To the Editor,
We read with great interest the comment published by Andrianopoulos et al 1 in which the authors advocate for cautious use of tocilizumab in coronavirus disease‐2019 (COVID‐19) patients. Tocilizumab is a monoclonal antibody against the interleukin‐6 (IL‐6) receptor that has immunosuppressive properties. Whereas accumulating results from uncontrolled trials present tocilizumab as an effective agent blocker of disease progression, some contrasting studies also progressively appear in COVID‐19 literature. 2 , 3 , 4 , 5 This muddies the waters and makes the situation more confused. Therefore, we would like to reinforce the purpose of Andrianopoulos et al 1 by commenting on the cytokine storm proposed in COVID‐19 as it constituted the rationale for most tocilizumab trials.
To this end, we referred to recent articles regarding the two main clinical conditions traditionally linked with the term “cytokine storm,” that is, septic shock and cytokine release syndrome (CRS) after CAR‐T cells infusion to compare mean/median plasmatic IL‐6 levels in these contexts and in COVID‐19 patients. Results are presented in Figure 1. The majority of median COVID‐19 IL‐6 values are far lower than those seen in septic shock or CRS (P < .0002, analysis of variance). In agreement, a recent meta‐analysis of IL‐6 values in 1426 COVID‐19 patients reported mean IL‐6 values at 57 pg/mL in severe patients and 17 pg/mL in less severe patients. 6 Although clearly elevated above normal range (generally <10 pg/mL depending on kits providers); those values remain far lower from those seen in usual clinical contexts associated with cytokine storms. Actually, despite rare extreme values, these mean concentrations are similar to those described in various inflammatory diseases (chronic infections, Crohn's disease, rheumatoid arthritis, and multiple sclerosis) which are not usually characterized or defined by the occurrence of cytokine storm. 7
Figure 1.
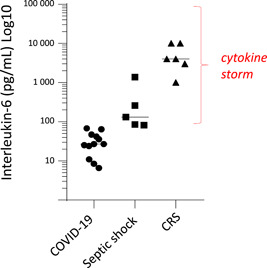
Median/mean IL‐6 values in COVID‐19, septic shock, and cytokine release syndrome (CRS). Black horizontal lines depict medians for each group. CRS refers to cytokine release syndrome after CAR‐T cell infusion (at peaks of cytokine release). Values from recent/illustrative studies. COVID‐19 studies: Chen G et al. J Clin Invest. 2020;130:2620‐2629; Mo P et al. Clin Infect Dis. 2020 (Online); Zhou F. Lancet. 2020;395:1054‐1062; Zhu. Int J Infect Dis. 2020;95:332‐339; Monneret G et al. Intensive Care Med. 2020 (Online); Cai Q et al. Allergy. 2020; Gao Y et al. J Med Virol. 2020;92:791‐796; Qin C et al. Clin Infect Dis. 2020 (Online); Wang C et al. Intensive Care Med. 2020 (Online); Chen R et al. J Allergy Clin Immunol. 2020 (Online). CRS studies: Maude SL et al. N Engl J Med. 2014;371:1507‐1517; Lee DW et al. Blood. 2014;124:188‐195; Xu J et al. Proc Natl Acad Sci USA. 2019;116:9543‐9551; Teachey DT et al. Cancer Discov. 2016;6:664‐679; Hay KA et al. Blood. 2017;130:2295‐2306. Septic shock studies: Feng M et al. J Clin Lab Anal. 2016;30:1037‐1043; Song J et al. BMC Infect Dis. 2019;19:968; Tsalik EL et al. J Emerg Med. 2012;43:97‐106; Lin WC et al. PLoS One. 2017;12:e0178387; Ríos‐Toro JJ et al PLoS One. 2017;12:e0175254. Differences between groups were as follows: all groups (P < .0002, ANOVA), COVID‐19 vs septic shock (P < .002, Mann‐Whitney), COVID‐19 vs CRS (P < .002, Mann‐Whitney). ANOVA, analysis of variance; COVID, coronavirus disease; IL, interleukin‐6
Of note, by recent international definition, severe COVID‐19 can be classified as a viral sepsis, 8 that is, organ failure (acute respiratory distress syndrome) induced by a dysregulated response to an infection (SARS‐CoV‐2). In sepsis, on a general basis, anti‐inflammatory strategies did not show any significant efficacy despite numerous clinical trials. 9 With that said, one study identified a protective effect of immunomodulatory treatment in a subgroup of septic patients when stratified based on circulating IL‐6 values. In this study, the threshold to highlight this effect was 1000 pg/mL. 10
