Abstract
The outbreak of coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, has become a major health crisis and a worldwide pandemic. COVID‐19 is characterized by high infectivity, long incubation period, diverse clinical presentations, and strong transmission intensity. COVID‐19 can cause myocardial injury as well as other cardiovascular complications, particularly in senior patients with pre‐existing medical conditions. The current review summarizes the epidemiological characteristics, potential mechanisms, clinical manifestations, and recent progress in the management of COVID‐19 cardiovascular complications.
Keywords: COVID‐19, SARS‐CoV‐2, Heart, Blood vessels, Inflammation
Introduction
In December 2019, a virus‐associated disease, predominately characterized by pneumonia, emerged and quickly spread around the world. The disease outbreak has triggered a major health crisis in many countries throughout the world and is now named coronavirus disease 2019 (COVID‐19) officially by the World Health Organization. 1 The pathogen causing COVID‐19 has been attributed to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), a novel coronavirus closely related to severe acute respiratory syndrome coronavirus (SARS‐CoV). 2 SARS‐CoV‐2 is the third member of coronaviruses family known to cause life‐threatening disease, following SARS‐CoV and Middle East respiratory syndrome coronavirus (MERS‐CoV). 3 The morbidity and mortality vary in patients with COVID‐19, largely depending upon underlying conditions. Elderly patients with cardiovascular disease (CVD) are particularly vulnerable. Globally, as of 7 July 2020, there have been 11 590 195 confirmed cases of COVID‐19, including 537 429 deaths, far exceeding those in previous coronavirus‐related outbreaks. 4
SARS‐CoV‐2 mainly attacks the respiratory system, clinically characterized by the rapidly progressive pneumonia, acute respiratory distress syndrome (ARDS), and multiple organ dysfunction syndrome. 5 While much of the focus has been on pulmonary manifestations, it is essential to be aware of cardiovascular complications, which increase the severity and mortality of COVID‐19. Cardiovascular manifestations are previously reported in the setting of SARS and MERS. Acute myocarditis has been reported in MERS patients without pre‐existing cardiac conditions. 6 About 10% of SARS patients have reversible cardiomegaly without any sign of heart failure. 7 Some SARS patients who died from cardiac arrest exhibited elevated levels of myocardial biomarkers, also indicative of myocardial damage. 8 Compared with SARS and MERS, COVID‐19 exerts more adverse impacts on the cardiovascular system, leading to an elevated incidence of cardiovascular events, most notably myocardial injury (Table 1 ). 5 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 In the current review, we summarize the epidemiological features, underlying mechanisms, and clinical characteristics of COVID‐19‐related myocardial injury. We also review and discuss recent progress in management and therapeutic strategies for COVID‐19 cardiovascular complications.
Table 1.
Cardiovascular complications of COVID‐19
| Incidence | Manifestations in COVID‐19 patients | Ref. | |
|---|---|---|---|
| Myocardial injury | 7.2–40.9% |
Elevation of myocardial biomarker levels (e.g. TnI/T) Non‐specific changes on electrocardiography and echocardiography |
(Table 2 ) 5, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 |
| Acute myocarditis | – a |
Elevation of myocardial biomarker levels (e.g. TnI/T) Diffuse or focal ST‐segment elevation on electrocardiography Myocardial oedema, ventricular hypokinesis and late gadolinium enhancement on echocardiography or magnetic resonance imaging Myocardial inflammation and SARS‐CoV‐2 genome confirmed by autopsy or biopsy |
22, 23, 24, 25, 26 |
| Pulmonary embolism | 27–33% b |
Elevation of D‐dimer levels Acute pulmonary embolism on computed tomography pulmonary angiography Deep vein thrombosis on ultrasonography |
27, 28, 29, 30 |
| Disseminated intravascular coagulation | 22–97% |
Haemorrhagic tendency and microcirculation disturbance Elevation of D‐dimer and fibrin degradation product levels Decrease in platelet counts and fibrinogen levels Prolonged activated partial thromboplastin time and prothrombin time |
29, 31 |
| Stroke | 25% |
Hemiplegia, dysarthria gaze preference and facial weakness Infarct lesion on computed tomography |
16, 29, 32 |
| Acute heart failure | 19.4–52% c |
Elevation of NT‐proBNP levels Pulmonary oedema on chest radiography Enlarged ventricle and reduced left ventricular ejection fraction on echocardiography |
11, 16, 33 |
| Malignant arrhythmia/cardiac arrest | 7–11.1% |
Rapid ventricular tachycardia lasting >30 s or ventricular fibrillation on electrocardiography Haemodynamic instability Syncope Sudden death |
17, 34 |
COVID‐19, coronavirus disease 2019; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TnI/T, troponin I/T.
No incidence data available.
Only patients suspected of having pulmonary embolism underwent computed tomography pulmonary angiography, so the real incidence may be lower.
Incidence in severe or deceased cohorts.
COVID‐19‐associated myocardial injury
The cardiovascular system is highly vulnerable to the tissue injury caused by COVID‐19. The pathogenesis of myocardial injury has been demonstrated by recent autopsy reports from different investigators. 9 , 35 The exact mechanism for the development of COVID‐19 cardiovascular complications has not been fully understood. COVID‐19 may damage the heart directly or indirectly or both (Figure 1 ). The occurrence of myocardial injury is generally diagnosed when the serum levels of troponin I/T (TnI/T) increase above the 99th percentile upper reference limit after excluding TnI/T elevation related to obstructive coronary artery disease, according to the fourth universal definition of myocardial infarction. 35 The incidence of myocardial injury in COVID‐19 ranges from 7.2% to 40.9% in general cohorts. 5 , 9 , 11 , 12 , 13 , 14 , 17 , 20 , 21 , 33 TnI/T elevation appears much more striking in severe patients and non‐survivors. 10 , 18 , 19 Recent reports on the prevalence and description of myocardial injury are summarized in Table 2 . 5 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 In a recent study enrolling 191 patients, myocardial injury occurred in 46% of non‐survivors, compared with only about 1% of discharged patients. 11 Myocardial injury appears to serve as an independent risk factor for the severity and mortality of COVID‐19, with reported hazard ratio ranging from 4.3 to 8.9, 12 , 13 , 16 and odds ratio from 6.6 to 26.9 14 , 15 , 36 in different studies. Furthermore, some of the deceased patients show dynamic elevation of TnT levels during hospitalization, whereas discharged patients or survivors showed no change in TnT levels, suggesting that aggravated myocardial injury is associated with adverse COVID‐19 prognosis. 17 , 18
Figure 1.
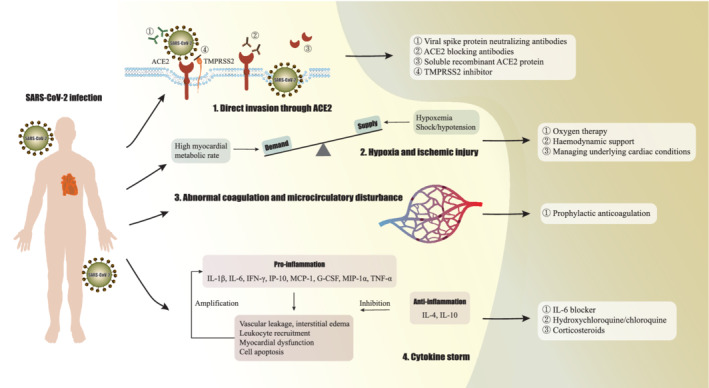
Schematic representation of the mechanisms underlying COVID‐19‐related myocardial injury and potential treatment strategies: (1) direct infection through angiotensin‐converting enzyme 2 (ACE2); (2) myocardial oxygen supply/demand imbalance; (3) abnormal coagulation and microcirculatory disturbance; (4) cytokine storm. G‐CSF, granulocyte colony‐stimulating factor; IFN, interferon; IL, interleukin; IP‐10, interferon‐γ inducible protein 10; MCP‐1, monocyte chemoattractant protein 1; MIP‐1α, macrophage inflammatory protein 1α; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, type II transmembrane serine protease; TNF, tumour necrosis factor.
