Abstract
Objectives/Hypothesis
To investigate clinical and radiological features of olfactory clefts of patients with mild coronavirus disease 2019 (COVID‐19).
Study Design
Prospective non controlled study.
Methods
Sixteen COVID‐19 patients were recruited. The epidemiological and clinical data were extracted. Nasal complaints were assessed through the 22‐item Sino‐Nasal Outcome Test. Patients underwent psychophysical olfactory testing, olfactory cleft examination, and computed tomography (CT) scans.
Results
Sixteen anosmic patients were included. The mean Sniffin' Sticks score was 4.6 ± 1.7. The majority of patients had no endoscopical abnormality, with a mean olfactory cleft endoscopy score of 0.6 ± 0.9. The olfactory clefts were opacified in three patients on the CT scan. The mean radiological olfactory cleft score was 0.7 ± 0.8. There were no significant correlations between clinical, radiological, and psychophysical olfactory testing.
Conclusions
The olfactory cleft of anosmic COVID‐19 patients is free regarding endoscopic examination and imaging. The anosmia etiology is not related to edema of the olfactory cleft.
Level of Evidence
4 Laryngoscope, 130:2526–2531, 2020
Keywords: COVID‐19, computed tomography scan, imaging, anosmia, olfaction, olfactory, smell, evaluation, taste, coronavirus
INTRODUCTION
Since the onset of the coronavirus disease 2019 (COVID‐19) pandemic in Europe, many otolaryngologists have reported a significant increase in patients with a sudden loss of smell. 1 , 2 The olfactory dysfunction may be associated with respiratory symptoms and fever, but also in isolation or in paucisymptomatic disease. Olfactory dysfunction has been recognized as a key symptom of COVID‐19, with more than 66% of patients in Europe and the United States reporting some degree of hyposmia, 1 , 3 leading to an inclusion of olfactory dysfunction in the diagnostic criteria by the World Health Organization (WHO). The olfactory disorder has been reported to occur before (11.8%), at the same time (22.8%), or after (65.4%) the presentation of other symptoms. 1
Since this recognition of the high prevalence of loss of sense of smell reported by COVID‐19 patients, there has been much speculation regarding the underlying pathophysiological mechanism. Different theories have been proposed ranging from conductive loss due to obstruction of the olfactory cleft 4 to central mechanisms relating to the known neurotropic properties of the human coronavirus. 5
Due to restrictions on travel and overwhelming demands placed on healthcare resources, there have been few reports in the literature of clinical and radiological investigations in these patients. We set out to undertake a detailed evaluation of a series of patients with COVID‐19 anosmia with the aim of further elucidating the underlying cause of the anosmia.
MATERIALS AND METHODS
The Jules Bordet Ethics Committee approved the study protocol (Central Ethics Committee, IJB‐0 M011‐3137). Patients were invited to participate, and informed consent was obtained once inclusion criteria were met.
Setting
Adults with confirmed COVID‐19 and self‐reported sudden‐onset olfactory dysfunction were recruited through a public call from the Department of Human Anatomy of the University of Mons (Mons, Belgium). Only mild‐to‐moderate patients were included. The patients were defined as mild‐to‐moderate if they did not require hospitalization to manage the infection. The COVID‐19 diagnosis was based on the WHO interim guidance and symptoms of disease. The diagnosis was confirmed through nasopharyngeal swab to identify severe acute respiratory coronavirus‐2 (SARS‐CoV‐2) and related reverse transcription polymerase chain reaction (RT‐PCR) analysis. In case of a negative RT‐PCR, serology was performed (Zentech; University of Liege Lab, Liege, Belgium). The details about the diagnosis procedure were reported in a previous publication. 6 Individuals with a history of olfactory dysfunction before the pandemic (e.g., chronic rhinosinusitis, history of nasal surgery, head and neck trauma, or degenerative neurological disease) were carefully excluded.
