Abstract
Objectives
To determine whether there is an overall survival (OS) benefit to the addition of thoracic radiation therapy (RT) following R0 resection of pathologic (p) T1 or pT2 N0 M0 small cell lung cancer.
Methods
Using the National Cancer Database, we performed a retrospective cohort analysis. Patients who underwent R0 resection for pT1 or p2 N0 M0 small cell lung cancer, stratified by receipt of adjuvant thoracic RT, were compared on the basis of OS using hierarchical Cox Proportional hazards models.
Results
Of 4969 patients diagnosed with pT1or pT2 N0 M0 SCLC from 2004 to 2014, 1617 (33%) underwent R0 resection of their primary tumor; of these resected patients, 146 (9.0%) had adjuvant thoracic RT. In unadjusted analysis, there was no significant difference in OS between groups (median survival: surgery alone, 62.2 months vs surgery+RT, 43.8 months; P = .1436). In multivariable analysis, RT was not associated with improved survival (P = .099). There was no significant difference in unadjusted or adjusted survival associated with receipt of RT in both a young and healthy cohort (P = .647 for unadjusted and P = .858 for adjusted) and a matched cohort (P = .867 and P = .954). In the matched cohort, improved OS was associated with younger patient age (adjusted hazard ratio, 1.07; 95% confidence interval, 1.04–1.10; P < .001), female sex (adjusted hazard ratio, 0.68, 95% confidence interval, 0.47–0.97; P = .035), and smaller tumors (adjusted hazard ratio, 1.02; 95% confidence interval, 1.01–1.03; P = .005). Having 2 or more comorbidities was associated with worse OS (adjusted hazard ratio, 2.16; 95% confidence interval, 1.21–3.86; P = .009).
Conclusions
Although complete resection was accomplished in a minority of patients, for these patients, survival was good. The addition of thoracic RT to complete resection does not appear to confer additional survival benefit.
Keywords: small cell lung cancer, radiation, surgical resection, adjuvant treatment
Lung cancer is the leading cause of cancer-related death in the United States with small cell lung cancer (SCLC) representing 13% of these diagnoses.1 This disease is characterized by early distant metastases and high response rate to chemoradiation therapy. Despite these early favorable responses, there is a near universal relapse rate, leading to an overall 5-year survival of <7% for all stages combined.2 The incidence of SCLC in the United States has decreased in recent years, mirroring the decline in tobacco use nationwide. However, there are still an estimated 31,000 cases annually.2
Until 1973, all lung cancer was treated with surgical resection where possible. That treatment paradigm changed when Fox and colleagues3 published a clinical trial comparing surgery to radiation therapy for SCLC. That practice-changing article created the perception of SCLC as a nonsurgical disease. Since that time, pretreatment staging has become more accurate thanks to the introduction of positron emission tomography and advanced tissue procurement procedures (eg, navigational bronchoscopy and endobronchial ultrasound). Additionally, the development of video-assisted thoracic surgery has reduced the morbidity of thoracic resections. Current guidelines recommend surgical resection, preferably lobectomy, for patients found to have localized, node-negative SCLC (T1 or T2 N0 M0) after thorough evaluation for distant metastasis as well as invasive staging of the mediastinum to exclude nodal disease. Level I evidence in support of these guidelines is lacking4; however, several large database studies have suggested that there may be a survival benefit to surgical resection.5–10
Although there is increasing evidence to support surgical resection for localized SCLC, the role of adjuvant radiation therapy (RT) for completely resected (R0), node-negative (N0) cancers after resection is unclear due to limited data. We sought to determine whether there is an overall survival (OS) benefit to the addition of thoracic RT following complete resection of pathologic (p) T1 or pT2 N0 M0 SCLC.
METHODS
We performed a retrospective cohort analysis of patients with early stage SCLC after complete resection to determine the effect on OS of adjuvant thoracic RT.
