Abstract
A newly identified coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which causes the infectious coronavirus disease 2019 (COVID‐19), emerged in December 2019 in Wuhan, Hubei Province, China, and now poses a major threat to global public health. Previous studies have observed highly variable alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in patients with COVID‐19. However, circulating levels of the cholangiocyte injury biomarker gamma‐glutamyltransferase (GGT) have yet to be reported in the existing COVID‐19 case studies. Herein, we describe the relationship between GGT levels and clinical and biochemical characteristics of patients with COVID‐19. Our study is a retrospective case series of 98 consecutive hospitalized patients with confirmed COVID‐19 at Wenzhou Central Hospital in Wenzhou, China, from January 17 to February 5, 2020. Clinical data were collected using a standardized case report form. Diagnosis of COVID‐19 was assessed by symptomatology, reverse‐transcription polymerase chain reaction (RT‐PCR), and computed tomography scan. The medical records of patients were analyzed by the research team. Of the 98 patients evaluated, elevated GGT levels were observed in 32.7%; increased C‐reactive protein (CRP) and elevated ALT and AST levels were observed in 22.5%, 13.3%, and 20.4%, respectively; and elevated alkaline phosphatase (ALP) and triglycerides (TGs) were found in 2% and 21.4%, respectively. Initially, in the 82 patients without chronic liver disease and alcohol history, age older than 40 years (P = 0.027); male sex (P = 0.0145); elevated CRP (P = 0.0366), ALT (P < 0.0001), and ALP (P = 0.0003); and increased TGs (P = 0.0002) were found to be associated with elevated GGT levels. Elevated GGT (P = 0.0086) and CRP (P = 0.0162) levels had a longer length of hospital stay. Conclusion: A sizable number of patients with COVID‐19 infection have elevated serum GGT levels. This elevation supports involvement of the liver in persons with COVID‐19.
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- GGT
gamma‐glutamyltransferase
- RT‐PCR
reverse‐transcription polymerase chain reaction
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- TBIL
total bilirubin
- TG
triglyceride
Coronavirus disease 2019 (COVID‐19), an infectious disease characterized by fever and pneumonia, is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Deep‐sequencing analysis from lower respiratory tract samples indicated that this pathogen is a novel coronavirus.( 1 ) COVID‐19 may progress rapidly to acute respiratory distress syndrome with considerable morbidity and mortality.( 2 ) The disease was initially identified in an outbreak that occurred in Wuhan, Hubei Province, China.( 3 ) In late January 2020, large numbers of people across China returned to their hometowns after visiting Wuhan for the Chinese Lunar New Year. Consequently, the virus has extended rapidly to most parts of China as well as to other countries.
Liver function test abnormalities have been reported in up to 40% of patients suffering from COVID‐19; 1 patient was reported to develop severe acute liver injury.( 1 , 4 , 5 ) Liver biopsy specimens of patients with COVID‐19 exhibit moderate microvesicular steatosis and mild lobular and portal activity.( 6 ) However, it is uncertain whether the injury was induced by SARS‐CoV‐2 infection or hepatotoxicity. Liver damage has been reported in patients infected with SARS‐CoV‐2 and Middle Eastern respiratory syndrome coronavirus (MERS‐CoV).( 7 , 8 )
COVID‐19 is closely related to SARS and has recently been demonstrated to share the same host cell receptor, angiotensin‐converting enzyme 2 (ACE2).( 9 , 10 ) Single‐cell RNA sequencing analyses identified significant enrichment of ACE2 expression in a major portion of the cholangiocyte cluster (59.7% of cells).( 11 ) In the adult liver, gamma‐glutamyltransferase (GGT), a diagnostic biomarker for cholangiocyte injury, is localized in the biliary pole of hepatocytes and cholangiocytes.( 12 , 13 ) In one study, GGT levels were reported to be elevated in subjects with COVID‐19; however, this study did not provide other granular results or correlation with other clinical variables.( 14 ) In this study, we describe the clinical characteristics and laboratory findings of patients in Wenzhou, Zhejiang Province, China, infected with SARS‐CoV‐2 to provide insight into the relationship between changes in GGT levels and infection and also to consider diagnostic strategies for patients with COVID‐19 with liver dysfunction.