Epidemiological and Clinical Outcomes
To minimize the risk of exposure for study personnel, the clinical and epidemiological characteristics of patients were electronically collected via an online questionnaire developed with professional SurveyMonkey (San Mateo, CA). Demographic data including gender, age, and patient comorbidities were collected. Symptoms were evaluated through a five‐point scale ranging from 0 (no symptom) to 4 (severe symptoms). The nasal symptoms were evaluated through the French version of the 22‐item Sino‐Nasal Outcome Test (SNOT‐22). 7 All patients were asked to complete the short version of the Questionnaire of Olfactory Disorders–Negative Statements. 8
Psychophysical Olfactory Evaluation
The psychophysical olfactory evaluations were performed using the identification Sniffin' Sticks test (Medisense, Groningen, the Netherlands), which is a validated objective test of olfactory dysfunction. 9 Sixteen scents were presented via a pen device to patients for 3 seconds followed by a forced choice from four given options, with a total possible score of 16 points. Regarding results, patients were classified as anosmic (score of 8 or below), hyposmic (score between 9 and 11), or normosmic (score between 12 and16).
Computed Tomography
Computed tomography (CT) images were independently analyzed by three raters, applying a Lund‐Mackay–style scoring system 10 , 11 : 0 for completely clear, 1 for partially opacified, and 2 for completely opacified. The olfactory cleft radiological evaluations were made through a semiquantitative 0 to 3 Likert scale. 12 , 13 Where there was disagreement between scores, the modal score was used. Each side was scored separately, with a total score ranging from 0 to 4.
Olfactory Cleft Endoscopy
Patients underwent rigid endoscopy with findings scored using the Olfactory Cleft Endoscopy Scale, 12 , 14 which is a validated scale reporting the findings of discharge, polyps, edema, scarring, or crusting on a scale of 0, 1, or 2 on each side, giving a total score ranging from 0 to 40. Endoscopies were performed by authors j.r.l. and s.s. using full personal protective equipment.
Statistical Analyses
Statistical analyses were performed using the Statistical Package for the Social Sciences for Windows (SPSS version 22,0; IBM, Armonk, NY). The relationship between clinical and olfactory outcomes was analyzed through a nonparametric test using Spearman correlation for scale data. We investigated all potential associations between nasal obstruction, rhinorrhea, endoscopy, and radiology scores and the occurrence of olfactory disorder (Sniffin' Sticks test). A level of significance of P < .05 was used.
RESULTS
A total of 16 patients underwent complete evaluation with regard to endoscopy, CT scan, and objective olfactory testing. The mean age was 36 ± 10.1 years. There were eight females (50%) and eight males (Table I). All were nonsmokers. Ten patients (62.5%) did not report nasal obstruction, five (31.3%) reported mild obstruction, and one (6.3%) moderate obstruction (Table II). The mean SNOT‐22 score was 28.8 ± 18.0, with a mean score of 4.4 ± 0.7 for the decreased sense of smell/taste item (Table III). All patients self‐reported complete loss of sense of smell at presentation, which was confirmed through Sniffin' Sticks tests, with a mean Sniffin' Sticks score of 4.6 ± 1.7 (Table IV).
TABLE I.
Patient Characteristics.
| Characteristic | N (%) |
|---|---|
| Age, yr, mean ± SD | 36.0 ± 10.1 |
| Gender, n (%) | |
| Female | 8 (50.0) |
| Male | 8 (50.0) |
| Addictions, n (%) | |
| Nonsmoker | 16 (100) |
| Allergic patients | 1 (6.3) |
| Comorbidities, n (%) | |
| Hypothyroidism | 1 (6.3) |
| Psoriasis | 1 (6.3) |
| Obstructive apnea syndrome | 1 (6.3) |
| Diabetes | 1 (6.3) |
| Hepatic insufficiency | 1 (6.3) |
| General symptoms, n (%) | |
| Asthenia | 7 (44.) |
| Loss of appetite | 5 (31.3) |
| Headache | 5 (31.3) |
| Arthralgia | 4 (25.0) |
| Myalgia | 4 (25.0) |
| Diarrhea | 4 (25.0) |
| Abdominal pain | 3 (18.8) |
| Cough | 3 (18.8) |
| Fever | 2 (12.5) |
| Chest pain | 2 (12.5) |
| Nausea/vomiting | 2 (12.5) |
| Urticaria | 2 (12.5) |
| Sticky sputum | 1 (6.3) |
| Conjunctivitis | 1 (6.3) |
SD = standard deviation.