Data Source and Variables
Data were obtained from the National Cancer Database (NCDB), a joint project of the American College of Surgeons and American Cancer Society. The largest cancer registry in the world, the NCDB is estimated to capture approximately 70% of all new cancer diagnoses in the United States and Puerto Rico, including 82% of lung cancer diagnoses.11 Data are collected by certified tumor registrars who undergo extensive training and are audited to ensure accuracy of the database. Hospital and patient identity are protected and not included in the Participant Use File (PUF). Data released in the PUF are in compliance with the privacy requirements of the Health Information Portability and Accountability Act. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer (CoC) have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators. The institutional review board at Northwestern University determined this study was exempt as it uses publicly available de-identified data.
Treatment components were entered into the model as dichotomized variables for adjuvant thoracic RT and chemotherapy indicating that the patient either received or did not receive each therapy. Patients were considered to have undergone adjuvant thoracic RT if they received external beam radiation to the thorax after definitive surgery for the primary cancer site. Patients were considered to have undergone chemotherapy if they received single- or multiagent chemotherapy. Although there were many details available regarding chemotherapy regimens, for model parsimony, chemotherapy was included in the Cox proportional hazards models as a dichotomous variable. However, to increase the accuracy of matching, we further specified whether chemotherapy was neoadjuvant or adjuvant for the propensity score model. Adjuvant versus neoadjuvant status was determined based on relationship of the date of definitive surgery relative to the date that chemotherapy was initiated. Sex was dichotomized into male and female categories. Race and ethnicity were grouped into 4 categories, as defined by the CoC: non-Hispanic white, non-Hispanic black, Hispanic, and other. Data regarding race and ethnicity were abstracted from patient charts by NCDB abstractors; because each hospital may vary with regard to how patient race and ethnicity was entered into charts, it was not possible to determine whether these data were self-reported or not. Patients were grouped into quartiles for income based on census data for their Zip code, as defined by the CoC. We included income to adjust for social determinants of health, which have been shown to be associated with health states.12,13 A modified Charlson-Deyo comorbidity score was included as a categorical variable as defined by CoC: 0, 1, and ≤2. Facility characteristics were accounted for in the model with a variable representing volume and academic status. For this variable, high volume refers to hospitals in the top quartile for number of unique patients treated for SCLC, averaged over the study time period. Academic hospitals were defined by CoC as ≥500 newly diagnosed cancer types per year and offering graduate medical education programs in >4 disciplines. We included these facility characteristics because hospital structure factors have been shown to have an influence on surgical outcomes.14,15 Age (in years), year of diagnosis, tumor size (in millimeters), and distance to treating facility (in miles) were included as continuous variables. Surgery type was grouped into 3 categories: lobectomy (including sleeve lobectomies and bilobectomies), sublobar or nonanatomic (includes segmentectomy and wedge resections), and pneumonectomy.
