Abstract
The rediscovery of the medical uses of silver provides another noticeable example, this time at the interface of chemistry and medicine, of the real (and nonlinear) progress of scientific research. Several new silver‐based antimicrobial products have thus been commercialized in the last two decades. Next‐generation antibacterials and antivirals of broad scope, low toxicity and affordable cost, we argue in this study, will be based on microencapsulated Ag nanoparticles.
Keywords: antibacterial agents, antiviral agents, microencapsulation, nanoparticles, silver, sol-gel
Silver lining: The rediscovery of the medical uses of silver provides a noticeable example at the interface of chemistry and medicine of the nonlinear progress of scientific research. Next‐generation antibacterials and antivirals of broad scope, low toxicity and affordable cost could soon use silver in the form of AgNPs microencapsulated in widely different coating materials.

1. Introduction
Noting that silver nanoparticles (AgNPs) had proven to be active against several types of viruses including human immunodeficiency virus, hepatitis B virus, herpes simplex virus, and respiratory syncytial virus, scholars in Italy reviewing the potential use of AgNPs as antivirals in 2011 concluded that their therapeutic or prophylactic use required better understanding of their in vivo toxicity and potential for long‐term sequelae associated with the exposure to these metal species.1
A similar conclusion was reached almost a decade later concerning novel antimicrobials based on nanoparticulate silver by India's scholars, calling for a more complete understanding of toxic potential of AgNPs to open the route to new antimicrobials based on AgNPs “with desired properties to ensure their safe use, exposure over extended period and fate in human body and environment”.2
For over 6000 years and until the introduction of penicillin in the early 1940s, silver was the main antimicrobial used by mankind.3 Since the early 2000s, facing the increasing resistance of microbial strains to antibiotics, the use of colloidal silver as antimicrobial has been rediscovered. In both antiviral and antibacterial applications, indeed, silver ions (Ag+) and metal nanoparticles attack bacteria4 and viruses1 in multiple ways thereby limiting the ability of both viruses and bacteria to develop resistance.
Covering topics from their use in antimicrobial wound‐healing dressings5 to the current status of AgNP technology for antimicrobial treatment and associated infectious diseases,6 several excellent accounts have recently appeared in the literature.
After a brief discussion of antimicrobial AgNPs, in the following we discuss how and why microencapsulated AgNPs are likely to shortly emerge as next‐generation antibacterials and antivirals with broad scope, low toxicity and affordable cost. We identify the remaining hurdles to be overcome prior to their widespread uptake for the treatment and the prevention of illness related to bacterial or viral contamination. The conclusions highlight how the important lessons learned from the rediscovery of silver as an antimicrobial can be incorporated into new chemistry education aimed to foster student creativity.
2. Antimicrobial Silver Nanoparticles
The broad scope biological activity of AgNPs, effective against over 650 microorganisms including bacteria (both Gram‐positive and ‐negative), fungi, and viruses,7 is primarily due to leaching of Ag+ ions from the nanoparticle outer surface.
In 2012, indeed, Alvarez and co‐workers demonstrated that under anaerobic conditions (i. e., in the absence of oxygen) that preclude Ag0 oxidation and Ag+ leaching [favored in acid environments, Equations (1) and (2)], AgNPs have no detectable effects on Escherichia coli up to concentration 7665 times higher than the minimum lethal concentration of Ag+ ions (0.025 mg/L) under the same exposure conditions and thousands of times higher than the AgNPs minimum lethal concentration observed under aerobic conditions:8
| (1) |
| (2) |
This discovery led the team to conclude that the antibacterial activity could be controlled by modulating the Ag+ release kinetics by tailoring the AgNP size, shape, and surface characteristic including the presence of a coating.8
Subsequent studies will show that both Ag+ and AgNPs enter the bacterial cell membrane by porin proteins causing cell membrane rupture, pore formation and cytoplasmic leakage.[4] Within the cell membrane, the Ag+ ions drive the formation of several highly oxidizing species such as hydroxyl and superoxide free radicals, and H2O2 which quickly oxidize DNA, RNA and denature proteins.[4]
In the case of viruses, on the other hand, the antiviral activity of AgNPs can be due to direct virucidal action of the nanoparticles. For example, in the case of human immunodeficiency virus (HIV‐1), the Ag nanoparticles bind to the sulfur groups of gp120 protein spikes over the viral membrane, thereby preventing infectivity due to fusion of the viral and host cell membranes.[9]
Similarly, the attachment and entry of herpes simplex virus type 1 (HSV‐1) into cells involving interaction between viral envelope glycoproteins and cell surface heparan sulfate (HS) for viral entry can be prevented by AgNPs capped with mercaptoethane sulfonate targeting the virus and competing for its binding to cellular HS through their sulfonate end groups.[10]
Even in the case of bacteria, the discovery that the antibacterial activity is chiefly due to Ag+ nonetheless led Alvarez and co‐workers to recommend the use of AgNPs in antimicrobial formulations because NPs are less prone than Ag+ to binding by naturally occurring ligands11 and thus they might better deliver Ag+ to the bacteria cytoplasm via the acidic cell membrane.