TABLE II.
Severity of Otolaryngological Symptoms Developed Over the Clinical Course of the Disease.
| Symptoms | No Symptom, n (%) | Mild, n (%) | Moderate, n (%) | Severe, n (%) | Very Severe, n (%) |
|---|---|---|---|---|---|
| Nasal obstruction | 10 (62.5) | 5 (31.3) | 1 (6.3) | 0 (0) | 0 (0) |
| Rhinorrhea | 14 (87.5) | 1 (6.3) | 1 (6.3) | 0 (0) | 0 (0) |
| Postnasal drip | 12 (75.0) | 2 (12.5) | 1 (6.3) | 0 (0) | 0 (0) |
| Throat pain | 10 (62.5) | 2 (12.5) | 1 (6.3) | 1 (6.3) | 0 (0) |
| Facial pain | 16 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Ear pain | 14 (87.5) | 1 (6.3) | 0 (0) | 1 (6.3) | 0 (0) |
| Dysphagia | 16 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dyspnea | 16 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dysphonia | 15 (93.75) | 0 (0) | 0 (0) | 1 (6.3) | 0 (0) |
Symptom severity was assessed with a five‐point scale (from 0 = no problem to 4 = severe problem).
TABLE III.
Sino‐Nasal Complaints of Patients With Olfactory Dysfunction.
| SNOT‐22 | |
| Need to blow nose | 1.6 ± 1.1 |
| Nasal blockage | 1.2 ± 1.0 |
| Sneezing | 1.6 ± 1.2 |
| Runny nose | 1.0 ± 1.3 |
| Cough | 1.0 ± 1.2 |
| Postnasal discharge | 0.2 ± 0.4 |
| Thick nasal discharge | 0.2 ± 0.4 |
| Ear fullness | 0.4 ± 0.6 |
| Dizziness | 0.4 ± 0.6 |
| Ear pain | 0.4 ± 0.7 |
| Facial pain/pressure | 0.6 ± 1.1 |
| Decreased sense of smell/taste | 4.4 ± 0.7 |
| Difficulty falling asleep | 1.2 ± 1.7 |
| Wake up at night | 1.4 ± 1.5 |
| Lack of a good night's sleep | 2.0 ± 1.6 |
| Wake up tired | 1.6 ± 1.6 |
| Fatigue | 1.8 ± 1.5 |
| Reduced productivity | 1.5 ± 1.6 |
| Reduced concentration | 1.4 ± 1.2 |
| Frustrated/restless/irritable | 1.8 ± 1.7 |
| Sad | 1.4 ± 1.7 |
| Embarrassed | 1.3 ± 1.5 |
| SNOT‐22 total score | 28.8 ± 18.0 |
| Short version QOD‐NS items | |
| Changes in my sense of smell isolate me socially. | 1.3 ± 1.0 |
| The problems with my sense of smell have a negative impact on my daily social activities. | 1.1 ± 1.1 |
| The problems with my sense of smell make me more irritable. | 1.5 ± 1.1 |
| Because of the problems with my sense of smell, I eat out less. | 1.1 ± 1.1 |
| Because of the problems with my sense of smell, I eat less than before (loss of appetite). | 1.3 ± 1.0 |
| Because of the problems with my sense of smell, I have to make more effort to relax. | 1.6 ± 1.0 |
| I'm afraid I'll never be able to get used to the problems with my sense of smell. | 0.5 ± 0.7 |
| Short version QOD‐NS total score | 8.1 ± 4.0 |
QOD‐NS = short version of Questionnaire of Olfactory Disorders–Negative Statements; SNOT‐22 = 22‐item Sino‐Nasal Outcome Test.
TABLE IV.