Table 2.
Clinical studies on COVID‐19 with myocardial injury information
| Cohort | Myocardial injury | Severity/prognosis | Troponin and other evidence | Ref. |
|---|---|---|---|---|
|
Wuhan, China Mortality: 15.0% n = 41 |
5/41 (12.2%) Definitions: blood levels of hs‐TnI above the 99th percentile upper reference limit or new abnormalities on electrocardiography and echocardiography |
30.8% ICU vs. 3.6% non‐ICU patients with myocardial injury |
|
5 |
|
Wuhan, China Mortality: 4.3% n = 138 |
10/138 (7.2%) Definitions: blood levels of TnI above the 99th percentile upper reference limit or new abnormalities on electrocardiography and echocardiography |
22.2% ICU vs. 2.0% non‐ICU patients with myocardial injury |
|
9 |
|
Wuhan, China Mortality: 61.5% n = 53 |
12/52 (23.1%) Definitions: blood levels of hs‐TnI above the 99th percentile upper reference limit |
28.1% non‐survivors vs. 15.0% survivors with myocardial injury |
|
10 |
|
Wuhan, China Mortality: 28.3% n = 191 |
24/145 (16.6%) Definitions: blood levels of hs‐TnI above the 99th percentile upper reference limit or new abnormalities on electrocardiography and echocardiography |
46.0% non‐survivors vs. 11% survivors with myocardial injury |
|
11 |
|
Wuhan, China Mortality: 57/97 (58.8%) a n = 416 |
82/416 (19.7%) Definitions: blood levels of hs‐TnI above the 99th percentile upper reference limit, regardless of electrocardiographic and echocardiographic findings |
Myocardial injury is an independent risk factor for mortality with COVID‐19 (HR 4.26, 95% CI 1.92–9.49) |
|
12 |
|
Wuhan, China Mortality: 12.5% n = 112 |
42/112 (37.5%) b Definitions: blood levels of TnI above the 99th percentile upper reference limit |
|
|
13 |
|
Wuhan, China Mortality: 7.3% n = 150 |
22/150 (14.7%) Definitions: blood levels of TnI above the 99th percentile upper reference limit |
Myocardial injury is an independent risk factor for mortality with COVID‐19 (HR 26.91, 95% CI 4.09–177.23) |
|
14 |
|
Wuhan, China Mortality: 34.1% n = 176 |
49/176 (27.8%) Definitions: blood levels of TnI above the 99th percentile upper reference limit |
Myocardial injury is an independent risk factor for mortality with COVID‐19 (OR 6.93, 95% CI 1.83–26.22) |
|
15 |
|
Wuhan, China Mortality: 9.2% n = 671 |
106/671 (15.8%) Definitions: blood levels of TnI above the 99th percentile upper reference limit |
TnI >0.026 ng/mL (HR 4.56, 95% CI 1.28–16.28) and NT‐proBNP >900 pg/mL (HR 3.12, 95% CI 1.25–7.80) are independent risk factors for mortality with COVID‐19 |
|
16 |
|
Wuhan, China Mortality: 23.0% n = 187 |
52/187 (27.8%) Definitions: blood levels of TnT above the 99th percentile upper reference limit |
Dynamic increase of TnI and NT‐proBNP levels observed during hospitalization in non‐survivors |
|
17 |
|
Wuhan, China Mortality: 10.0% n = 25 |
11/15 (73.3%) Definitions: blood levels of hs‐TnI above the 99th percentile upper reference limit |
77.8% of hs‐TnI levels in the last test increased compared with that in the first test |
|
18 |
|
Wuhan, China Mortality: 10.0% n = 92 |
31/91 (34.1%) c Definitions: blood levels of TnI above the 99th percentile upper reference limit |
Myocardial injury is common in non‐survivors |
|
19 |
|
Multi‐centre in China Mortality: 8.0% n = 476 |
86/384 (22.4%) Definitions: blood levels of TnI/T above the 99th percentile upper reference limit |
36.2%, 24.4% and 19.9% patients with myocardial injury in critical, severe and moderate groups |
|
20 |
|
New York, USA Mortality: 553/2634 (21.0%) a n = 5700 |
801/3533 (22.6%) Definitions: blood levels of TnI/T above the 99th percentile upper reference limit |
– |
|
21 |
CI, confidence interval; COVID‐19, coronavirus disease 2019; HR, hazard ratio; hs‐TnI, high‐sensitivity troponin I; ICU, intensive care unit; IQR, interquartile range; NT‐proBNP, N‐terminal pro B‐type natriuretic peptide; OR, odds ratio; TnI/T, troponin I/T.
Mortality is counted in patients with known outcome (discharged or deceased). Patients who remained hospitalized at the final follow‐up date are not included.
One of 42 COVID‐19 patients with elevated biomarkers was diagnosed as myocardial infarction.
Six of 31 COVID‐19 patients with elevated biomarkers were diagnosed as myocardial infarction.
Electrocardiogram was performed during the periods of cardiac biomarker elevation.
Reference ranges were different in separate centres.
Electrocardiographic and echocardiographic findings for COVID‐19 patients with myocardial injury are generally normal. 13 Specific electrocardiographic and imaging abnormalities are usually diagnosed as acute myocarditis. 22 , 23 , 24 , 25 Inciardi et al. 23 reported a case of COVID‐19 with myocarditis in the absence of respiratory symptoms. Magnetic resonance imaging (MRI) revealed typical changes of myocarditis, such as diffuse myocardial oedema and late gadolinium enhancement. The patient had severe systolic dysfunction, indicated by refractory hypotension and diffuse biventricular hypokinesis (left ventricular ejection fraction of 35%) on MRI. The pathological evidence for acute myocarditis has been obtained from another COVID‐19 patient 22 who also presented with typical manifestations of fulminant myocarditis. Myocardial inflammation was confirmed, and coronavirus particles were detected in the patient's endomyocardial biopsy specimens, although in macrophages and not in myocardial or endothelial cells.
Other adverse cardiovascular events in COVID‐19
In addition to the direct myocardial injury caused by viral infection, other cardiovascular complications may occur in COVID‐19, in particular acute vascular events, which may contribute to the development of myocardial injury and dysfunction.