Population
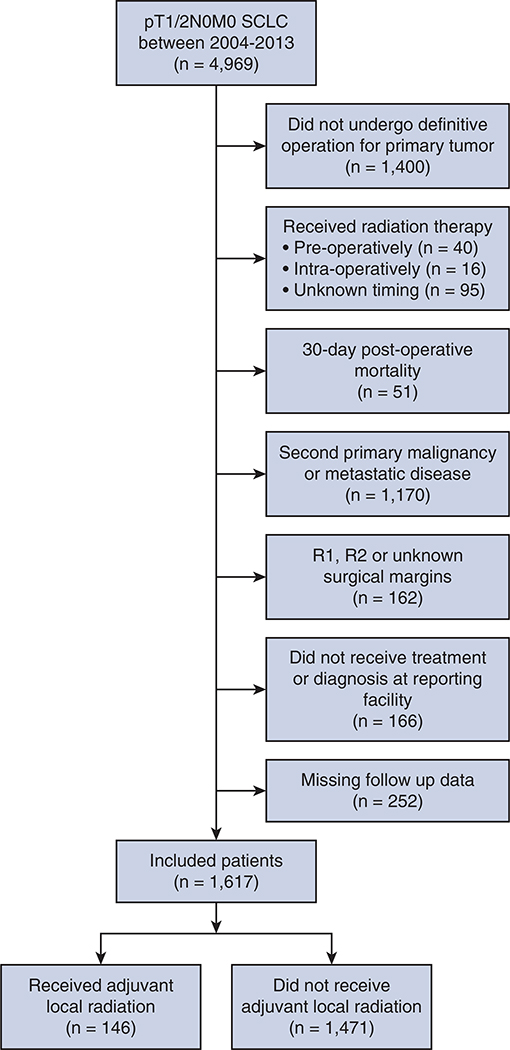
We queried the NCDB PUF for patients diagnosed with pT1 or p2 N0 M0 SCLC between 2004 and 2013 (N = 4969). We excluded patients who did not have a definitive operation for their primary tumor (n = 1400) (see Table E1 for a characterization of these patients); patients who received radiation either preoperatively (n = 40), intraoperatively (n = 16), or at an unknown time period (n = 95); patients who died within 30 days postoperatively because these deaths are likely treatment related and not cancer related (n = 51); patients for whom their SCLC diagnosis represents a second primary or metastatic disease (n = 1170); patients with incompletely resected primary tumors (R1 or R2) or unknown margins (n = 162), patients who were not treated or diagnosed at the reporting facility (n = 166), and patients who were missing follow-up data (n = 252). Only patients who received thoracic RT were considered to have undergone adjuvant local radiation. Thoracic RT is defined by the NCDB as RT directed at the region of the lung without nodal irradiation. Specifically, this definition excludes hilar, mediastinal, or supraclavicular nodes. Those who received radiation targeted elsewhere (eg, prophylactic cranial irradiation) were included in the surgery-only cohort (Figure 1).
TABLE E1.
Comparison of operative (n = 1617) and nonoperative (n = 1289) patients
| Variable | No surgery | Surgery | P value |
|---|---|---|---|
| No thoracic radiation | 692 (53.7) | 1471 (91.0) | <.001 |
| Thoracic radiation | 597 (46.3) | 146 (9.0) | |
| No chemotherapy | 308 (23.9) | 566 (35.0) | <.001 |
| Chemotherapy | 981 (76.1) | 1051 (65.0) | |
| Age (y) | 67 (60–75) | 68 (61–73) | .662 |
| Sex | .349 | ||
| Male | 602 (46.7) | 727 (45.0) | |
| Female | 687 (53.3) | 890 (55.0) | |
| Race/ethnicity | .151 | ||
| Non-Hispanic white | 1049 (81.7) | 1360 (84.4) | |
| Non-Hispanic black | 193 (15.0) | 196 (12.2) | |
| Hispanic | 23 (1.8) | 28 (1.7) | |
| Other or unknown | 19 (1.5) | 28 (1.7) | |
| Charlson-Deyo score | <.001 | ||
| 0 | 763 (59.2) | 730 (45.2) | |
| 1 | 349 (27.1) | 633 (39.2) | |
| ≥2 | 177 (13.7) | 254 (15.7) | |
| Year of diagnosis | 2007 (2005–2009) | 2009 (2006–2011) | <.001 |
| Median income ($) | .012 | ||
| <38,000 | 257 (20.6) | 289 (18.2) | |
| 38,000–47,999 | 357 (28.6) | 409 (25.8) | |
| 48,000–62,999 | 349 (28.0) | 446 (28.1) | |
| 63,000+ | 285 (22.8) | 442 (27.9) | |
| Facility location | .451 | ||
| Urban | 1137 (93.4) | 92 (6.0) | |
| Nonurban | 81 (6.6) | 1454 (94.0) | |
| Tumor size (mm) | 47 (28–90) | 20 (15–30) | <.001 |
| Hospital characteristic | <.001 | ||
| Academic and high volume | 24 (1.9) | 43 (2.7) | |
| Other academic | 289 (22.5) | 520 (32.3) | |
| Community | 973 (75.7) | 1050 (65.1) | |
| Distance to hospital (miles) | 7.9 (3.5–18.7) | 10.7 (4.9–26.4) | <.001 |
Values are presented as n (%) or median (interquartile range).
FIGURE 1.
Consolidated standards of reporting trials flow diagram of patient selection. SCLC, Small cell lung cancer.
Outcomes and Analysis
Separate Cox proportional hazards models were constructed for 3 different cohorts of patients with pT1 or pT2 N0 M0 SCLC after complete resection: all patients, only young (age <70 years) and healthy (Charlson-Deyo score = 0) patients, and a propensity score-matched cohort of patients (all 3 included receipt of adjuvant radiation as the primary predictor of interest).