Patients and Methods
Patients
We recruited patients with symptoms from January 17 to February 5, 2020, at Wenzhou Central Hospital in Wenzhou, China, in this retrospective study. Our hospital treated only mild patients; severe/intensive care unit patients were transferred to other advanced care hospitals. All patients had been discharged by January 28 to March 3, 2020. Patients with suspected SARS‐CoV‐2 infection were admitted centrally to the hospital from the whole of Wenzhou without selectivity. All patients at Wenzhou Central Hospital who were diagnosed with COVID‐19 pneumonia by symptomatology, reverse‐transcription polymerase chain reaction (RT‐PCR), and computed tomography scan according to World Health Organization interim guidance were enrolled in this study. The study was approved by the Wenzhou Central Hospital ethics committee, and written informed consent was obtained from patients at the time of retrospective data collection.
Data Collection
On the first day of admission, the results of laboratory tests were collected. Data were extracted from electronic medical records of patients using a standardized data collection form. Variables included patient epidemiologic, clinical, laboratory, and radiologic characteristics and treatment. The data were reviewed by a trained team of physicians. Information recorded included demographic data, medical history, exposure history, underlying comorbidities, symptoms, signs, and laboratory findings. The medical records of patients were analyzed by the research team.
RT‐PCR Assay for COVID‐19
The RT‐PCR test was performed using nasal and pharyngeal swab specimens or induced sputum at Wenzhou Municipal Center for Disease Control and Prevention and Wenzhou Central Hospital.
Statistical Analysis
Categorical variables were described as frequency rates and percentages, and continuous variables were described using mean values. Means for continuous variables were compared using independent group t tests when the data were normally distributed. For comparison, patients were classified into two groups according to GGT levels: group 1 (G1), normal level; and group 2 (G2), elevated level. To obtain associations between G1 and G2 and the variables listed in the study, the χ2 test or the unpaired t test, as appropriate, was applied. Differences were considered significant at P < 0.05.
Results
Epidemiologic Characteristics
A total of 98 patients with COVID‐19 were studied; their clinical, biomedical, and characteristics are shown in Table 1. The mean age was 44.5 years (range, 2‐85 years), and more than half (58.2%) were male patients. Among the 98 patients, 53 (54%) had exposure history in Wuhan and 3 (3%) had a history of exposure to the Wuhan Huanan seafood market. All patients were hospitalized at the time of diagnosis. Of the patients, 15 (15.3%) had a history of chronic liver disease (hepatitis B virus, 7 [7.1%]; steatosis, 8 [8.2%]), 4 (4%) had acute kidney injury, 1 (1%) had myocardial damage, and 1 (1%) had a history of drinking. The most common symptoms at onset were fever in 83 patients (84.7%) and cough in 59 patients (60.2%). Other nonspecific symptoms included expectoration (15 [15.3%]), diarrhea (8 [8.2%]), chest pain (6 [6.1%]), and fatigue (3 [3%]); 1 patient exhibited no symptoms (Table 1). Regarding the infection index, most patients (73 [74.5%]) had high levels of C‐reactive protein (CRP). On admission, leukocytes were below the normal range in 27 patients (27.5%) and above the normal range in 1 patient (1%) and 33 patients (33.7%) had neutrophils above the normal range. Serum creatinine and hemoglobin were below the normal range in 26.5% and 11.2%, respectively, of patients (Table 2).
Table 1.