Thromboembolic complications
In a study of 184 intensive care unit patients with COVID‐19, up to 31% of them presented with thromboembolic complications, including 27% with venous thromboembolism and 3.7% with arterial thrombotic events, even though all patients had received standard doses of thromboprophylaxis. 27 In a New York hospital, reportedly, several young and previously healthy COVID‐19 patients were found to suffer from an abnormally high incidence of stroke. 32 In another study, patients diagnosed as disseminated intravascular coagulation (DIC) accounted for 71.4% of non‐survivors but occurred only in 0.6% of survivors. 31 Activated partial thromboplastin time and prothrombin time were decreased in 16% and 30% of COVID‐19 patients. 37 Moreover, levels of D‐dimer were significantly higher among those who died of COVID‐19 vs. those who survived and a subsequent study identified an abnormal D‐dimer level (>1 µg/L) as a major risk factor for death, 11 also suggesting that coagulation abnormality contributes to mortality. The above findings reveal a high prevalence of thromboembolic events during the development of COVID‐19, and support the necessity of closely monitoring the status of coagulation and thrombosis in hospitalized patients. 38
Acute heart failure
Acute heart failure represents another common cardiovascular complication of COVID‐19, especially in patients undergoing clinical deterioration. 11 , 26 , 33 In the history of deceased patients, plasma N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) levels often showed dynamic elevation during hospitalization, revealing the close relationship between cardiac dysfunction and disease severity. 17 The new‐onset myocardial injury is partly responsible for acute cardiac failure, described by several case reports as discussed above. 22 , 23 Clearly, with COVID‐19 progression, there is a positive correlation between the levels of NT‐proBNP and TnT (R2 = 0.376, P < 0.001). 17 Nearly 50% of COVID‐19 patients developing heart failure had a history of CVD, suggesting that the decompensation of underlying cardiovascular conditions also strongly contributes to the acute cardiac dysfunction. 33
Arrhythmia and cardiac arrest
An increased risk of arrhythmia has recently been reported in patients with SARS‐CoV‐2 infection. 13 Electrophysiologically, COVID‐19 patients are prone to the development of tachycardia. A study of 112 COVID‐19 patients reported that 29.5% of patients presented with tachycardia, and other evidence for myocardial injury. 13 The heart rate of COVID‐19 patients appeared to be correlated with troponin levels, suggesting the link of tachycardia to myocardial injury in COVID‐19. In addition to tachycardia, malignant arrhythmia and following cardiac arrest may occur in COVID‐19 patients, specifically in those with myocardial injury, 17 which might lead to sudden cardiac death, reportedly responsible for 11.1% of deaths. 34 A French population‐based study found that the out‐of‐hospital cardiac arrest incidence in the COVID‐19 pandemic increased two times over that in the same weeks in the non‐pandemic period, 39 and a third of the increase was caused by suspected or confirmed COVID‐19.
Mechanism underlying myocardial injury in COVID‐19
Despite the high incidence of myocardial injury in COVID‐19 patients, the exact mechanisms underlying the pathogenesis of cardiac injury and dysfunction remain largely unclear. Molecular and cellular evidence and clinical data have disclosed multifactorial events and pathways which likely trigger or accelerate the micro‐ and macro‐process of myocardial injury. The viral infection may provoke multiple pathogenic factors, which may directly or indirectly cause the impairment of cardiovascular cells by SARS‐CoV‐2 infection, as illustrated in Figure 1 .
SARS‐CoV‐2 host cell invasion through surface angiotensin‐converting enzyme 2 receptor
Similar to SARS‐CoV, SARS‐CoV‐2 invades host cells through viral spike protein (S protein) binding to the surface angiotensin‐converting enzyme 2 (ACE2) receptor 40 (Figure 2 ). Known as a negative regulator of the renin–angiotensin system (RAS), ACE2 plays a regulatory role in counter‐balancing the bioactivity of ACE. 41 It can also initiate outside‐in signalling as a membrane protein. 42
Figure 2.
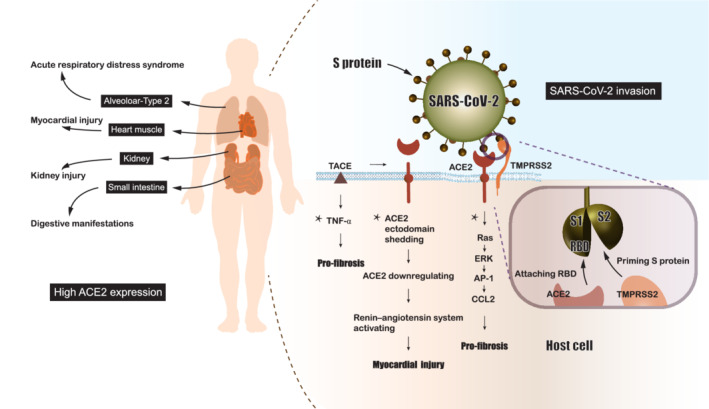
Schematic representation of molecular pathways underlying the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) cellular invasion and injury. SARS‐CoV‐2 invasion is mediated by the S protein binding to its ligand angiotensin‐converting enzyme 2 (ACE2), which is primed by type II transmembrane serine proteases (TMPRSS2) through cleaving S protein into S1 and S2 subunits to facilitate the exposure of receptor‐binding domain (RBD) on S1 subunit. The binding of RBD to ACE2 is followed by receptor‐mediated endocytosis. Activation of the renin–angiotensin system, up‐regulation of the tumour necrosis factor (TNF)‐α pathway and the Ras pathway following ACE2 attachment can injure cells/tissues that highly express ACE2. *These mechanisms are speculated based on SARS‐CoV studies and the similarity of two viruses. AP‐1, activator protein 1; CCL2, C‐C motif chemokine ligand 2; ERK, extracellular signal‐regulated kinase; TACE, tumour necrosis factor‐α‐converting enzyme.
The specific cellular mechanism, by which SARS‐CoV‐2 damages cardiomyocytes, has not been clarified completely. SARS‐CoV‐2 shares a similar biological pathway with SARS‐CoV. Both viruses rely on type II transmembrane serine proteases, another protein expressed on the cellular membrane, to cleave S protein and expose the receptor‐binding domain to bind with ACE2. This S protein–ACE2 binding leads to the endocytosis of virus particles 43 and may be followed by the down‐regulation of ACE2 expression in cardiomyocytes, 44 , 45 and the over‐activation of RAS. The down‐regulation of ACE2 associated with SARS‐CoV infection is partly caused by the shedding of ACE2 ectodomain, mediated by tumour necrosis factor (TNF)‐α‐converting enzyme in coupling with the production of TNF‐α, a well‐known factor for pro‐fibrosis and myocardial damage. 46 The Ras–ERK–AP‐1 pathway may be triggered, as well as the activation of the C‐C motif chemokine ligand 2 (a pro‐fibrosis factor). 47
The above theories are supported by an autopsy report regarding SARS, showing the presence of SARS‐CoV in the heart associated with marked down‐regulation of ACE2 expression. 45 The interstitial fibrosis was observed in the heart tissues of SARS and COVID‐19 patients with myocardial injury, implying the involvement of following pro‐fibrotic effect. 22 , 45 However, although the identification of viral particles and viral genetic materials in the myocardium of COVID‐19 cases has offered pathological evidence of viral myocarditis, 22 , 48 further investigation is needed to determine expression of ACE2 and its interactions with the virus and host cell components, which may help clarify the impacts of changed cellular ACE2 levels on the myocardial injury driven by SARS‐CoV‐2 infection.