Our primary outcome of interest in each model was overall survival. The factors associated with OS were examined using 2 level hierarchical Cox proportional hazards models with treatment components; patient age, sex, and race/ethnicity; Charlson-Deyo score; year of diagnosis; income; facility location and characteristics; tumor size; distance to the hospital; and procedure performed included as fixed effects. In models 1 and 2, we accounted for clustering by including random intercepts for treating facility and in model 3, we accounted for clustering by including random intercepts for each pair.
Propensity Score Matching
We matched patients based on their propensity to have received adjuvant radiation. The propensity scores were estimated based on a logistic regression model, including the following variables determined a priori to be associated with the receipt of adjuvant RT: receipt of neoadjuvant or adjuvant chemotherapy, age, sex, race, comorbidities, year of diagnosis, income, facility location and characteristics, tumor size, distance to treating facility, and surgery type. Patients treated with and without adjuvant RT were matched using greedy matching based on the odds ratio of the propensity score using one-to-one matching, caliper distance of 0.1, with no replacements allowed. Differences between treatment groups in the matched cohort were assessed using cluster-corrected χ2 or Wilcoxon rank-sum tests, where appropriate.
Sensitivity Analyses
We completed 2 sensitivity analyses. First, because we assessed the type of surgery performed as a potential independent variable, and because surgical approach may influence the number of lymph nodes harvested, we included a categorical variable for number of lymph nodes harvested. We categorized this variable into ≥10 (recommended), 5 to 9, 1 to 4, and 0 lymph nodes examined. We entered this variable into our Cox proportional hazards model with 0 lymph nodes as the referent category. Second, we chose the time point of 30 days to address potential immortal time bias. To assess whether a different choice may have affected our results, we also examined 0 days and 90 days as potential landmarks.
Analyses were performed using Stata version 14 (StataCorp, College Station, Tex). Propensity matching was performed based on a user-written program module of Stata version 14 (PSMATCH2).
RESULTS
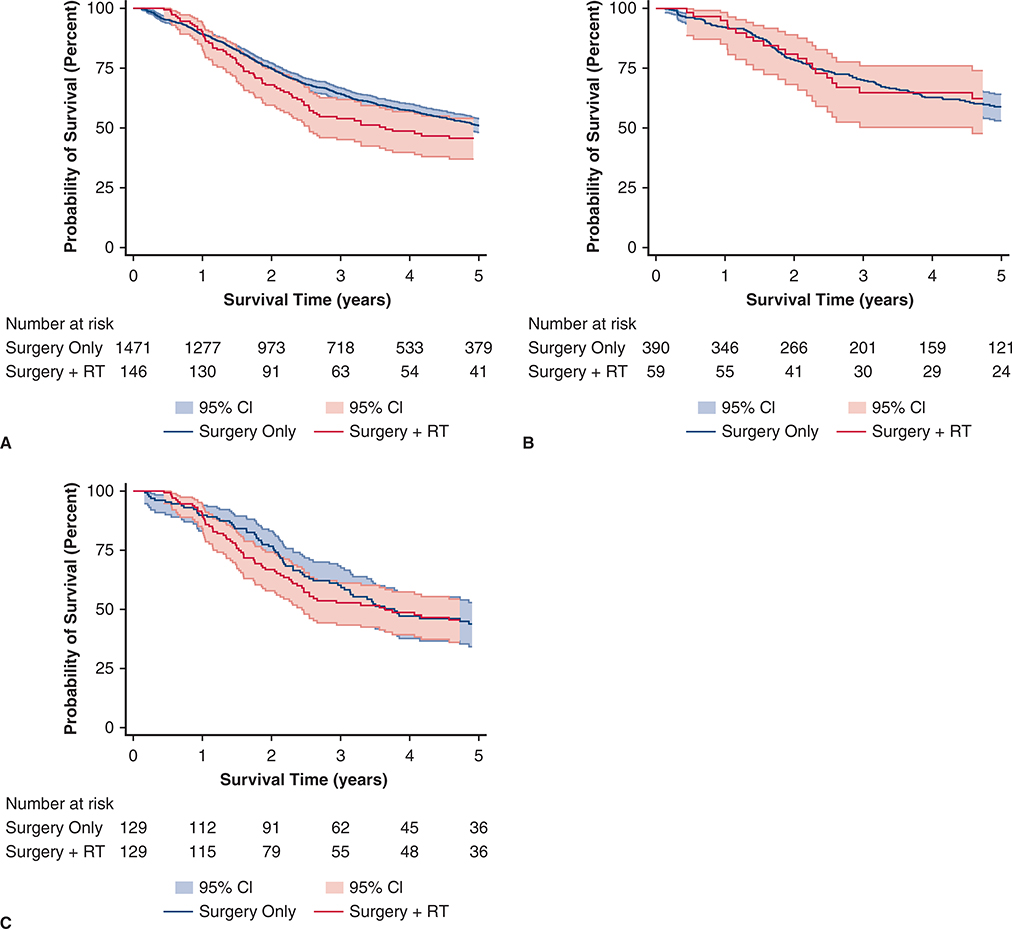
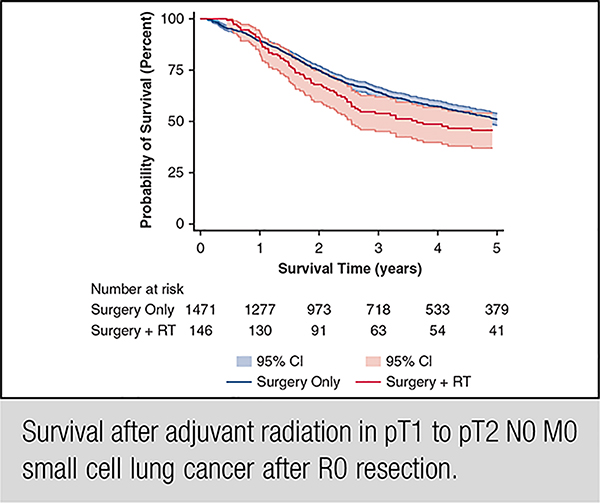
We identified 1617 patients who underwent complete resection for pT1 or pT2 N0 M0 SCLC from 2004 to 2014; of these resected patients, 146 (9.0%) had adjuvant thoracic RT and 1417 (91.0%) did not have thoracic radiation. In unadjusted analysis (Figure 1, A), there was no significant difference in OS between groups (median survival, surgery alone 62.2 months vs surgery and RT 43.8 months; P = .1436). In multivariable analysis (Table 1), RT was not associated with better survival (P = .099). In addition, increasing age, ≥2 comorbid conditions, sublobar resection (compared with lobectomy), and treatment at a high-volume academic center were associated with worse survival, whereas receipt of chemotherapy and black race were associated with improved survival. After limiting our sample to healthy young patients (Figure 2, B, and Table 2), there was still no significant difference in unadjusted (P = .647) or adjusted survival (P = .858) associated with receipt of RT. In this healthy young cohort, median OS for surgery-only patients was 87.5 months compared with 120.8 for those who underwent surgery and RT (P = .8419).
Table 1.