From the nanochemistry viewpoint adopted in this study, it should be noted that the polydispersed spherical capped AgNPs with an average size of 4 nm highly effective in inhibiting infectivity of the herpes virus of HSV‐1 were sonochemically synthesized in order to be fully covered with a mercaptoethane sulfonate monolayer.10
Similarly, the strong disinfectant activity of AgNPs against both Salmonella and highly contagious African swine fever virus (AFSV, an enveloped DNA virus with particles in the 170–190 nm range with more than 50 proteins, including glycoprotein) respectively observed at the nanoparticle concentration of 25 and 0.78 ppm and at the bacterial concentration of 108 colony‐forming units (CFU) mL−1 and viral titer of 103 median hemadsorption units (HAd50) required the use of 14 nm spherical AgNPs synthesized in the presence of chitosan as a stabilizer by dropwise addition of NaBH4 to an AgNO3 solution under fast stirring (500 rpm).12 At the concentration of 0.78 ppm the aforementioned AgNPs did not show cytotoxicity to the primary porcine alveolar macrophages (PAM) cells making the treatment suitable for real trials in pig farms.
Again, the scholars ascribed the antiviral activity of AgNPs to interaction of the NP surface with viral capsid gp120 glycoprotein, preventing the interaction between virus and host cell required for cellular entry.
Then, in 2015 Avnir reported a major discovery concerning the antibacterial activity of silver in solution that, surprisingly, had never occurred to microbiologists, namely the silver ability to be a catalytic antibacterial agent (all others, except enzymes, are reagents, not catalysts), that is, to kill bacteria, generation after generation with the same silver being not consumed during the bacteria deactivation cycle.13
Aptly called the “zombie effect”, and demonstrated by first killing Pseudomonas aeruginosa with silver nitrate and then using the dead bacteria to kill a culture of the same living bacterium, the efficient antibacterial activity of the dead bacteria is due to their action as a reservoir of Ag+ ions, which upon contact with living bacteria are transferred to the silver‐free living microorganisms. Therein, the bacteria accumulate silver as small AgNPs (5–10 nm in size) evenly distributed throughout the bacterium (Figure 1) with Ag0 nanoparticles being formed through the reduction of Ag+ cations by the reductive enzymes and reducing sugar molecules of the living cell acting as reducing agents for the incorporated metal cations.
Figure 1.
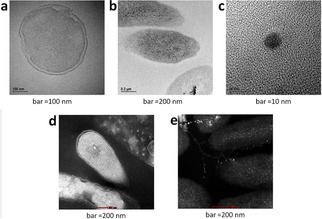
TEM (bright background) and STEM (dark background) of P. aeruginosa before (a and d) and after (b, c and e) treatment with silver; the black (b) and white (e) granules represent silver deposition which account for the zombies biocidal action [Reproduced from ref. [13], under Creative Commons 4.0 Licence].