Hypoxia and ischaemic injury
Pulmonary inflammation and dysfunction caused by SARS‐CoV‐2 infection limits the oxygen–blood exchange, and triggers hypoxaemia, hypotension, and even septic shock. 49 Consequently, insufficient oxygen supply may occur in vital organs, including the heart. Concomitantly, myocardial oxygen demand may be elevated by heightened temperature and high myocardial metabolic rate that augments the inflammatory burden and imbalance between oxygen supply and consumption. 50 Along with COVID‐19 progression, this imbalance is increasingly aggravated and worsened by the development of metabolic acidosis, fluid or electrolyte disorder, and dysfunction of the neuro‐humoral system. 22 Thus, myocardial injury in COVID‐19 patients may be indirectly triggered or augmented, especially in those with pre‐existing cardiovascular disorders and compromising myocardial reserve capacity, which may have already exhausted on the supply side. 17
Abnormal coagulation and microcirculatory disturbance
In theory, SARS‐CoV‐2 may directly attack vascular endothelial cells, which also express high levels of ACE2, 51 leading to abnormal coagulation and microcirculatory disturbance. Intramural microvascular blood flow may be altered, causing regional ischaemia, followed by focal myocardial injury and cardiac dysfunction. 52 A recent study 53 has shown that COVID‐19 patients with DIC have a high incidence of myocardial injury. However, the detrimental effects of abnormal coagulation and microcirculatory disorders in myocardial injury need to be proved by further pathological evidence. Inflammation of small vessel walls and diffused microcirculatory thrombosis have been identified in liver and lung biopsy specimens from COVID‐19 patients, However, so far, there has been a lack of convincing evidence in the heart. 54 , 55
Cytokine storm
Previous studies have confirmed that immune abnormalities contribute to many pathological changes in SARS‐CoV and MERS‐CoV infection. 7 , 56 Specifically, the cytokine storm represents excessive and uncontrollable cytokine production in response to virus invasion and one of the main contributors to the pathogenic injury to the heart. The levels of serum pro‐inflammatory cytokines [e.g. interleukin (IL)‐1β, IL‐6, interferon‐γ] are markedly increased in COVID‐19 patients and associated with disease progression. 5 Interestingly, in the cytokine storm, Th2 anti‐inflammatory cytokines, such as IL‐4 and IL‐10, are reportedly at high levels, and even related to COVID‐19 severity. 33 Asymptomatic patients exhibited lower levels of both pro‐ and anti‐inflammatory cytokines than the symptomatic group, suggesting the pathogenic role of cytokines. 57
Among the inflammatory cytokines from anti‐viral immune responses, IL‐6 serves as a core component of the cytokine storm, expressing at significantly higher levels in COVID‐19 patients with severe conditions and adverse prognosis, compared with those without. 11 , 26 , 33 IL‐6 not only amplifies the cytokine storm by stimulating the production of other pro‐inflammatory cytokines but also promoting vascular leakage and interstitial oedema. 58 Moreover, IL‐6 weakens papillary muscle contraction and causes myocardial dysfunction. 59 Increased levels of IL‐6 occurred in many hospitalized COVID‐19 patients, significantly associated with elevated high‐sensitivity TnI levels. 60 C‐reactive protein, a popular inflammatory biomarker and indicator of the cardiovascular inflammation heavily regulated by IL‐6, has been reported to be positively correlated with TnT levels in COVID‐19 patients. 17 These findings point to the IL‐6 predominant cytokine storm's potential contributing role in the development of myocardial injury, and warrants further study of IL‐6 expression in cardiomyocytes.
Overall, the pathogenic changes in COVID‐19‐associated myocardial injury are multiple. The direct harmful effect of the viral infection on host cells, the renin–ACE axis disorder mediated by S protein–ACE2 receptor binding, the imbalance between myocardial oxygen supply and demand, dysfunctional microcirculation, and abnormal immune responses may all serve as adverse factors for the pathogenesis of myocardial injury in COVID‐19. Of note, the above speculations are mostly based on clinical observation, and in‐depth research may help further understanding of SARS‐CoV‐2‐induced myocardial injury, and facilitate the development of preventive and/or therapeutic agents.
Clinical profiles of COVID‐19‐associated myocardial injury
The COVID‐19‐associated myocardial injury occurs more frequently in the elderly with pre‐existing cardiovascular comorbidities or risk factors, e.g. diabetes, hypertension, coronary heart disease, and chronic kidney disease, 12 , 17 which are known independent risk factors for heart disease. 16 Given that pre‐existing CVD and in‐hospital myocardial injury are both key determinants of COVID‐19 fatality, 12 , 61 it is not surprising that COVID‐19 patients with the adverse conditions possess the highest mortality (69.4%), compared with patients without myocardial injury but with underlying CVD (13.3%) and patients with myocardial injury but without underlying CVD (37.5%), while the mortality in patients without myocardial injury or underlying CVD is the lowest (7.62%). 17
The general symptoms of COVID‐19 patients are mostly atypical, similar to those observed in SARS, MERS, and even other respiratory infections, such as fever (87.9%), cough (67.7%), fatigue (38.1%), expectoration (33.4%). 49 Confirmed cases with myocardial injury show more specific symptoms, such as chest tightness and pain. 12 , 13 , 62 More than 13% of COVID‐19 patients with myocardial injury have reported chest pain, while less than 1% of non‐myocardial injury patients did the same. 12 Notably, the majority of COVID‐19 patients with myocardial injury do not show any difference in non‐cardiac symptoms from those in ordinary COVID‐19 patients.
The electrocardiographic findings of COVID‐19‐related myocardial injury sometimes resemble those seen in cardiac ischaemia. In a study of 14 patients who underwent an electrocardiogram examination during the period of cardiac biomarker elevation, there were electrocardiographic changes, such as T‐wave depression and inversion, ST‐segment depression, and Q waves, all compatible with myocardial ischaemia. 12 Bangalore et al. 62 reported 10 COVID‐19 patients who were presumed to have acute myocardial infarction at the beginning, according to the ST‐segment elevations on the electrocardiogram (40% diffuse and 60% focal). However, they were later diagnosed as myocardial injury since coronary angiography and echocardiography did not show abnormality.
With regard to the imaging features, echocardiography can morphologically evaluate the structural and functional changes in the myocardium injured by SARS‐CoV‐2 infection. Reduced left ventricular ejection fraction and abnormal wall motion shown on the echocardiogram have been reported in hospitalized COVID‐19 patients with myocardial injury. However, these cardiac image changes may be, to a certain degree, attributable to pre‐existing cardiac disorders. 13 Besides that, the abnormalities on echocardiography were mainly a small amount of pericardial effusion. 13 The sign of myocardial oedema and ventricular hypokinesis found by echocardiography or MRI suggests a critical state of COVID‐19 patients. Computed tomography is also leveraged to explore the occurrence of myocardial injury in COVID‐19 patients. Epicardial adipose tissue density evaluated by the chest computed tomography scan may serve as a valuable parameter of myocardial injury with heightened cytokine production and inflammatory activation. 63
Recent autopsy reports have demonstrated several anatomical features of COVID‐19‐induced myocardial injury. Liu et al. 64 showed that a COVID‐19 patient's cardiac tissue was greyish‐red and infiltrated with inflammatory cells, indicating the myocardial injury associated with SARS‐CoV‐2 infection. The autopsy also found a moderate amount of light‐yellow pericardial effusion and mild epicardial oedema, further suggesting the occurrence of the pericardial inflammatory response in COVID‐19 patients. Another pathologic case found focal myofibrillar lysis and lipid droplets in endomyocardial specimens of COVID‐19 patients. 22 Focal, mainly perivascular interstitial fibrosis, and large (>20 µm), vacuolated, CD68‐positive macrophages with coronavirus particles inside were also found in the myocardium. However, so far, there has been no convincing evidence of cardiac intramural microcirculation dysfunction or thrombosis in COVID‐19. Future researches are required to clarify the histopathologic characteristics of COVID‐19‐related myocardial injury.