Endoscopy and Psychophysical Test Features of Patients.
| Anosmia Features | |
|---|---|
| Sniffin’ Sticks tests, mean ± SD | 4.6 ± 1.7 |
| Olfactory Cleft Endoscopy Scale, mean ± SD | |
| Discharge | 0.2 ± 0.6 |
| Polyps | 0.0 ± 0.0 |
| Edema | 0.3 ± 0.7 |
| Crusting | 0.1 ± 0.3 |
| Scarring | 0.0 ± 0.0 |
| Total Olfactory Cleft Endoscopy Scale | 0.6 ± 0.9 |
| Lund‐Mackay score, mean ± SD | 0.8 ± 0.9 |
*The Sniffin’ Sticks test is an olfactory performance test using 16 pens.
SD = standard deviation.
The majority of patients had the CT scan, endoscopy, and olfactory testing performed within 48 to 72 hours of each other. The longest interval between tests was 3 days. There was a variable interval from the onset of loss of smell and testing due to patient recruitment (mean = 19.8 ± 12.8 days). At the time of testing, patients had persistent olfactory dysfunction, whereas other symptoms had resolved. The mean Lund‐Mackay score was 0.8 ± 0.9.
The mean radiological olfactory cleft score was 0.7 ± 0.8; 13 patients were rated as having the olfactory clefts completely (seven) or partly (six) clear, whereas three patients were completely opacified on CT imaging (Fig. 1).
Fig 1.
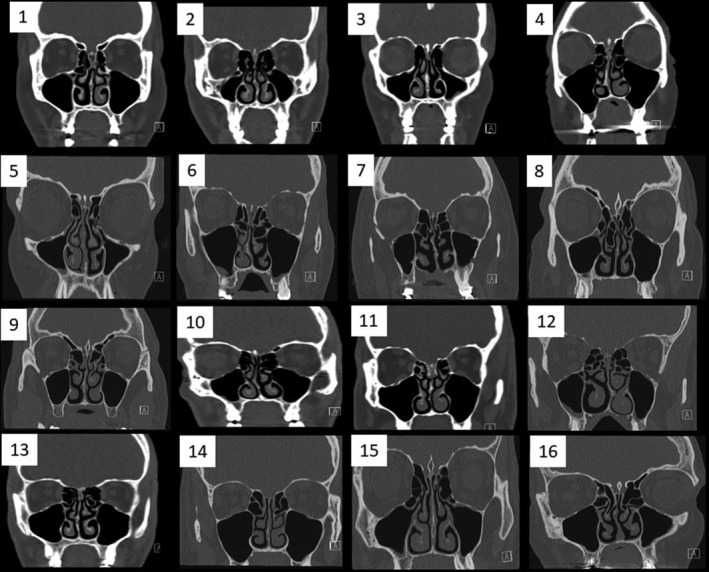
Computed tomography (CT) scan findings of patients. Sinus CT scans of our clinical series. Each number corresponds to a patient.
Endoscopy findings showed that the majority of patients had no visible abnormality, with a mean total olfactory cleft endoscopy score of 0.6 ± 0.9, with edema being the most common finding (mean = 0.3 ± 0.7) (Table IV). There were no significant correlations between endoscopy, CT scan findings, SNOT‐22, nasal obstruction, or Sniffin' Sticks scores. Of all univariate comparisons, only the correlation between CT scan and Sniffin' Sticks scores approached significance (ρ = −0.46, P = .09).