More recently, these findings were applied and extended using silver‐killed E. coli and its supernatant against fresh viable cells of several other bacterial, including multidrug‐resistant P. aeruginosa and methicillin‐resistant Staphylococcus aureus (MRSA) with both silver‐killed bacteria and supernatant showing prolonged antimicrobial activity against the tested strains that extended to 40 days, leading the team to promote the use of silver‐killed bacteria as both disinfectant for polluted waters and as antibacterial to be included in wound dressings.14
Even more, this discovery and the mechanism behind it (with sulfhydryl groups in various proteins, DNA and RNA acting as the adsorbent and Ag+ as the adsorbate)14 further support the development of next‐generation silver‐based antimicrobials and antivirals based on microencapsulated Ag nanoparticles.
3. Microencapsulated Silver Nanoparticles
Widely used in the pharmaceutical, food, cosmetic and textile industries for protection, compatibility of the encapsulated substance, and controlled release, microencapsulation is the process of enclosing micron‐sized particles of solid, liquid or gas substances in an inert shell to isolate and protect them from the external environment.15
The microencapsulated substance can either be located at the core of the microencapsulant matrix or in its outer surface, while the microencapsulant can be an organic, inorganic or hybrid organic‐inorganic material. For example, presenting their commercial polymeric microencapsules functionalized with AgNPs in the outer wall of the microcapsules (SilverMaster) with both antibacterial and deodorizing effects, a company based in South Korea emphasizes how “the existing silver nanoparticles on sale have very low concentration and have difficulty in keeping colloid state stable”.16
Prepared in a five‐step process involving use of an outer‐shell‐forming substance selected from a melanin precondensate, gelatin, urethane and epoxy to the resulting emulsion to form a microcapsule followed by dissolving water‐soluble styrene‐maleic anhydride polymer in water,17, 18 the latter antimicrobial microcapsules are functionalized with AgNPs on the outer shell of the microcapsule.
Most studies devoted to the microencapsulation of AgNPs deal with biocompatible polysaccharides and proteins in sight of medical (wound‐healing) applications. For example, chitosan microparticles functionalized with 60 nm AgNPs show excellent antibacterial activity against Gram‐negative E. coli and Gram positive Micrococcus luteus and Staphylococcus epidermidis.19
The bioadhesive character of the chitosan carrier particles leading to formation of stable aggregates with the bacteria enhances the antibacterial efficacy of the AgNPs and Ag+ ions released through a contact mechanism.19 The mechanism, in other words, is similar to that subsequently identified by Avnir discovering the catalytic effect of by Ag+ ions contained in dead bacteria killed by Ag+ ions.13
Research rapidly progressed and in a review published in 2015 scholars could already list over 35 different commercially available wound‐care products containing silver formulated in cloth, film, foam, gauze, hydrocolloid, hydrofiber, hydrogel, powder and wash formulations.20
Being nontoxic, biocompatible, versatile chemical and easily moisturized, both proteins and polysaccharides are intrinsically well suited to embed AgNPs in hydrophilic functional materials used to manufacture wound dressings in which nanoscale silver is the active pharmaceutical ingredient. Indeed, chitin, chitosan, starch, pullulan, alginate, cellulose, hyaluronic acid, collagen, gelatin, silk, keratin and natural rubber latex are extensively used in the fabrication of Ag nanoparticle‐loaded biomaterials for wound‐healing applications.21
The debate concerning the mechanism of action of the antibacterial Ag@polysaccharide coatings is revealing. Finding a a 58 ppm concentration of Ag+ in solution due to release of the ions from AgNPs microencapsulated in a chitosan‐alginate hydrogel at 2.6 % load, scholars in 2009 ascribed the antimicrobial activity of the material to contact of the microencapsulated AgNPs with the bacterial membrane noting that silver nanoparticles “can not be uptaken and internalized by eukaryotic cells”.22
On the other hand, strong disinfectant activity of free AgNPs against both Salmonella and highly contagious African swine fever virus was lately reported by spraying a pig house with a AgNP solution at nanoparticle concentration of 25 ppm.12
In other words, the former Ag@polysaccharide hydrogel coating22 and other microencapsulants embedding AgNPs most likely act as reservoirs of extremely bioactive Ag+ ions readily released in solution upon contact with water and O2 dissolved in the aqueous phase.