Characteristics of Patients With COVID‐19
| Characteristic | Number |
|---|---|
| Age, years | |
| <18 | 2 |
| 19‐40 | 33 |
| 41‐60 | 54 |
| >61 | 9 |
| Sex | |
| Female | 41 |
| Male | 57 |
| Chronic medical illness | |
| Hypertension | 27 |
| Liver disease* | 15 |
| Hepatitis B virus | 7 |
| Steatosis | 8 |
| Diabetes | 8 |
| Malignancy | 3 |
| Gastropathy | 2 |
| Complication | |
| Acute kidney injury | 4 |
| Cardiac injury | 1 |
| Drinking history* | 1 |
| Medication history | |
| Antihypertensive drug | 15 |
| Antiviral drug | 7 |
| Antidiabetic medication | 8 |
| Antibiotic drug | 10 |
| Antipyretics and analgesic | 12 |
| Cough syrup | 8 |
| Exposure history in Wuhan | |
| Yes | 45 |
| No | 53 |
| Signs and symptoms | |
| Fever | 83 |
| Cough | 59 |
| Expectoration | 15 |
| Diarrhea | 8 |
| 6 | |
| Chest pain | 3 |
| Fatigue | 1 |
| No symptoms | 0 |
Because liver disease and alcohol use impact liver function results, 16 patients with liver disease and drinking history were excluded, and only 82 cases were statistical.
Table 2.
Laboratory Findings of Patients Infected With COVID‐19 on the First Day of Admission
| Parameter | Mean ± SD |
|---|---|
| International normalized ratio (reference, 0.8‐1.2) | 1.2 (±0.098) |
| increased (39.6%) | |
| Leukocytes (×109/L; 3.5‐9.5) | 4.7 (±2.03) |
| increased (1%) | |
| decreased (27.5%) | |
| Neutrophils (50%‐70%) | 65.0 (±0.105) |
| increased (33.7%) | |
| decreased (7.1%) | |
| Erythrocytes (×1012/L; 3.8‐5.1) | 4.7 (±0.499) |
| decreased (38.8%) | |
| increased (2%) | |
| Platelets (×109/L; 125‐350) | 184.5 (±71.46) |
| increased (4%) | |
| decreased (14.3%) | |
| Hemoglobin (g/L; 120‐160) | 138.4 (±17.43) |
| decreased (11.2%) | |
| increased (1%) | |
| Serum creatinine (μmol/L; 57‐111) | 68.2 (±15.79) |
| decreased (26.5%) | |
| increased (1%) | |
| Lactate dehydrogenase (U/L; 120‐250) | 225.1 (±70.85) |
| decreased (2%) | |
| increased (32.7%) | |
| CRP (mg/L; 0‐5.0) | 22.5 (±24.57) |
| increased (74.5%) | |
| ALT (U/L; 9‐50) | 30.71 (±30.85) |
| increased (13.3%) | |
| AST (U/L; 15‐40) | 32.6 (±20.11) |
| increased (20.4%) | |
| GGT (U/L; 7‐45) | 53.6 (±80.33) |
| increased (32.7%) | |
| ALP (U/L; 50‐135) | 53.8 (±22.63) |
| decreased (43.9%) | |
| increased (2%) | |
| Low‐density lipoprotein cholesterol (mmol/L; 2.86‐4.38) | 1.85 (±0.75) |
| decreased (89.8%) | |
| High‐density lipoprotein cholesterol (mmol/L; 1.0‐2.1) | 1.26 (±0.387) |
| decreased (24.5%) | |
| increased (4%) | |
| Cholesterol (mmol/L; <5.17) | 3.69 (±0.898) |
| increased (5%) | |
| TG (mmol/L; <1.7) | 1.28 (±0.527) |
| increased (21.4%) | |
| Albumin (g/L; 40‐55) | 41.7 (±4.09) |
| decreased (29.6%) | |
| TBIL (μmol/L; 3‐22) | 12.3 (±5.88) |
| increased (5.1%) |
Characteristics of Liver Function Tests