DISCUSSION
To date, there have been very few published cases of COVID‐19 anosmia with radiological investigation. The first presented the magnetic resonance imaging (MRI) of a 27‐year‐old male with sudden onset complete anosmia, but found normal volume and signal intensity of the olfactory bulb with no inflammatory changes in the nasal cavity or olfactory cleft. 15 The report did not specify at what time point the scan was performed, or if the patient has persistent anosmia at the time of imaging. We therefore read with interest the case report detailing CT scan findings in a young female with COVID‐19–related anosmia. 4 The CT scan demonstrated bilateral opacification of the olfactory clefts; this was proposed as the mechanism of underlying anosmia, with the obstructive inflammation preventing the passage of odorant molecules to the olfactory epithelium. The syndrome of inflammatory obstruction of the olfactory clefts has previously been described by the same team. In their study of 34 patients with complete obstruction of the olfactory clefts, patients showed severe deficits in olfactory function, both in terms of detection and identification. 16
There is further support for their hypothesis in the literature. In patients with chronic rhinosinusitis with nasal polyps, there is a significant correlation between olfactory cleft opacification and results of psychophysical measures of olfactory function. 17 Furthermore, a study using self‐reported reduction in sense of smell reported that total, but not partial, opacity of the olfactory cleft correlated with decreased sense of smell. 17 In a previous experimental model of coronavirus‐induced common cold in young adults, impairment in olfactory function was correlated to nasal obstruction. 18 However in this study, although the subjects developed hyposmia, none became anosmic. 18
We have previously evaluated a group of 86 patients presenting with initial onset loss of sense of smell, with confirmed COVID‐19 infection on either polymerase chain reaction or serology. 6 On psychophysical olfactory testing, 52% of patients were anosmic. Although 46% of our cohort reported nasal obstruction to some degree, we found no significant correlation between the severity of nasal obstruction and that of the olfactory loss. 19 However, we note that the patient reported by Eliezer et al., also failed to report nasal obstruction, and therefore sought to confirm whether our cohort may have localized obstruction of the olfactory cleft. 4 Due to fear of contamination and ongoing travel restrictions, we have been able to undertake complete evaluation in only 16 patients. Although all patients were confirmed to be anosmic on testing, seven patients had completely or partly clear olfactory clefts on both sides, six had partial bilateral opacification, and only three patients had bilateral complete opacification of the olfactory cleft. Endoscopy did not demonstrate congestion of the olfactory cleft. We were unable to obtain CT scans in COVID‐19 patients without anosmia. In one study analyzing opacification of the olfactory cleft in chronic rhinosinusitis, a control group undergoing CT imaging for nonrhinological indications was found to have 47% opacification of the cleft. 13 In a group of normosmic adults included as a control, where the olfactory cleft was scored for edema on endoscopy using a similar three‐point scale (0, 1, and 2), the mean score was 0.3 and 0.4 on the left and right, respectively. 14 Although the radiological scores are not directly comparable, it is clear that opacification of the olfactory cleft is a common incidental finding.
Thus, the mechanism proposed by Eliezer et al. 4 cannot account for the anosmia in the majority of our patients, likely supporting a sensorineural etiology. There was a trend toward significance suggesting a weak correlation between olfactory cleft obstruction and olfactory dysfunction, suggesting that, when present, the obstruction may increase the severity of olfactory dysfunction in some patients. How much the observed olfactory opacification contributes to the severity of olfactory dysfunction and how long it persists deserves further investigation, as it may help determine the potential role, if any, for treatments such as topical steroids in the management of olfactory loss. However, Trotier et al. report that such patients are usually unresponsive to topical therapies. 16
Other potential mechanisms for olfactory loss have been proposed. Expression of angiotensin‐converting enzyme 2 (ACE2) has been demonstrated by the sustentacular cells of the olfactory epithelium, although not by the olfactory neurons themselves. 20 Thus, it has been proposed that damage to the supporting epithelium may be responsible for the observed, and often transient, olfactory loss reported by many patients after COVID‐19. 21 It is possible that these patients have a different mechanism of olfactory loss when compared with those with olfactory dysfunction persisting beyond this time. These patients may have a higher prevalence of olfactory cleft edema that resolves within the first 7 to 10 days. There are logistical challenges with scanning patients during the first 7 days due to need for self‐isolation, and therefore we were unable to perform scans in this early phase.