Management and therapeutic strategy for COVID‐19 cardiac injury
Strategies for targeting cardiovascular complications
To date, treatment of COVID‐19 has been mostly restricted to supportive care measures as few specific therapeutics have been available to treat this disease. Pre‐existing poor health conditions make patients more vulnerable to infection‐induced cardiovascular complications, thus increasing related mortality risk. 17 Therefore, senior patients who have underlying cardiac conditions are highly vulnerable to COVID‐19 cardiac injury, and they should be prioritized for clinical care.
Regarding diagnostic criteria, the abnormal levels of myocardial biomarkers, especially TnI/T, constitute the main criteria to identify COVID‐19 patients with myocardial injury. However, TnI/T changes may be affected by other determinants, such as the infection status, hypoxia, and renal insufficiency, which are commonly observed with the development of COVID‐19. The ‘rise‐and‐fall’ pattern of TnI/T is also seen in patients with acute coronary syndrome (ACS). There may be a longer waiting period from the first symptom onset to receiving medical care during the COVID‐19 pandemic than in the non‐pandemic period. Hence, a comprehensive assessment of the heart function in COVID‐19 patients should be performed using electrocardiography, imaging, and laboratory testing for proper clinical judgment in patients with abnormal TnI/T levels. However, even after comprehensive examinations, sometimes it remains hard to differentiate ACS from other TnI/T elevating conditions associated with COVID‐19. 62 Therefore, it is essential to promptly perform coronary angiography and continue necessary primary percutaneous coronary intervention (PCI) for patients with suspected ACS. The primary PCI procedures used for ACS patients is favourably suitable to COVID‐19 patients, even though elective coronary procedures in the catheterization laboratory are recommended to be temporarily suspended due to the COVID‐19 pandemic. 65 , 66
Considering the high prevalence of thromboembolic complications in COVID‐19 patients, it is essential to actively take prophylactic anticoagulation during hospitalization for the management of COVID‐19, especially for severe COVID‐19. A recent study involving 449 severe COVID‐19 patients found that treating with unfractionated heparin or low molecular weight heparin for at least 7 days could significantly reduce mortality in patients meeting the criteria for sepsis‐induced DIC, or in patients with markedly elevated D‐dimer. 67 Instead of direct oral anticoagulants, parenteral anticoagulation is recommended, to avoid the possible drug–drug interactions with anti‐viral and anti‐bacterial treatment. 68
Benign arrhythmias, particularly tachycardia, are a common clinical signal secondary to fever. They may reflect the state of sympathetic activation, following the development of hypoxaemia and cardiac output decline. There is no particular need for the treatment of benign arrhythmias. For life‐threatening arrhythmias, prevention is of great importance. Several candidate treatment options (e.g. chloroquine, hydroxychloroquine, azithromycin, and lopinavir/ritonavir) for COVID‐19 are known to pose a risk of malignant arrhythmias. 69 Therefore, for patients with inherited long QT syndrome, unexplained syncope, or family history of sudden cardiac death, these drugs should be undertaken only if necessary. If there is any indication, careful control of dosage and duration is essential. Arrhythmia monitoring or repeated QTc interval checks should be conducted if patients are in clinical deterioration or are taking multiple medications that may cause QTc elongation. In the event of malignant arrhythmias that have led to haemodynamic instability, cardioversion should be immediately performed. Intravenous administration of amiodarone needs to be considered in order to terminate incessant ventricular arrhythmia and reduce recurrence during resuscitation. Implantable cardioverter‐defibrillator therapy may reduce the mortality of COVID‐19 patients with life‐threatening ventricular arrhythmia, particularly if the arrhythmia is drug‐resistant and uncorrectable. For the arrhythmia triggered by transient or reversible factors emerging during the COVID‐19 progression, such as pro‐arrhythmic medication effects, or electrolyte disturbances, identification and correction of potential risk factors should be done in a high priority.
Anti‐viral therapies
Since the COVID‐19 outbreak, several anti‐virus agents have been proposed and are currently under clinical investigation. Among them, the most hopeful one is remdesivir. This broad‐spectrum investigational anti‐viral agent was initially developed for treating Ebola virus infection but failed to show satisfactory efficacy in clinical trials. 70 In the first randomized controlled trial (RCT) regarding COVID‐19, remdesivir showed little clinical benefit compared with placebo for serious COVID‐19 patients. 71 However, this trial was terminated early, so it is underpowered to draw any definite conclusion. The second RCT enrolling 1063 participants showed that remdesivir is superior to control treatment in shortening the time to recovery (11 days vs. 15 days, P < 0.001) and alleviating respiratory tract infection in adults hospitalized with COVID‐19. 72 There is no significant difference in mortality between the groups receiving remdesivir and placebo. 72 Nonetheless, remdesivir has offered new insight into the therapeutic approaches against the current global COVID‐19 crisis.
Anti‐inflammatory and immunoregulatory agents
The pivotal role of immunologic over‐response in COVID‐19 prompts anti‐inflammatory therapy to be studied for treating COVID‐19. Hydroxychloroquine and chloroquine are traditional anti‐malarial drugs that can efficiently control the SARS‐Cov‐2 replication in vitro. 73 , 74 The first study about hydroxychloroquine treatment in COVID‐19 patients is a small open‐label, non‐randomized study, in which hydroxychloroquine administration was significantly associated with viral load reduction/disappearance. 75 However, a double‐masked non‐randomized trial has yielded conflicting results, 76 and increased prolongation of the QT interval was observed in patients who underwent hydroxychloroquine treatment. 76 , 77 Hydroxychloroquine administration was not associated with a lowered risk of intubation or death in an observational study involving 1446 patients. 78 Moreover, it could not prevent SARS‐CoV‐2 infection when used as post‐exposure prophylaxis, reported by a RCT conducted in North America. 79
Corticosteroids have previously been used in the settings of SARS and MERS to control infection‐associated ARDS. However, it has been debated whether corticosteroids exert protective 80 , 81 or adverse 82 effects, since studies of SARS and MERS cases have come to conflicting conclusions. The steroids may increase the incidence of in‐hospital secondary infection and delay virus clearance, as reported in a COVID‐19 study. 83 However, a small dose of steroid treatment can help control fulminant myocarditis and reduce ARDS‐related mortality. 84 , 85 Based on the Recovery trial, one of the biggest studies of corticoids on COVID‐19 to date, dexamethasone could be the first drug shown to reduce the death rates of COVID‐19 patients. 86 Compared with those receiving standard care, a low dose of dexamethasone for 10 days reduced mortality by one‐third in patients on ventilators and by one‐fifth in patients receiving supplemental oxygen in other ways. 86 Therefore, short‐duration administration of low‐dose corticosteroids may practically serve as a therapeutic option for treating COVID‐19.
Targeted anti‐inflammatory therapies, such as IL‐6 blockade, have also been viewed as a potential treatment option, given the pivotal role of cytokine storm in the pathogenesis of COVID‐19 and its cardiovascular complications. The anti‐IL‐6 receptor monoclonal antibody, tocilizumab, has been reported to quickly control fever and improve respiratory function of 21 severe COVID‐19 patients. 87 However, an Italian RCT found that treatment with tocilizumab failed to reduce severe respiratory symptoms, intensive care visits, or death in patients with early‐stage COVID‐19. 88 Thus, there appears to be controversy regarding the efficacy of anti‐IL‐6 therapy in the COVID‐19 cohorts. More data from patients in advanced stage and severe conditions are hopefully coming up from ongoing RCT. 89
Regulators of angiotensin activities
The structural evidence of SARS‐CoV‐2 entering the cell via ACE2 has led to the hypothesis that angiotensin‐converting enzyme inhibitors (ACEI) and angiotensin receptor blockers (ARB) may potentially induce the overexpression of ACE2, and subsequently increase susceptibility to SARS‐CoV‐2 infection and aggravate disease severity. 90 However, ACEI/ARB appears to play a protective rather than a harmful role in COVID‐19, given that the SARS‐CoV‐2 invasion may result in the activation of the RAS axis, which is partly responsible for severe organ injury of COVID‐19. 44 Potential mechanisms in detail have been summarized elsewhere. 91 Growing evidence showed that COVID‐19 patients under ACEI/ARB treatment had similar and even better clinical prognosis than those not. 17 , 20 , 92 , 93 While several clinical trials are looking for compelling evidence proving the usefulness and safety of ACEI/ARB in COVID‐19, 94 , 95 it is not recommendable to alter the routine anti‐hypertensive therapy in COVID‐19 patients.