Cox proportional hazards model for overall survival for all patients with pathologic (p)T1 or pT2 N0 M0 small cell lung cancer after R0 resection (N = 1617)
| Variable | Result | Hazard ratio (95% confidence interval) | P value | Adjusted hazard ratio (95% confidence interval) | P value |
|---|---|---|---|---|---|
| No adjuvant radiation | 1471 (91.0) | 1.00 (Ref) | – | 1.00 (Ref) | – |
| Adjuvant radiation | 146 (9.0) | 1.18 (0.93–1.50) | .182 | 1.25 (0.96–1.63) | .099 |
| No chemotherapy | 566 (35.0) | 1.00 (Ref) | – | ||
| Chemotherapy | 1051 (65.0) | 0.69 (0.59–0.80) | <.001 | 0.75 (0.64–0.89) | .001 |
| Age (y) | 68 (61–73) | 1.04 (1.03–1.05) | <.001 | 1.03 (1.02–1.04) | <.001 |
| Sex | |||||
| Male | 727 (45.0) | 1.00 (Ref) | – | ||
| Female | 890 (55.0) | 0.86 (0.74–1.00) | .044 | 0.89 (0.76–1.03) | .127 |
| Race/ethnicity* | |||||
| Non-Hispanic white | 1360 (84.4) | 1.00 (Ref) | – | ||
| Non-Hispanic black | 196 (12.2) | 0.83 (0.66–1.05) | .118 | 0.76 (0.60–0.97) | .027 |
| Hispanic | 28 (1.7) | 0.97 (0.53–1.76) | .919 | 0.93 (0.51–1.69) | .804 |
| Other or unknown | 28 (1.7) | 1.17 (0.67–2.04) | .578 | 1.17 (0.66–2.05) | .592 |
| Charlson-Deyo score | |||||
| 0 | 730 (45.2) | 1.00 (Ref) | – | ||
| 1 | 633 (39.2) | 1.09 (0.92–1.28) | .316 | 1.07 (0.91–1.27) | .410 |
| ≥2 | 254 (15.7) | 1.37 (1.12–1.69) | .002 | 1.41 (1.14–1.75) | .002 |
| Year of diagnosis | 2009 (2006–2011) | 0.99 (0.96–1.02) | .609 | 0.99 (0.96–1.02) | .498 |
| Median income ($)* | |||||
| <38,000 | 289 (18.2) | 1.00 (Ref) | – | ||
| 38,000–47,999 | 409 (25.8) | 1.00 (0.81–1.25) | .980 | 1.07 (0.86–1.34) | .545 |
| 48,000–62,999 | 446 (28.1) | 0.94 (0.75–1.16) | .558 | 0.95 (0.76–1.19) | .639 |
| 63,000+ | 442 (27.9) | 0.87 (0.70–1.09) | .222 | 0.82 (0.65–1.04) | .098 |
| Facility location* | |||||
| Urban | 1454 (94.1) | 1.00 (Ref) | – | ||
| Nonurban | 92 (5.9) | 1.02 (0.74–1.40) | .915 | 0.99 (0.71–1.38) | .950 |
| Tumor size (mm)* | 20 (15–30) | 1.00 (1.00–1.00) | .176 | 1.00 (1.00–1.00) | .224 |
| Hospital characteristic* | |||||
| Academic and high volume | 43 (2.7) | 1.48 (0.95–2.32) | .082 | 1.69 (1.07–2.67) | .024 |
| Other academic | 520 (32.2) | 1.07 (0.90–1.27) | .451 | 1.13 (0.94–1.35) | .191 |
| Community | 1050 (65.1) | 1.00 (Ref) | – | ||
| Distance to hospital (miles)* | 10.7 (4.9–26.4) | 1.00 (1.00–1.00) | .542 | 1.00 (1.00–1.00) | .214 |
| Procedure performed | |||||
| Lobectomy | 1111 (68.7) | 1.00 (Ref) | – | ||
| Sublobar or nonanatomic | 483 (29.9) | 1.59 (1.37–1.86) | <.001 | 1.48 (1.25–1.74) | <.001 |
| Pneumonectomy | 23 (1.4) | 0.93 (0.49–1.77) | .826 | 1.27 (0.66–2.45) | .471 |
Values are presented as n (%) or median (interquartile range). Ref, Reference category.
Five patients (0.3%) were missing race data, 31 patients (1.9%) were missing income data, 71 patients (4.3%) were missing facility location data, 10 patients (0.6%) were missing tumor size data, 4 patients (0.2%) were missing hospital characteristic data, and 28 patients (1.7%) were missing distance to hospital data.
FIGURE 2.
Unadjusted overall survival (Kaplan-Meier survival curves) for all patients diagnosed between 2004 and 2013 with pathologic (p) T1 or pT2 N0 M0 small cell lung cancer treated with surgery only compared with surgery and adjuvant thoracic radiation. A, All patients underwent complete resection (N = 1617). There was no difference when the survival functions were compared using the log-rank test (P = .144). B, All patients underwent complete resection (N = 449). There was no difference when the survival functions were compared using the log-rank test (P = .647). C, All patients underwent complete resection (N = 258). There was no difference when the survival functions were compared using the log-rank test (P = .867). RT, Adjuvant radiation therapy; CI, confidence interval.