Similarly to what happens in catalysis for synthetic organic chemistry with solid catalysts showing modest leaching of the entrapped metal, far from being an useless replacement for their homogeneous counterparts,23 materials heterogenized with AgNPs are ideally suited for practical application as antimicrobials as they leach low or ultralow amounts of overly reactive Ag+.
Another recent example is the SilverSil xerogel coating recently introduced as broad‐spectrum antibacterial showing outstanding antibacterial activity against S. aureus and E. coli.24 Washed with water in seven consecutive washing cycles, the xerogel leached 3.46 ppm of silver. Regardless of such modest leaching, both the washing solution and the xerogel showed very high antibacterial activity
Accordingly, the high antimicrobial activity of the washing solutions was ascribed to the lethal effect of the Ag+ present in solution in ultralow concentration, in a similar fashion to the 2 ppb Ag residual on textiles after prolonged washing inhibiting >99.9 % of E. coli growth.25
4. Advancing Ag‐Based Antimicrobials and Antivirals
A meta‐analysis of 157 clinical studies published from 2000 to 2015 concluded that the evidence base for silver in wound management was significantly more favorable than commonly perceived in the clinical community, with evidence suggesting that the use of silver in wound management is also cost‐effective and improves the patient quality of life.26
Amid silver‐based antimicrobials, silver NPs are broad‐spectrum bactericidal and virucidal species.27 Furthermore, as shown for instance by the AgNPs capped with mercaptoethane sulfonate targeting the herpes virus,10 they can be tailed to target specific microorganisms by surface functionalization.
Hence, once stabilized via effective microencapsulation and made available at affordable cost, antimicrobials based on silver nanoparticles could become ubiquitous. Research targets both microencapsulated AgNPs and the microencapsulant material.
For instance, to improve the antimicrobial activity of silver NPs synthesized via reduction of Ag+ ions with NaBH4 in water (in the presence of stabilizing sodium citrate), four factors were recently found to crucially affect the antimicrobial activity: the pH, and the concentration of AgNO3, sodium citrate, and NaBH4.28
Optimal antibacterial silver nanoparticles have an average size of 9.94 nm, are spherical and hemispherical in shape, and are obtained in the synthesis at pH 8 starting from [AgNO3] 0.06 M, [Na3C6H5O7] 0.01 M and [NaBH4] 0.01 M. Figure 2 shows the significant improvement in the antibacterial activity when using such optimized NPs in place of other nanoparticles prepared without having carried out the optimization driven by the design of experiments.
Figure 2.
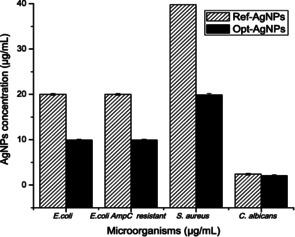
Comparison between the minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC) of the reference AgNPs and the optimized AgNPs. [Reproduced from ref. [28], under Creative Commons 4.0 Licence].
The pH largely affects the particle size distribution, with the polydispersity decreasing by reducing the pH; the concentration of AgNO3 affects the production yield of the NPs; citrate chiefly affects the size by preventing NP agglomeration; while BH4 − has a significant effect on the polydispersity of the NPs ensuring lesser time for the Ag+ to generate clusters of variable size.28
Once optimized, the antimicrobial activity of thoroughly synthesized Ag nanoparticles, the microencapsulation technology will be selected on the basis of the application requirements.
Examples include hydrogel composites embedding AgNPs for wound dressing and antibacterial applications,29 biopolymer hydrogels of sodium alginate and gelatin doped with silver nanoparticles for healing cutaneous lesions,30 and the aforementioned low cost, entrapment in the inner mesoporosity of organically modified silicas24 easily deposited as a thin xerogel layer with excellent adhesion to a range of widely different substrates.31
5. Conclusions and Outlook
The rediscovery of silver as a powerful and broad‐spectrum antimicrobial since the early 2000s has several lessons to teach us. Few people today are aware that by 1940, prior to the introduction of penicillin, in the USA alone more than 50 silver‐based antimicrobial products were marketed in different formulations (solutions, ointments, colloids or foils) for topical, oral, and intramuscular injection.3 In brief, between 1900 and 1940, tens of thousands of patients assumed colloidal silver, with several million doses of silver administered intravenously.3
The rediscovery of silver, this time in the form of silver nanoparticles, against drug resistant bacteria such as P. aeruginosa, ampicillin‐resistant E. coli, erythromycin‐resistant Streptococcus pyogenes, and MRSA has also been driven by the continuous increase in multidrug‐resistant human pathogenic microbes.32
Research advances with new medical uses of silver, including nanocrystalline silver, have been rapid and numerous products are marketed, especially for healing wounds. Adding to several rediscoveries typical of the real process of scientific progress,33 the rediscovery of the medical uses of silver provides another noticeable example, this time at the interface of chemistry and medicine, of the real (and nonlinear) progress of scientific research.