In our small cohort, the majority of patients remain anosmic at their second assessment with psychophysical tests (duration from onset of anosmia of 27–49 days), and we find low rates of olfactory cleft opacification, suggesting the role of a central mechanism. The neurotropic potential for human coronavirus has been previously described and is supported by recent publications. Netland et al. demonstrated on transgenic mice expressing the SARS‐CoV receptor (ACE2) that SARS‐CoV may enter the brain through the olfactory bulb, 5 and from there propagate by direct axonal transmission. 22 Emerging autopsy reports have shown SARS‐CoV‐2 tracking along the olfactory bulb, gyrus rectus, and medulla of a patient with COVID‐19–related anosmia who subsequently died. 23 , 24 More recent imaging reports in the literature show evidence of hyperintense signal and edema of the olfactory bulb, which subsequently resolved, 25 , 26 giving further support to a central mechanism of anosmia in this group.
The limitation of this study is the small number and variable duration from onset of symptoms to CT scan and endoscopy, unavoidable due to the restrictions on both travel and access to imaging and endoscopy. To mitigate against this, Sniffin' Sticks testing was repeated within 10 days of imaging, and on the day of endoscopy. Furthermore, to minimize contact time and reduce risk of contamination, only the identification part of the Sniffin' Sticks assessment was made. However, as identification was shown to be severely impaired in obstructed olfactory cleft disease, 16 we believed this would have sufficient sensitivity. As all of our patients were anosmic, and therefore scored poorly in the identification test, likely needing to guess at answers, it is unlikely that any correlation would be demonstrated between Sniffin’ Sticks and olfactory cleft scores. Future studies should ideally include MRI, although both reported cases have failed to demonstrate any significant findings in the acute phase. Because changes in the olfactory bulb and cortex are related to the duration of postviral olfactory loss, 27 such changes will likely only be detected with delayed MRI.
CONCLUSION
This is the first study to report both endoscopic and radiologic imaging in a series of patients with COVID‐19–related anosmia. Our findings suggest that although obstruction of the olfactory cleft may play a small role in increasing the severity of the olfactory dysfunction, it does not appear to be the primary underlying mechanism.
Editor's Note: This Manuscript was accepted for publication on July 7, 2020.
This work was funded by grants from Fond de la Recherche Médicale dans le Hainaut (FRMH) and University of Mons (UMONS).
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLOGRAPHY
- 1. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol 2020;277:2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hopkins C, Surda P, Kumar N. Presentation of new onset anosmia during the COVID‐19 pandemic. Rhinology 2020;58:295–298. [DOI] [PubMed] [Google Scholar]
- 3. Lechien JR, Chiesa‐Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1,420 European patients with mild‐to‐moderate coronavirus disease 2019 [published online April 30, 2020]. J Intern Med . 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eliezer M, Hautefort C, Hamel AL, et al. Sudden and complete olfactory loss function as a possible symptom of COVID‐19 [published online April 8, 2020]. JAMA Otolaryngol Head Neck Surg doi: 10.1001/jamaoto.2020.0832. [DOI] [PubMed] [Google Scholar]
- 5. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008;82:7264–7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lechien JR, Cabaraux P, Chiesa‐Estomba CM, et al. Objective olfactory evaluation of self‐reported loss of smell in a case series of 86 COVID‐19 patients. Head Neck 2020;42;1583–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Dorlodot C, Horoi M, Lefebvre P, et al. French adaptation and validation of the Sino‐Nasal Outcome Test‐22: a prospective cohort study on quality of life among 422 subjects. Clin Otolaryngol 2015;40:29–35. [DOI] [PubMed] [Google Scholar]