Conclusion
In the COVID‐19 pandemic, patients with pre‐existing medical conditions are vulnerable to myocardial injury as well as other cardiovascular complications. Individuals with elevated risk factors, such as advanced age, diabetes and obesity, are highly vulnerable to COVID‐19‐associated myocardial injury. Many direct and indirect pathogenic factors induced by the viral infection, such as ACE2‐mediated SARS‐CoV‐2 infection of cardiomyocytes, hypoxia, microcirculatory disturbance, heightened coagulation and thrombogenesis, and cytokine storm, may contribute to the development of myocardial injury in COVID‐19. It is important to closely monitor cardiovascular biomarkers, conduct early diagnosis, and take preventive measures of cardiac injury and dysfunction by COVID‐19. It is also essential to continue medications for controlling pre‐existing medical conditions. To date, the treatment of COVID‐19 is largely restricted to supportive care measures. Cardiovascular considerations for the management of COVID‐19 patients are of great importance and should continuously evolve in future researches.
Funding
This work was supported by grants from the National Natural Science Foundation of China(grant no. 81670337) and from the Clinical and Translational Medicine Research Foundation of the Chinese Academy of Medical Sciences (grant no. 2019XK320061).
Conflict of interest: none declared.
References
- 1. World Health Organization . Coronavirus disease (COVID‐19) outbreak. https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019 (8 February 2020).
- 2. Sun P, Lu X, Xu C, Sun W, Pan B. Understanding of COVID‐19 based on current evidence. J Med Virol 2020;92:548–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell 2020;181:281–292.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Johns Hopkins University and Medicine . COVID‐19 map. Johns Hopkins Coronavirus Resource Centre. https://coronavirus.jhu.edu/map.html (23 January 2020).
- 5. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alhogbani T. Acute myocarditis associated with novel middle east respiratory syndrome coronavirus. Ann Saudi Med 2016;36:78–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yu CM, Wong RS, Wu EB, Kong SL, Wong J, Yip GW, Soo YO, Chiu ML, Chan YS, Hui D, Lee N, Wu A, Leung CB, Sung JJ. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J 2006;82:140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan SF, Zhang HY, Li CS, Wang C. Cardiac arrest in severe acute respiratory syndrome: analysis of 15 cases. Zhonghua Jie He He Hu Xi Za Zhi 2003;26:602–605. [PubMed] [Google Scholar]
- 9. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respir Med 2020;8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult in patients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol 2020;5:802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng Q, Hu B, Zhang Y, Wang H, Zhou X, Hu W, Cheng Y, Yan J, Ping H, Zhou Q. Suspected myocardial injury in patients with COVID‐19: evidence from front‐line clinical observation in Wuhan, China. Int J Cardiol 2020;311:116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen C, Chen C, Yan JT, Zhou N, Zhao JP, Wang DW. Analysis of myocardial injury in patients with COVID‐19 and association between concomitant cardiovascular diseases and severity of COVID‐19. Zhonghua Xin Xue Guan Bing Za Zhi 2020;48:E008. [DOI] [PubMed] [Google Scholar]
- 15. Ni W, Yang X, Liu J, Bao J, Li R, Xu Y, Guo W, Hu Y, Gao Z. Acute myocardial injury at hospital admission is associated with all‐cause mortality in COVID‐19. J Am Coll Cardiol 2020;76:124–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, Huang H, Yang B, Huang C. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J 2020;41:2070–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li X, Wang L, Yan S, Yang F, Xiang L, Zhu J, Shen B, Gong Z. Clinical characteristics of 25 death cases with COVID‐19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis 2020;94:128–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Analysis of 92 deceased patients with COVID‐19. J Med Virol 2020. Apr 15. 10.1002/jmv.25891 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feng Y, Ling Y, Bai T, Xie Y, Huang J, Li J, Xiong W, Yang D, Chen R, Lu F, Lu Y, Liu X, Chen Y, Li X, Li Y, Summah HD, Lin H, Yan J, Zhou M, Lu H, Qu J. COVID‐19 with different severity: a multi‐center study of clinical features. Am J Respir Crit Care Med 2020;201:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer‐Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City area. JAMA 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, Sepe PA, Resasco T, Camporotondo R, Bruno R, Baldanti F, Paolucci S, Pelenghi S, Iotti GA, Mojoli F, Arbustini E. Myocardial localization of coronavirus in COVID‐19 cardiogenic shock. Eur J Heart Fail 2020;22:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warchoł I, Dębska‐Kozłowska A, Karcz‐Socha I, Książczyk M, Szymańska K, Lubiński A. Terra incognita: clinically suspected myocarditis in a patient with severe acure respiratory syndrome coronavirus 2 infection. Pol Arch Intern Med 2020;130:446–448. [DOI] [PubMed] [Google Scholar]
- 25. Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, Godino C, Esposito A. Acute myocarditis presenting as a reverse Tako‐Tsubo syndrome in a patient with SARS‐CoV‐2 respiratory infection. Eur Heart J 2020;41:1861–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med 2020;46:1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klok FA, Kruip M, van der Meer NJ, Arbous MS, Gommers D, Kant KM, Kaptein FH, van Paassen J, Stals MA, Huisman MV, Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res 2020;191:145–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ullah W, Saeed R, Sarwar U, Patel R, Fischman DL. COVID‐19 complicated by acute pulmonary embolism and right‐sided heart failure. JACC Case Rep 2020;2:1379–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, Kucher N, Studt JD, Sacco C, Alexia B, Sandri MT, Barco S; Humanitas COVID‐19 Task Force . Venous and arterial thromboembolic complications in COVID‐19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020;191:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID‐19 pneumonia: a random association? Eur Heart J 2020;41:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, De Leacy RA, Shigematsu T, Ladner TR, Yaeger KA, Skliut M, Weinberger J, Dangayach NS, Bederson JB, Tuhrim S, Fifi JT. Large‐vessel stroke as a presenting feature of Covid‐19 in the young. N Engl J Med 2020;382:e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, Ma K, Xu D, Yu H, Wang H, Wang T, Guo W, Chen J, Ding C, Zhang X, Huang J, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Du Y, Tu L, Zhu P, Mu M, Wang R, Yang P, Wang X, Hu C, Ping R, Hu P, Li T, Cao F, Chang C, Hu Q, Jin Y, Xu G. Clinical features of 85 fatal cases of COVID‐19 from Wuhan. A retrospective observational study. Am J Respir Crit Care Med 2020;201:1372–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018;72:2231–2264. [DOI] [PubMed] [Google Scholar]