Elevated serum GGT levels were observed in 32 patients (32.7%), and univariate analysis showed a statistically significant association between increased GGT levels and patients older than 40 years of age (P = 0.027) (Tables 2 and 3; Fig. 1). Moreover, there was a significant relationship between high GGT levels and male sex (P = 0.0145). Among the patients, 22 (22.5%) had increased CRP levels, 13 (13.3%) had elevated serum alanine aminotransferase (ALT) levels, and 20 (20.4%) had high aspartate aminotransferase (AST) levels. Interestingly, there was a significant association between elevated GGT and elevated CRP levels. There was also a positive relationship between elevated ALT and elevated GGT (P < 0.0001). Similarly, there was a positive association between elevated GGT and elevated AST (P = 0.008). Although only 2 patients (2%) had increased alkaline phosphatase (ALP) levels, a significant positive correlation between elevated GGT and ALP levels (P = 0.0003) was identified. Likewise, there was a significant association between elevated total bilirubin (TBIL) levels (5.1% of patients) and elevated GGT (P = 0.0423). In addition, 21 patients (21.4%) had increased triglyceride (TG) levels, and there was a significant positive relationship between elevated GGT levels and high TG levels (P = 0.0002). Finally, a significant positive association was found between elevated GGT and length of hospital stay (Fig. 1J). Patients with elevated GGT levels experienced a significantly longer hospitalization compared with those with normal GGT levels (P = 0.0086), and a similar result was found for those with elevated CRP levels (P = 0.0162).
Table 3.
Univariate Analysis of Clinical Factors Associated With GGT Level
| Parameter | Normal (n = 56) | Elevated ( n = 26) | P < 0.05* |
|---|---|---|---|
| Age (years) | |||
| ≤40/>40 | 26/30 | 5/21 | 0.027 |
| Sex (female/male) | 29/27 | 6/20 | 0.0145 |
| CRP (mean × ULN) | 3.7 | 6.196 | 0.0366 |
| ALT (mean × ULN) | 0.404 | 1.028 | <0.0001 |
| AST (mean × ULN) | 0.671 | 1.014 | 0.0008 |
| ALP (mean × ULN) | 0.354 | 0.501 | 0.0003 |
| TG (mean × ULN) | 0.676 | 0.946 | 0.0002 |
| TBIL (mean × ULN) | 0.514 | 0.634 | 0.0423 |
Indicates significant difference.
FIG. 1.
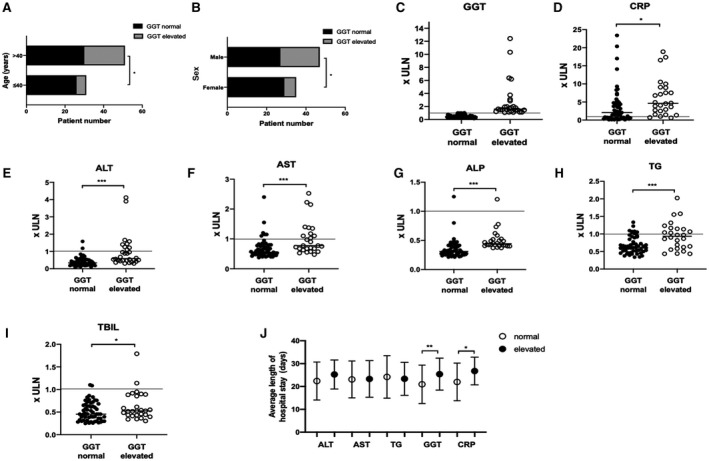
Univariate analysis of clinical and biochemical factors associated with GGT levels. Relationship between (A) age and (B) sex and elevated GGT levels. Relationship between elevated GGT levels and other liver enzymes and injury markers: (C) GGT, (D) CRP, (E) ALT, (F) AST, (G) ALP, (H) TG, (I) TBIL. (J) Relationship between elevated GGT levels and length of hospital stay. (C‐I) Data show individual readings. (J) Data show mean ± SD. * P < 0.05, ** P < 0.01, *** P < 0.001. Abbreviation: ULN, upper limit of normal.