Table 2.
Cox proportional hazards model for overall survival for young, healthy patients with pathologic (p) T1 or pT2 N0 M0 small cell lung cancer after R0 resection (N = 449)
| Variable | Result | Hazard ratio | P value | Adjusted hazard ratio | P value |
|---|---|---|---|---|---|
| No adjuvant radiation | 390 (86.9) | 1.00 (Ref) | – | 1.00 (Ref) | – |
| Adjuvant radiation | 59 (13.1) | 0.89 (0.55–1.44) | .647 | 1.05 (0.63–1.75) | .858 |
| No chemotherapy | 138 (30.7) | 1.00 (Ref) | – | ||
| Chemotherapy | 311 (69.3) | 0.65 (0.46–0.92) | .003 | 0.74 (0.52–1.07) | .113 |
| Age (y) | 62 (56–66) | 1.04 (1.01–1.07) | .006 | 1.04 (1.01–1.07) | .005 |
| Sex | |||||
| Male | 200 (44.5) | 1.00 (Ref) | – | ||
| Female | 249 (55.5) | 0.69 (0.49–0.96) | .027 | 0.74 (0.53–1.02) | .068 |
| Race/ethnicity | |||||
| Non-Hispanic white | 382 (85.1) | 1.00 (Ref) | – | ||
| Non-Hispanic black | 51 (11.4) | 1.08 (0.64–1.82) | .777 | 1.06 (0.64–1.77) | .817 |
| Hispanic | 9 (2.0) | 1.23 (0.35–4.32) | .750 | 0.90 (0.26–3.05) | .859 |
| Other or unknown | 7 (1.6) | 1.17 (0.33–4.10) | .810 | 1.33 (0.39–4.5) | .644 |
| Year of diagnosis | 2009 (2006–2011) | 1.02 (0.95–1.08) | .625 | 1.03 (0.97–1.1) | .309 |
| Median income ($)* | |||||
| <38,000 | 67 (15.1) | 1.00 (Ref) | – | ||
| 38,000–47,999 | 113 (25.5) | 0.69 (0.41–1.16) | .160 | 0.55 (0.33–0.91) | .021 |
| 48,000–62,999 | 140 (31.6) | 0.85 (0.52–1.40) | .535 | 0.72 (0.45–1.17) | .187 |
| 63,000+ | 123 (27.8) | 0.70 (0.40–1.13) | .132 | 0.52 (0.31–0.87) | .013 |
| Facility location* | |||||
| Urban | 404 (94.6) | 1.00 (Ref) | – | ||
| Nonurban | 23 (5.4) | 0.98 (0.46–2.10) | .959 | 1.04 (0.5–2.16) | .926 |
| Tumor size (mm)* | 21 (15–30) | 1.00 (1.00–1.00) | .524 | 1.00 (1.00–1.00) | .747 |
| Hospital characteristic* | |||||
| Academic and high volume | 14 (3.1) | 1.88 (0.76–4.66) | .170 | 2.03 (0.83–4.97) | .121 |
| Other academic | 163 (36.5) | 1.53 (1.08–2.17) | .016 | 1.62 (1.14–2.30) | .007 |
| Community | 270 (60.4) | 1.00 (Ref) | – | ||
| Distance to hospital (miles)* | 10.6 (4.5–26.7) | 1.00 (1.00–1.00) | .588 | 1.00 (1.00–1.00) | .805 |
| Procedure performed | |||||
| Lobectomy | 329 (73.3) | 1.00 (Ref) | – | ||
| Sublobar or nonanatomic | 106 (23.6) | 1.60 (1.11–2.29) | .011 | 1.53 (1.05–2.23) | .028 |
| Pneumonectomy | 14 (3.1) | 0.88 (0.34–2.29) | .800 | 0.97 (0.38–2.47) | .955 |
Values are presented as n (%) or median (interquartile range). Ref, Reference category.