Said progress was not halted when, in 2009, a randomized controlled trial on silver‐based antimicrobial dressings for venous leg ulcers study concluded that there was “no evidence to support the routine use of silver‐donating dressings beneath compression for venous ulceration”,34 leading to withdrawal of several silver‐based antimicrobials, and spreading doubts amid patients and physicians.
Shortly afterwards, however, a group of academic and clinical scholars from universities and hospitals based in Portugal, the UK, Sweden, Spain, the USA, Ireland, Thailand, South Africa and Australia published an expert working‐group consensus for the proper use of silver dressings in wound management based “on experience in clinical practice and available evidence”35 wherein they showed how following said guidelines, wound management based on silver dressings becomes cost‐effective.
Today, new results pointing to the exceptional utility of silver, and of AgNPs in particular, to prevent and control infections caused by pathogenic fungi, bacteria and viruses are reported on regular basis, almost invariably with long‐lasting protection of the functionalized surfaces.
For instance, medical surface silicone elastomers and environmental surface bandage dressings functionalized with AgNPs inhibit the growth of Candida auris biofilms at very low concentrations.36 More than 50 % inhibition is observed with 2.3 to 0.28 ppm on functionalized silicone, and more than 80 % inhibition with 2.3 to 0.017 ppm for dressings. Dressings loaded with 0.036 ppm AgNPs retain protection against C. auris growth during washing cycles 1 to 3 (>80 % inhibition) and from washing cycles 4 to 6 (>50 % inhibition).
It is now clear that the exceptional antimicrobial activity of nanoscale silver is due to the release of Ag+ from the nanoparticle surface driven by the action of O2 [Eq. (1)].8 On the other hand, the antiviral activity of AgNPs in the case of certain viruses such as HIV‐1 is due to the direct interaction of the NPs with the viral spike proteins.9
In any case, the effective microencapsulation of AgNPs protects the valued NPs from rapid loss in solution and ensures prolonged and sustained release of the bactericidal Ag+ ions. The latter ions are reduced by the reducing sugar moieties and reducing enzymes at the cell membrane again forming AgNPs that end up within the dead bacteria.13
In early 2020, for example, a Swiss company commercialized an antiviral and antimicrobial textile treatment effective against human coronavirus, that also led to a dramatic reduction in infectivity against several influenza viruses and respiratory syncytial virus.37 The technology combines vesicle microencapsulation and silver antimicrobial technologies with vesicles easing deactivation of lipophilic viruses by silver‐based active microparticles which also inhibit the replication of viruses and bacteria.
Physically and chemically robust mesoporous silica and organosilica, not swelling in any organic solvent and featuring large surface area and pore specific volume, are ideally suited for said microencapsulation. For example, silica nanoparticles functionalized with AgNPs formed upon reaction of Ag+ with the green tea biophenols adsorbed on the mesoporous silica shows prolonged antimicrobial activity against S. aureus and E. coli.38
The main argument of this study, namely that effective microencapsulation is the key technology enabling the development of new and long‐lasting metal‐based antimicrobials, is also valid for copper NPs. A cheap and powerful metal‐based antimicrobial alternative to nanoparticulate silver is, indeed, nanoscale copper.39
In conclusion, many lessons may be learned from the rediscovery of silver as an antimicrobial. The nanochemistry aspects of AgNPs, their controlled synthesis and tailored microencapsulation to develop low‐cost antifungal, antibacterial and antiviral coatings of general applicability are purposeful topics to develop new lectures and laboratory activities in renewed chemistry education curricula using recent research outcomes.40
Conflict of interest
The authors declare no conflict of interest.