Discussion
Cases of COVID‐19 first emerged in December 2019 when a mysterious illness was reported in Wuhan, China. By January 29, 2020, there were 172 confirmed cases of COVID‐19 in Wenzhou, the highest figure for any city outside Hubei Province.( 15 ) A diagnosis of COVID‐19 is generally associated with SARS, including dry cough, fever, and breathing difficulties. However, results from this study suggest that hepatobiliary complications are prevalent in patients with COVID‐19 as well, supporting the hypothesis that high levels of ACE2 expression in cholangiocytes may render them more susceptible to SARS‐CoV‐2 infection.
Studies have noted that increased ALT levels are observed in 24%‐39% of patients with COVID‐19, and 31%‐34% of patients have elevated levels of AST.( 1 , 5 , 16 ) Our results (elevated level of ALT in 13.3%; elevated level of AST in 20.4%) suggest a lower frequency of aminotransferase elevations. However, in this study, elevated GGT levels were observed in 32 patients (32.7%) with COVID‐19, ranging from 46 to 559 U/L. Moreover, in the 98 patients evaluated, the average GGT level (53.8 U/L) was higher than the normal range (0‐45 U/L), indicating that GGT alterations frequently accompany SARS‐CoV‐2 infection. To elucidate the implications of these changes, we subsequently analyzed the relationship between serum GGT levels and several variables related to patient characteristics and liver function. A significant association between high GGT levels and age older than 40 years was demonstrated by univariate analysis (Fig. 1A). Despite the fact that age affects the activity of serum GGT,( 17 ) this result in our study seems to be related to SARS‐CoV‐2 infection as previous studies suggest that older age may be a risk factor for poor outcome of COVID‐19.( 18 ) There was also a significant positive correlation between high GGT level and male sex (Fig. 1B); indeed, select studies have shown that the progression of COVID‐19 is worse in men.( 18 ) Another finding suggesting a relationship between liver injury (high GGT level) and COVID‐19 progression is that of a significant association between elevated GGT levels and elevated CRP levels (Fig. 1D); high levels of CRP are an important predictive marker for COVID‐19 outcome.( 19 ) Moreover, elevated CRP levels indicated longer hospitalization (Fig. 1J). Similarly, patients with increased GGT levels had a longer hospital stay compared with those who had normal GGT levels. These results suggest that high GGT levels at entry are associated with longer hospitalizations.
Although there is a close association between GGT and ALP, we observed only one case of elevated ALP levels in the GGT‐elevated group (Fig. 1G). Cholestasis in COVID‐19 patients is rare (GGT and ALP levels simultaneously increase in cases of chronic cholestatic liver disease( 20 )). Given these findings, the isolated increase of GGT observed in most patients may indicate that SARS‐CoV‐2 directly induces cholangiocyte damage, and patients with elevated GGT may be susceptible to the development of more severe liver injury given the trend toward increased ALP compared to patients with normal GGT. In our study, there was a significant association between elevated GGT levels and increased ALT levels (P < 0.0001), and this relationship was also observed between GGT levels and AST (P = 0.0008) (Fig. 1E,F). These findings suggest specificity of GGT for hepatocyte injury. Just as with ALP, although nearly all TBIL levels were within normal range, our results suggest that there is a significant relationship between increased GGT level and TBIL level (P = 0.0423).
Our study had some limitations. It is a single‐center retrospective study, and therefore epidemiologic data may not be representative of other countries and races. Although 46% of individuals included in this study resided in or previously traveled to Wuhan, 54% did not. Thus, the inclusion of patients with community‐acquired SARS‐CoV‐2 will reflect similar phenomena that will drive the spread of the disease in other countries. Moreover, only 4 patients had acute kidney injury and 1 patient had myocardial damage, both of which are also related to elevated GGT levels.