- 36. Wei JF, Huang FY, Xiong TY, Liu Q, Chen H, Wang H, Huang H, Luo YC, Zhou X, Liu ZY, Peng Y, Xu YN, Wang B, Yang YY, Liang ZA, Lei XZ, Ge Y, Yang M, Zhang L, Zeng MQ, Yu H, Liu K, Jia YH, Prendergast BD, Li WM, Chen M. Acute myocardial injury is common in patients with Covid‐19 and impairs their prognosis. Heart 2020;106:1154–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia Ja YT, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang Y, Stewart Coats AJ, Zheng Z, Adamo M, Ambrosio G, Anker SD, Butler J, Xu D, Mao J, Khan MS, Bai L, Mebazaa A, Ponikowski P, Tang Q, Ruschitzka F, Seferovic P, Tschöpe C, Zhang S, Gao C, Zhou S, Senni M, Zhang J, Metra M. Management of heart failure patients with COVID‐19: a joint position paper of the Chinese Heart Failure Association & National Heart Failure Committee and the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:941–956. [DOI] [PubMed] [Google Scholar]
- 39. Marijon E, Karam N, Jost D, Perrot D, Frattini B, Derkenne C, Sharifzadehgan A, Waldmann V, Beganton F, Narayanan K, Lafont A, Bougouin W, Jouven X. Out‐of‐hospital cardiac arrest during the COVID‐19 pandemic in Paris, France: a population‐based, observational study. Lancet Public Health 2020;5:e437–e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hoffmann M, Kleine‐Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuba K, Imai Y, Ohto‐Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin‐angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther 2010;128:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kohlstedt K, Brandes RP, Muller‐Esterl W, Busse R, Fleming I. Angiotensin‐converting enzyme is involved in outside‐in signaling in endothelial cells. Circ Res 2004;94:60–67. [DOI] [PubMed] [Google Scholar]
- 43. Li F, Li W, Farzan M, Harrison SC. Structure of SARS coronavirus spike receptor‐binding domain complexed with receptor. Science 2005;309:1864–1868. [DOI] [PubMed] [Google Scholar]
- 44. Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med 2005;11:875–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oudit GY, Kassiri Z, Jiang C, Liu PP, Poutanen SM, Penninger JM, Butany J. SARS‐coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest 2009;39:618–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haga S, Yamamoto N, Nakai‐Murakami C, Osawa Y, Tokunaga K, Sata T, Yamamoto N, Sasazuki T, Ishizaka Y. Modulation of TNF‐alpha‐converting enzyme by the spike protein of SARS‐CoV and ACE2 induces TNF‐alpha production and facilitates viral entry. Proc Natl Acad Sci U S A 2008;105:7809–7814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen IY, Chang SC, Wu HY, Yu TC, Wei WC, Lin S, Chien CL, Chang MF. Upregulation of the chemokine (C‐C motif) ligand 2 via a severe acute respiratory syndrome coronavirus spike‐ACE2 signaling pathway. J Virol 2010;84:7703–7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao S‐Y. Pathological study of the 2019 novel coronavirus disease (COVID‐19) through postmortem core biopsies. Mod Pathol 2020;33:1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for Covid‐19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Heusch G. Myocardial ischemia: lack of coronary blood flow or myocardial oxygen supply/demand imbalance? Circ Res 2016;119:194–196. [DOI] [PubMed] [Google Scholar]
- 51. Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sugiura M, Hiraoka K, Ohkawa S, Ueda K, Matsuda T. A clinicopathological study on cardiac lesions in 64 cases of disseminated intravascular coagulation. Jpn Heart J 1977;18:57–69. [DOI] [PubMed] [Google Scholar]
- 53. Wang YD, Zhang SP, Wei QZ, Zhao MM, Mei H, Zhang ZL, Hu Y. COVID‐19 complicated with DIC: 2 cases report and literatures review. Zhonghua Xue Ye Xue Za Zhi 2020;41:245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Li QR, Huang X, Cui Y, Li XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. A pathological report of three COVID‐19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi 2020;49:E009. [DOI] [PubMed] [Google Scholar]
- 55. Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID‐19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunopathol 2017;39:529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, Hu JL, Xu W, Zhang Y, Lv FJ, Su K, Zhang F, Gong J, Wu B, Liu XM, Li JJ, Qiu JF, Chen J, Huang AL. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med 2020;26:1200–1204. [DOI] [PubMed] [Google Scholar]
- 58. Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL‐6 blockade for cytokine storm. Immunotherapy 2016;8:959–970. [DOI] [PubMed] [Google Scholar]
- 59. Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, McCabe C, Welch SB, Whitney A, O'Gara P, Nadel S, Relman DA, Harding SE, Levin M. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet 2004;363:203–209. [DOI] [PubMed] [Google Scholar]
- 60. Wu C, Hu X, Song J, Du C, Xu J, Yang D, Chen D, Zhong M, Jiang J, Xiong W, Lang K, Zhang Y, Shi G, Xu L, Song Y, Zhou X, Wei M, Zheng J. Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID‐19). medRxiv https://www.medrxiv.org/content/10.1101/2020.02.26.20028589v1 (26 February 2020).
- 61. Guan WJ, Liang WH, Zhao Y, Liang HR, Chen ZS, Li YM, Liu XQ, Chen RC, Tang CL, Wang T, Ou CQ, Li L, Chen PY, Sang L, Wang W, Li JF, Li CC, Ou LM, Cheng B, Xiong S, Ni ZY, Xiang J, Hu Y, Liu L, Shan H, Lei CL, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Cheng LL, Ye F, Li SY, Zheng JP, Zhang NF, Zhong NS, He JX. Comorbidity and its impact on 1590 patients with COVID‐19 in China: a nationwide analysis. Eur Respir J 2020;55:2000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B, Ibrahim H, Friedman GH, Thompson C, Alviar CL, Chadow HL, Fishman GI, Reynolds HR, Keller N, Hochman JS. ST‐segment elevation in patients with Covid‐19 – a case series. N Engl J Med 2020;382:2478–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hui H, Zhang Y, Yang X, Wang X, He B, Li L, Li H, Tian J, Chen Y. Clinical and radiographic features of cardiac injury in patients with 2019 novel coronavirus pneumonia. medRxiv https://www.medrxiv.org/content/10.1101/2020.02.24.20027052v1 (24 February 2020).