R. Ciriminna, Y. Albo, M. Pagliaro, ChemMedChem 2020, 15, 1619.
Dedicated to Francesco Graziano, gentleman and Sicily's leading food entrepreneur, on the occasion of his 50th birthday
Contributor Information
Dr. Yael Albo, Email: yaelyt@ariel.ac.il.
Dr. Mario Pagliaro, Email: mario.pagliaro@cnr.it.
References
- 1. Galdiero S., Falanga A., Vitiello M., Cantisani M., Marra V., Galdiero M., Molecules 2011, 16, 8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pareek V., Gupta R., Panwar J., Mater. Sci. Eng. C 2018, 90, 739–749. [DOI] [PubMed] [Google Scholar]
- 3. Alexander J. W., Surg. Infect. 2009, 10, 289–292. [DOI] [PubMed] [Google Scholar]
- 4. Gugala N., Lemire J., Chatfield-Reed K., Yan Y., Chua G., Turner R. J., Genes 2018, 9, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.J. Boateng, O. Catanzano in Therapeutic Dressings, Wound Healing Applications (Ed.: J. Boateng), Wiley, 2020, pp.157–184.
- 6. Kailasa S. K., Park T.-J., Rohit J. V., Koduru in J. R., Nanoparticles in Pharmacotherapy, (Ed.: A. Mihai Grumezescu), William Andrew, 2019, pp. 461–484. [Google Scholar]
- 7. Dakal T. C., Kumar A., Majumdar R. S., Yadav V., Front. Microbiol. 2016, 7, 1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiu Z. M., Zhang Q. B., Puppala H. L., Colvin V. L., Alvarez P. J. J., Nano Lett. 2012, 12, 4271–4275. [DOI] [PubMed] [Google Scholar]
- 9. Lara H. H., Ayala-Nuñez N. V., Ixtepan-Turrent L., Rodriguez-Padilla C., J. Nanobiotechnol. 2010, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baram-Pinto D., Shukla S., Perkas N., Gedanken A., Sarid R., Bioconjugate Chem. 2009, 20, 1497–1502. [DOI] [PubMed] [Google Scholar]
- 11. Xiu Z. M., Ma J., Alvarez P. J. J., Environ. Sci. Technol. 2011, 45, 9003–9008. [DOI] [PubMed] [Google Scholar]
- 12. Ngoc Dung T. T., Nam V. N., Nhan T. T., Bich Ngoc T. T., Minh L. Q., To Nga B. T., Le V. P., Quang D. V., Mater. Res. Express 2019, 6, 1250 g9. [Google Scholar]
- 13. Ben-Knaz Wakshlak R., Pedahzur R., Avnir D., Sci. Rep. 2015, 5, 9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohamed D. S., Abd El-Baky R. M., Sandle T., Mandour S. A., Ahmed E. F., Antibiotics 2020, 9, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghosh S. K., Functional Coatings and Microencapsulation: A General in Perspective in Functional Coatings (Ed.: S. K. Ghosh), Wiley-VCH, Weinheim: 2016; pp. 1–28. [Google Scholar]
- 16.InSilico, SilverMaster, 2020, www.insilico.co.kr/chemical/eng/functionality.html#silverMaster (last accessed 10 July 2020).
- 17.S.-Y. Yang, Method of Preparing Functional Microcapsule Incorporating Silver Nanoparticles, KR20010085893, 2001.
- 18.J. Yip, M. Y. A. Luk in Antimicrobial Textiles (Ed.: G. Sun), Woodhead, 2016, pp. 19–46.
