/abs/10.1111/ajt.15997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maggi U, De Carlis L, Yiu D et al. The impact of the COVID‐19 outbreak on liver transplantation programmes in Northern Italy. Am. J. Transplant. [Internet] 2020. Apr 24; 20: 1840–1848; Available from:. https://onlinelibrary.wiley.com/doi/abs/10.1111/ajt.15948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Liu H, Buhler LH, Deng S. Strategies to halt 2019 novel coronavirus (COVID‐19) spread for organ transplantation programs at the Sichuan Academy of Medical Science and Sichuan Provincial People's Hospital, China [Internet]. Am. J. Transplant. 2020; 20: 1837–1839. Available from:. 10.1111/ajt.15972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z. Patients with cancer appear more vulnerable to SARS‐COV‐2: a multi‐center study during the COVID‐19 outbreak. Canc. Discov. [Internet] 2020. Apr 28; 10 6:783–791; Available from:. 10.1158/2159-8290.CD-20-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miyashita H, Mikami T, Chopra N et al. Do patients with cancer have a poorer prognosis of COVID‐19? An experience in New York City. Ann. Oncol. [Internet] 2020. Apr 21. Available from:. 10.1016/j.annonc.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mehta V, Goel S, Kabarriti R et al. Case fatality rate of cancer patients with COVID‐19 in a New York Hospital System [Internet]. Cancer Discov. 2020; 10: 935–941. Available from:. 10.1158/2159-8290.cd-20-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meyer T, Chan S. Management of HCC during Covid‐19 pandemic: ILCA Guidance [Internet]. https://ilca-online.org/. 2020. [cited 2020 Apr 16]. Available from: https://ilca-online.org/covid19andlivercancer/
- 32. Leuty R. Clinical trials in the age of COVID: shelter‐in‐place, hospital restrictions cause drug study delays. [Internet]. [cited 2020 May 13]. Available from: https://www.bizjournals.com/sanfrancisco/news/2020/04/09/covid-19-coronavirus-clinical-trials.html
- 33. Lui RN, Wong SH, Sánchez‐Luna SA et al. Overview of guidance for endoscopy during the coronavirus disease 2019 pandemic. J Gastroenterol Hepatol [Internet] 2020. Mar 31; 35: 749–759; Available from:. 10.1111/jgh.15053 [DOI] [PubMed] [Google Scholar]
- 34. Sultan S, Lim JK, Altayar O et al. AGA Institute rapid recommendations for gastrointestinal procedures during the COVID‐19 pandemic. Gastroenterol. Int. 2020. Mar 31. Available from:. 10.1053/j.gastro.2020.03.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gralnek IM, Hassan C, Beilenhoff U et al. ESGE and ESGENA position statement on gastrointestinal endoscopy and the COVID‐19 pandemic. Endoscopy [Internet] 2020. Apr 17; 52: 483–490; Available from:. 10.1055/a-1155-6229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chiu PWY, Ng SC, Inoue H et al. Practice of endoscopy during COVID‐19 pandemic: position statements of the Asian Pacific Society for Digestive Endoscopy (APSDE‐COVID statements). Gut [Internet] 2020. Apr 2; 69: 991–996; Available from:. 10.1136/gutjnl-2020-321185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. BSG Guidance on recommencing GI Endoscopy in the deceleration & early recovery phases of the COVID‐19 pandemic | The British Society of Gastroenterology [Internet]. The British Society of Gastroenterology. 2020. [cited 2020 May 7]. Available from: https://www.bsg.org.uk/covid-19-advice/bsg-guidance-on-recommencing-gi-endoscopy-in-the-deceleration-early-recovery-phases-of-the-covid-19-pandemic/ [DOI] [PMC free article] [PubMed]
- 38.Joint AGA/DHPA Guidance: recommendations for resumption of elective endoscopy during the COVID‐19 pandemic [Internet]. 2020 [cited 2020 May 7]. Available from: https://www.dhpassociation.org/2020/04/27/aga-dhpa-resume-endoscopy-covid19/
- 39. American Society for Gastrointestinal Endoscopy . Guidance for resuming GI endoscopy and practice operations after the COVID‐19 pandemic. 2020; Available from: https://www.asge.org/docs/default-source/default-document-library/asge-guidance-for-reopeningl_4-28-2020.pdf [DOI] [PMC free article] [PubMed]
- 40. Han J, Wang Y, Zhu L et al. Preventing the spread of COVID‐19 in digestive endoscopy during the resuming period: meticulous execution of screening procedures. Gastrointest Endosc [Internet] 2020. Apr 5. Available from:. 10.1016/j.gie.2020.03.3855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Han J, Zhu L, Wang Y, Zeng Z, Zhang S. Resumption of daily services in a gastroenterology department in Guangzhou, China, in the wake of COVID‐19. Lancet Gastroenterol. Hepatol. [Internet] 2020. May 4. Available from:. 10.1016/S2468-1253(20)30133-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Franchis R, Baveno VI. Faculty. expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015. Sep; 63: 743–752. [DOI] [PubMed] [Google Scholar]
- 43. Villanueva C, Albillos A, Genescà J et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet 2019. Apr 20; 393: 1597–1608. [DOI] [PubMed] [Google Scholar]
- 44. Congly SE, Sadler MD, Abraldes JG, Tandon P, Lee SS, Burak KW. Practical management of esophageal varices in the context of SARS‐CoV‐2 (COVID‐19): the Alberta protocol. Can Liv J. 2020. May; 6: e20200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS‐CoV‐2 through the postpandemic period. Science 2020. May 22; 368: 860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toner E, Waldhorn R. What hospitals should do to prepare for an influenza pandemic [Internet]. Biosecur. Bioterror.:Biodefence Strat. Pract. Sci. 2006; 4: 397–402. Available from:. 10.1089/bsp.2006.4.397 [DOI] [PubMed] [Google Scholar]
- 47. Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID‐19)? [Internet]. Ann. Intern. Med. 2020; 172: 621–622. Available from:. 10.7326/m20-0907 [DOI] [PMC free article] [PubMed] [Google Scholar]