In conclusion, it is unquestionable that COVID‐19 presents with inflammatory characteristics. As such, there is likely a room for tocilizumab (or other anti‐inflammatory drugs) in subgroups of patients to avoid progression toward uncontrolled inflammation. That given, we suggest that such treatment should be envisaged in a more individualized manner and on short period not to amplify marked immunosuppression observed in intensive care unit COVID‐19 patients. 11 , 12 We thus agree with Andrianopoulos et al 1 to cautiously consider tocilizumab depending on disease chronology, occurrence of ARDS, IL‐6 level stratification and most importantly, the depth of lymphopenia.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
REFERENCES
- 1. Andrianopoulos I, Papathanasiou A, Papathanakos G, Chaidos A, Koulouras V. Tocilizumab's efficacy in COVID‐19 patients is determined by the presence of cytokine storm [published online ahead of print June 22, 2020]. J Med Virol. 2020. 10.1002/jmv.26209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Morena V, Milazzo L, Oreni L, et al. Off‐label use of tocilizumab for the treatment of SARS‐CoV‐2 pneumonia in Milan, Italy. Eur J Intern Med. 2020;76:36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Radbel J, Narayanan N, Bhatt PJ. Use of tocilizumab for COVID‐19‐induced cytokine release syndrome: a cautionary case report. Chest. 2020;158:e‐15‐e‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Colaneri M, Bogliolo L, Valsecchi P, et al. Tocilizumab for treatment of severe COVID‐19 patients: preliminary results from SMAtteo COvid19 REgistry (SMACORE). Microorganisms. 2020;8(5):695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Quartuccio L, Sonaglia A, McGonagle D, et al. Profiling COVID‐19 pneumonia progressing into the cytokine storm syndrome: Results from a single Italian Centre study on tocilizumab versus standard of care. J Clin Virol. 2020;129:104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aziz M, Fatima R, Assaly R. Elevated interleukin‐6 and severe COVID‐19: a meta‐analysis [published online ahead of print April 28, 2020]. J Med Virol. 2020. 10.1002/jmv.25948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coutant DE, Hall SD. Disease‐drug interactions in inflammatory states via effects on CYP‐mediated drug clearance. J Clin Pharmacol. 2018;58(7):849‐863. [DOI] [PubMed] [Google Scholar]
- 8. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis‐3). JAMA. 2016;315(8):801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Marshall JC. Such stuff as dreams are made on: mediator‐directed therapy in sepsis. Nat Rev Drug Discov. 2003;2(5):391‐405. [DOI] [PubMed] [Google Scholar]
- 10. Panacek EA, Marshall JC, Albertson TE, et al. Efficacy and safety of the monoclonal anti‐tumor necrosis factor antibody F(ab')2 fragment afelimomab in patients with severe sepsis and elevated interleukin‐6 levels. Crit Care Med. 2004;32(11):2173‐2182. [DOI] [PubMed] [Google Scholar]
- 11. Monneret G, Cour M, Viel S, Venet F, Argaud L. Coronavirus disease 2019 as a particular sepsis: a 2‐week follow‐up of standard immunological parameters in critically ill patients [published online ahead of print June 2, 2020]. Intensive Care Med. 2020. 10.1007/s00134-020-06123-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jeannet R, Daix T, Formento R, Feuillard J, Francois B. Severe COVID‐19 is associated with deep and sustained multifaceted cellular immunosuppression [published online ahead of print June 8, 2020]. Intensive Care Med. 2020. 10.1007/s00134-020-06127-x [DOI] [PMC free article] [PubMed] [Google Scholar]