In summary, we observed that a substantial number of patients with COVID‐19 had increased GGT levels. This finding suggests that enhanced ACE2 expression in cholangiocytes may increase vulnerability to SARS‐CoV‐2 infection. Furthermore, the positive relationships identified between elevated GGT levels and other markers of liver injury lead us to conclude that GGT activity may serve as a useful biomarker in COVID‐19‐related liver injury, either in isolation or in conjunction with other liver enzymes and histologic findings. Moreover, elevated levels of GGT can be used as a biomarker that, combined with other detection indexes of COVID‐19, could yield a much clearer picture of COVID‐19 screening at an early stage of disease and serve to stratify those destined for more prolonged hospitalization. Additional research is ongoing to further unveil the mechanism of association between GGT activity and COVID‐19 vulnerability.
Acknowledgment
We thank all physicians and nurses on the front line of health care provision, particularly in the COVID‐19 outbreak. They deserve everyone’s gratitude and admiration.
Supported by the Science and Technology Key Project of Wenzhou Government (grant ZY202004 to D.C.) and the Project of Wenzhou Science and Technology Bureau (grant Y20180290 to Y.T.).
Potential conflict of interest: Dr. Chung received grants from Gilead, AbbVie, Merck, BMS, Boehringer, Janssen, and Roche. The other authors have nothing to report.
Contributor Information
Raymond T. Chung, Email: rtchung@partners.org.
Dong Chen, Email: Chendong_wz@126.com.
References
Author names in bold designate shared co‐first authorship.
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, et al. A new coronavirus associated with human respiratory disease in China. Nature 2020;579:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet 2020;395:470‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al.; China Medical Treatment Expert Group for Covid‐19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420‐422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chau TN, Lee KC, Yao H, Tsang TY, Chow TC, Yeung YC, et al. SARS‐associated viral hepatitis caused by a novel coronavirus: report of three cases. Hepatology 2004;39:302‐310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee JY, Kim YJ, Chung EH, Kim DW, Jeong I, Kim Y, et al. The clinical and virological features of the first imported case causing MERS‐CoV outbreak in South Korea, 2015. BMC Infect Dis 2017;17:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffmann M, Kleine‐Weber H, Krüger N, Müller M, Drosten C, Pöhlmann S. The novel coronavirus 2019 (2019‐nCoV) uses the SARS‐coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv 2020. 10.1101/2020.01.31.929042. [DOI] [Google Scholar]
- 11. Chai X, Hu L, Zhang Y, Han W, Lu Z, Ke A, et al. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019‐nCoV infection. bioRxiv 2020. 10.1101/2020.02.03.931766. [DOI] [Google Scholar]
- 12. Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci 2001;38:263‐355. [DOI] [PubMed] [Google Scholar]
- 13. Hanigan MH, Frierson HF Jr. Immunohistochemical detection of gamma‐glutamyl transpeptidase in normal human tissue. J Histochem Cytochem 1996;44:1101‐1108. [DOI] [PubMed] [Google Scholar]
- 14. Zhang C, Shi L, Wang FS. Liver injury in COVID‐19: management and challenges. Lancet Gastroenterol Hepatol 2020;5:428‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu L, Yuan J, Zhang Y, Zhang G, Lu F, Su J, et al. Highland of COVID‐19 outside Hubei: epidemic characteristics, control and projections of Wenzhou, China. medRxiv 2020. 10.1101/2020.02.25.20024398. [DOI] [Google Scholar]
- 16. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daeppen JB, Smith TL, Schuckit MA. Influence of age and body mass index on gamma‐glutamyltransferase activity: a 15‐year follow‐up evaluation in a community sample. Alcohol Clin Exp Res 1998;22:941‐944. [PubMed] [Google Scholar]
- 18. Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020. 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang J, Zhou L, Yang Y, Peng W, Wang W, Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med 2020;8:e11‐e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology 2000;31:1005‐1013. [DOI] [PubMed] [Google Scholar]