- 64. Liu QW, Qu GQ, Wang YY, Liu P, Zhu YZ, Fei G, Reng L, Zhou Y, Liu L. An anatomical report of a coronavirus disease 2019 death case. J Forensic Med 2020;36:19–21. [Google Scholar]
- 65. Szerlip M, Anwaruddin S, Aronow HD, Cohen MG, Daniels MJ, Dehghani P, Drachman DE, Elmariah S, Feldman DN, Garcia S, Giri J, Kaul P, Kapur N, Kumbhani DJ, Meraj PM, Morray B, Nayak KR, Parikh SA, Sakhuja R, Schussler JM, Seto A, Shah B, Swaminathan RV, Zidar DA, Naidu SS. Considerations for cardiac catheterization laboratory procedures during the COVID‐19 pandemic perspectives from the Society for Cardiovascular Angiography and Interventions Emerging Leader Mentorship (SCAI ELM) Members and Graduates. Catheter Cardiovasc Interv 2020;96:586–597. [DOI] [PubMed] [Google Scholar]
- 66. Welt FG, Shah PB, Aronow HD, Bortnick AE, Henry TD, Sherwood MW, Young MN, Davidson LJ, Kadavath S, Mahmud E, Kirtane AJ. Catheterization laboratory considerations during the coronavirus (COVID‐19) pandemic: from the ACC's Interventional Council and SCAI. J Am Coll Cardiol 2020;75:2372–2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost 2020;18:1094–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID‐19. J Thromb Haemost 2020;18:1023–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sapp JL, Alqarawi W, MacIntyre CJ, Tadros R, Steinberg C, Roberts JD, Laksman Z, Healey JS, Krahn AD. Guidance on minimizing risk of drug‐induced ventricular arrhythmia during treatment of COVID‐19: a statement from the Canadian Heart Rhythm Society. Can J Cardiol 2020;36:948–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mulangu S, Dodd LE, Davey RT Jr, Tshiani Mbaya O, Proschan M, Mukadi D, Lusakibanza Manzo M, Nzolo D, Tshomba Oloma A, Ibanda A, Ali R, Coulibaly S, Levine AC, Grais R, Diaz J, Lane HC, Muyembe‐Tamfum JJ, Sivahera B, Camara M, Kojan R, Walker R, Dighero‐Kemp B, Cao H, Mukumbayi P, Mbala‐Kingebeni P, Ahuka S, Albert S, Bonnett T, Crozier I, Duvenhage M, Proffitt C, Teitelbaum M, Moench T, Aboulhab J, Barrett K, Cahill K, Cone K, Eckes R, Hensley L, Herpin B, Higgs E, Ledgerwood J, Pierson J, Smolskis M, Sow Y, Tierney J, Sivapalasingam S, Holman W, Gettinger N, Vallée D, Nordwall J. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 2019;381:2293–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, Fu S, Gao L, Cheng Z, Lu Q, Hu Y, Luo G, Wang K, Lu Y, Li H, Wang S, Ruan S, Yang C, Mei C, Wang Y, Ding D, Wu F, Tang X, Ye X, Ye Y, Liu B, Yang J, Yin W, Wang A, Fan G, Zhou F, Liu Z, Gu X, Xu J, Shang L, Zhang Y, Cao L, Guo T, Wan Y, Qin H, Jiang Y, Jaki T, Hayden FG, Horby PW, Cao B, Wang C. Remdesivir in adults with severe COVID‐19: a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet 2020;395:1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, Hohmann E, Chu HY, Luetkemeyer A, Kline S, Lopez de Castilla D, Finberg RW, Dierberg K, Tapson V, Hsieh L, Patterson TF, Paredes R, Sweeney DA, Short WR, Touloumi G, Lye DC, Ohmagari N, Oh MD, Ruiz‐Palacios GM, Benfield T, Fätkenheuer G, Kortepeter MG, Atmar RL, Creech CB, Lundgren J, Babiker AG, Pett S, Neaton JD, Burgess TH, Bonnett T, Green M, Makowski M, Osinusi A, Nayak S, Lane HC. Remdesivir for the treatment of Covid‐19 – preliminary report. N Engl J Med 2020. Mar 22. 10.1056/NEJMoa2007764 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res 2020;30:269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis 2020;71:732–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honore S, Colson P, Chabriere E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents 2020;56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76. Borba MG, Val FF, Sampaio VS, Alexandre MA, Melo GC, Brito M, Mourão MP, Brito‐Sousa JD, Baía‐da‐Silva D, Guerra MV, Hajjar LA, Pinto RC, Balieiro AA, Pacheco AG, Santos JD Jr, Naveca FG, Xavier MS, Siqueira AM, Schwarzbold A, Croda J, Nogueira ML, Romero GA, Bassat Q, Fontes CJ, Albuquerque BC, Daniel‐Ribeiro CT, Monteiro WM, Lacerda MV; CloroCovid‐19 Team . Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection: a randomized clinical trial. JAMA Netw Open 2020;3:e208857. [DOI] [PubMed] [Google Scholar]
- 77. Chorin E, Dai M, Shulman E, Wadhwani L, Bar‐Cohen R, Barbhaiya C, Aizer A, Holmes D, Bernstein S, Spinelli M, Park DS, Chinitz LA, Jankelson L. The QT interval in patients with COVID‐19 treated with hydroxychloroquine and azithromycin. Nat Med 2020;26:808–809. [DOI] [PubMed] [Google Scholar]
- 78. Geleris J, Sun Y, Platt J, Zucker J, Baldwin M, Hripcsak G, Labella A, Manson DK, Kubin C, Barr RG, Sobieszczyk ME, Schluger NW. Observational study of hydroxychloroquine in hospitalized patients with Covid‐19. N Engl J Med 2020;382:2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Boulware DR, Pullen MF, Bangdiwala AS, Pastick KA, Lofgren SM, Okafor EC, Skipper CP, Nascene AA, Nicol MR, Abassi M, Engen NW, Cheng MP, LaBar D, Lother SA, MacKenzie LJ, Drobot G, Marten N, Zarychanski R, Kelly LE, Schwartz IS, McDonald EG, Rajasingham R, Lee TC, Hullsiek KH. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid‐19. N Engl J Med 2020;383:517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Arabi YM, Mandourah Y, Al‐Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al‐Omari A, Kharaba A, Almotairi A, Al Khatib K, Alraddadi B, Shalhoub S, Abdulmomen A, Qushmaq I, Mady A, Solaiman O, Al‐Aithan AM, Al‐Raddadi R, Ragab A, Balkhy HH, Al Harthy A, Deeb AM, Al Mutairi H, Al‐Dawood A, Merson L, Hayden FG, Fowler RA. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018;197:757–767. [DOI] [PubMed] [Google Scholar]
- 81. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med 2006;3:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, Zhong N. Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest 2006;129:1441–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, Wu F, Song ZG, Huang W, Chen J, Hu BJ, Wang S, Mao EQ, Zhu L, Zhang WH, Lu HZ. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wu C, Chen X, Cai Y, Xia Ja, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hu H, Ma F, Wei X, Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur Heart J 2020. Mar 16. 10.1093/eurheartj/ehaa190 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kupferschmidt K. A cheap steroid is the first drug shown to reduce death in COVID‐19 patients. https://www.sciencemag.org/news/2020/06/cheap‐steroid‐first‐drug‐shown‐reduce‐death‐covid‐19‐patients (16 June 2020).
- 87. Xu X, Han M, Li T, Sun W, Wang D, Fu B, Zhou Y, Zheng X, Yang Y, Li X, Zhang X, Pan A, Wei H. Effective treatment of severe COVID‐19 patients with tocilizumab. Proc Natl Acad Sci U S A 2020;117:10970–10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. O'Donnell C, Parodi E. Roche rheumatoid arthritis drug fails to help COVID‐19 patients in Italian study. https://www.sharenet.co.za/views/views‐article.php?views‐article=440948 (18 June 2020).
- 89. ClinicalTrials.gov. A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID‐19 Pneumonia (COVACTA). Identifier: NCT04320615. https://clinicaltrials.gov/ct2/show/NCT04320615 (25 March 2020).
- 90. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation 2005;111:2605–2610. [DOI] [PubMed] [Google Scholar]
- 91. Vaduganathan M, Vardeny O, Michel T, McMurray JJ, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med 2020;382:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol 2020;5:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen J, She ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Huang X, Yuan Y, Rohit L, Liu PP, Li H. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res 2020;126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. ClinicalTrials.gov. Losartan for Patients With COVID‐19 Not Requiring Hospitalization. Identifier: NCT04311177. https://clinicaltrialsgov/ct2/show/NCT04311177 (17 March 2020).
- 95. ClinicalTrials.gov. Losartan for Patients With COVID‐19 Requiring Hospitalization. Identifier: NCT04312009. https://clinicaltrialsgov/ct2/show/NCT04312009 (17 March 2020).