- 8. Mattos JL, Edwards C, Schlosser RJ, et al. A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2019;9:1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hummel T, Kobal G, Gudziol H, Mackay‐Sim A. Normative data for the "Sniffin' Sticks" including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol 2007;264:237–243. [DOI] [PubMed] [Google Scholar]
- 10. Lund VJ, Kennedy DW. Quantification for staging sinusitis. The staging and therapy group. Ann Otol Rhinol Laryngol Suppl 1995;167:17–21. [PubMed] [Google Scholar]
- 11. Vandenhende‐Szymanski C, Hochet B, Chevalier D, Mortuaire G. Olfactory cleft opacity and CT score are predictive factors of smell recovery after surgery in nasal polyposis. Rhinology 2015;53:29–34. [DOI] [PubMed] [Google Scholar]
- 12. Soler ZM, Hyer JM, Karnezis TT, Schlosser RJ. The Olfactory Cleft Endoscopy Scale correlates with olfactory metrics in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2016;6:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loftus C, Schlosser RJ, Smith TL, Alt JA, et al. Olfactory cleft and sinus opacification differentially impact olfaction in chronic rhinosinusitis [published online October 11, 2019]. Laryngoscope . 10.1002/lary.28332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poletti SC, Murta G, Hahner A, Hummel T. Olfactory cleft evaluation: a predictor for olfactory function in smell‐impaired patients? Eur Arch Otorhinolaryngol 2018;275:1129–1137. [DOI] [PubMed] [Google Scholar]
- 15. Galougahi MK, Ghorbani J, Bakhshayeshkaram M, Naeini AS, Haseli S. Olfactory bulb magnetic resonance imaging in SARS‐CoV‐2‐induced anosmia: the first report. Acad Radiol 2020;27:892–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trotier D, Bensimon JL, Herman P, Tran Ba Huy P, Doving KB, Eloit C. Inflammatory obstruction of the olfactory clefts and olfactory loss in humans: a new syndrome? Chem Senses 2007;32:285–292. [DOI] [PubMed] [Google Scholar]
- 17. Hong SC, Leopold DA, Oliverio PJ, et al. Relation between CT scan findings and human sense of smell. Otolaryngol Head Neck Surg 1998;118:183–186. [DOI] [PubMed] [Google Scholar]
- 18. Akerlund A, Bende M, Murphy C. Olfactory threshold and nasal mucosal changes in experimentally induced common cold. Acta Otolaryngol 1995;115:88–92. [DOI] [PubMed] [Google Scholar]
- 19. Lechien JR, Cabaraux P, Chiesa‐Estomba CM, et al. Psychophysical olfactory tests and detection of COVID‐19 in patients with sudden onset olfactory dysfunction: a prospective study [published online May 29, 2020]. Ear Nose Throat J 2020. doi: 10.1177/0145561320929169. [DOI] [PubMed] [Google Scholar]
- 20. Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R. Expression of the SARS‐CoV‐2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci 2020;11:1555–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hopkins C, Surda P, Whitehead E, Kumar BN. Early recovery following new onset anosmia during the COVID‐19 pandemic—an observational cohort study. J Otolaryngol Head Neck Surg 2020;49:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dube M, Le Coupanec A, Wong AHM, Rini JM, Desforges M, Talbot PJ. Axonal transport enables neuron‐to‐neuron propagation of human coronavirus OC43. J Virol 2018;92:e00404–e00418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bulfamante G, Chiumello D, Canevini MP, et al. First ultrastructural autoptic findings of SARS‐Cov‐2 in olfactory pathways and brainstem. Minerva Anestesiol 2020;86:678–679. [DOI] [PubMed] [Google Scholar]
- 24. Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS‐Cov‐2 invasion as port of central nervous system entry in COVID‐19 patients. bioRxiv preprint. Available at: https://www.biorxiv.org/content/10.1101/2020.06.04.135012v1. Published June 4, 2020. [DOI] [PubMed]
- 25. Politi LS, Salsano E, Grimaldi M. Magnetic resonance imaging alteration of the brain in a patient with coronavirus disease 2019 (COVID‐19) and anosmia [published online May 29, 2020]. JAMA Neurol . 10.1001/jamaneurol.2020.2125. [DOI] [PubMed] [Google Scholar]
- 26. Laurendon T, Radulesco T, Mugnier J, et al. Bilateral transient olfactory bulbs edema during COVID‐19‐related anosmia [published online May 22, 2020]. Neurology . 10.1212/WNL.0000000000009850. [DOI] [PubMed] [Google Scholar]
- 27. Yao L, Yi X, Pinto JM, et al. Olfactory cortex and olfactory bulb volume alterations in patients with post‐infectious olfactory loss. Brain Imaging Behav 2018;12:1355–1362. [DOI] [PubMed] [Google Scholar]