- 19. Tokárová V., Kašpar O., Knejzlík Z., Ulbrich P., Štěpánek F., Powder Technol. 2013, 235, 797–805. [Google Scholar]
- 20. Boateng J., Catanzano O., J. Pharm. Sci. 2015, 104, 3653–3680. [DOI] [PubMed] [Google Scholar]
- 21. Kumar S. S. D., Rajendran N. K., Houreld N. N., Abrahamse H., Int. J. Biol. Macromol. 2018, 115, 165–175. [DOI] [PubMed] [Google Scholar]
- 22. Travan A., Pelillo C., Donati I., Marsich E., Benincasa M., Scarpa T., Semeraro S., Turco G., Gennaro R., Paoletti S., Biomacromolecules 2009, 10, 1429–1435. [DOI] [PubMed] [Google Scholar]
- 23. Pandarus V., Ciriminna R., Béland F., Pagliaro M., Appl. Mater. Today 2020, 20, 100661. [Google Scholar]
- 24. Trabelsi K., Ciriminna R., Albo Y., Pagliaro M., ChemistryOpen 2020, 9, 459–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reed R. B., Zaikova T., Barber A., Simonich M., Lankone R., Marco M., Hristovski K., Herckes P., Passantino L., Fairbrother D. H., Tanguay R., Ranville J. F., Hutchison J. E., Westerhoff P. K., Environ. Sci. Technol. 2016, 50, 4018–4026. [DOI] [PubMed] [Google Scholar]
- 26. Dissemond J., Böttrich J. G., Braunwarth H., Hilt J., Wilken P., Münter K.-C., J. Dtsch. Dermatol. Ges. 2017, 15, 524–535. [DOI] [PubMed] [Google Scholar]
- 27. Lara H. H., Garza-Treviño E. N., Ixtepan-Turrent L., Singh D. K., J. Nanobiotechnol. 2011, 9, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quintero-Quiroz C., Acevedo N., Zapata-Giraldo J., Botero L. E., Quintero J., Zárate-Triviño D., Saldarriaga J., Pérez V. Z., Biomater. Res. 2019, 23, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Varaprasad K., Mohan Y. M., Vimala K., Mohana Raju K., J. Appl. Polym. Sci. 2011, 121, 784–796. [Google Scholar]
- 30. Diniz F. R., Maia R. C. A. P., Andrade L. R., Nalone Andrade L., Chaud M. V., Ferreira da Silva C., Bani Corrêa C., R. Luiz C de Albuquerque Jr , Pereira da Costa L., Ryon Shin S., Hassan S., Sanchez-Lopez E., Barbosa Souto E., Severino P., Nanomaterials 2020, 10, 390. [Google Scholar]
- 31. Pagliaro M., Ciriminna R., Palmisano G., J. Mater. Chem. 2009, 19, 3116–3126. [Google Scholar]
- 32. Rai M., Deshmukh S., Ingle A., Gade A., J. Appl. Microbiol. 2012, 112, 841–852. [DOI] [PubMed] [Google Scholar]
- 33.J.-M. Lévy-Leblond in Science, Technology Awareness in Europe: New Insights (Ed.: M. Vitale), EUR-OP, 1998, pp. 143–151.
- 34. Michaels J. A., Campbell B., King B., Palfreyman S. J., Shackley P., Stevenson M., Br. J. Surg. 2009, 96, 1147–1156. [DOI] [PubMed] [Google Scholar]
- 35. Appropriate Use of Silver Dressings in Wounds. An Expert Working Group Consensus, International consensus, London, 2012, www.woundsinternational.com/download/resource/6010 (last accessed 10 July 2020).
- 36. Lara H. H., Ixtepan-Turrent L., Jose Yacaman M., Lopez-Ribot J., ACS Appl. Mater. Interfaces 2020, 12, 21183–21191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.HeiQ Materials, HeiQ Viroblock NPJ03 Antiviral Textile Technology Tested Effective against Coronavirus, 2020, https://heiq.com/2020/03/16/heiq-viroblock-antiviral-textiletechnology- against-coronavirus (last accessed 10 July 2020).
- 38. Otari S. V., Patel S. K. S., Kalia V. C., Kim I.-W., Lee J.-K., Indian J. Microbiol. 2019, 59, 379–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chatterjee A. K., Chakraborty R., Basu T., Nanotechnology 2014, 25, 135101. [DOI] [PubMed] [Google Scholar]
- 40. Pagliaro M., Isr. J. Chem. 2019, 59, 565–571. [Google Scholar]