Six patients (1.3%) were missing income data, 22 patients (4.7%) were missing facility location data, 6 patients (1.3%) were missing tumor size data, 2 patients (0.4%) were missing hospital characteristic data, and 6 patients (1.3%) were missing distance to hospital data.
To further reduce the risk of confounding bias, we created a propensity score-matched cohort (Table 3). After matching, there were no significant differences in baseline characteristics between patients treated with surgery alone and patients treated with adjuvant RT after surgery, indicating successful matching. Compared with our entire cohort, the matched cohort was younger, had an earlier date of diagnosis, and were more likely to have been treated with chemotherapy, RT, and a nonanatomic resection (Table E1). We found no significant association between adjuvant RT and OS in unadjusted (P = .867) or adjusted (P = .954) analysis between matched groups in a Cox proportional hazards model examining survival (Figure 1, C, and Table 4). Additionally, surgical approach was not associated with OS in the adjusted Cox proportional hazards model. However, in this matched cohort, increasing age (P < .001), having ≥2 comorbidities (P = .009), and increasing tumor size (P = .005) were associated with worse OS. Female sex was associated with improved OS (P = .035).
Table 3.
Baseline characteristics of propensity score-matched cohort (N = 258)
| Characteristic | Surgery only (n = 129) | Surgery and adjuvant radiation therapy (n = 129) | P value* |
|---|---|---|---|
| Neoadjuvant chemotherapy | .495 | ||
| No | 117 (90.7) | 120 (93.0) | |
| Yes | 12 (9.3) | 9 (7.0) | |
| Adjuvant chemotherapy | .259 | ||
| No | 27 (20.9) | 20 (15.5) | |
| Yes | 102 (79.1) | 109 (84.5) | |
| Age (y) | 65 (60–71) | 66 (60–71) | .937 |
| Sex | .802 | ||
| Male | 55 (42.6) | 57 (44.2) | |
| Female | 74 (57.4) | 72 (55.8) | |
| Race/ethnicity | .633 | ||
| Non-Hispanic white | 109 (84.5) | 111 (86.1) | |
| Non-Hispanic black | 18 (14.0) | 14 (10.9) | |
| Hispanic | 2 (1.6) | 3 (2.3) | |
| Other or unknown | 0 (0.0) | 1 (0.8) | |
| Charlson-Deyo score | .362 | ||
| 0 | 61 (47.3) | 69 (53.5) | |
| 1 | 54 (41.9) | 43 (33.3) | |
| ≥2 | 14 (10.9) | 17 (13.2) | |
| Year of diagnosis | 2007 (2005–2010) | 2007 (2005–2010) | .826 |
| Median income ($) | .953 | ||
| <38,000 | 23 (17.8) | 25 (19.4) | |
| 38,000–47,999 | 29 (22.5) | 31 (24.0) | |
| 48,000–62,999 | 32 (24.8) | 32 (24.8) | |
| 63,000+ | 45 (34.9) | 41 (31.8) | |
| Facility location | .373 | ||
| Urban | 120 (93.0) | 116 (89.9) | |
| Nonurban | 9 (7.0) | 13 (10.1) | |
| Tumor size (mm) | 20 (12–28) | 20 (12–31) | .468 |
| Hospital characteristic | .991 | ||
| Academic and high volume | 4 (3.1) | 4 (3.1) | |
| Other academic | 44 (34.1) | 45 (34.9) | |
| Community | 81 (62.8) | 80 (62.0) | |
| Distance to hospital (miles) | 7.8 (4–22.8) | 9.4 (4.7–20.7) | .374 |
| Procedure performed | .355 | ||
| Lobectomy | 51 (39.5) | 66 (51.2) | |
| Sublobar or nonanatomic | 77 (56.7) | 61 (47.3) | |
| Pneumonectomy | 1 (0.8) | 2 (1.6) |
Values are presented as n (%) or median (interquartile range).
Based on cluster-corrected χ2 or Kruskal-Wallace rank-sum tests, where appropriate.
Table 4.
Cox proportional hazards model for overall survival for propensity score-matched patients with pathologic (p) T1 or pT2 N0 M0 small cell lung cancer after complete resection (N = 258) accounting for clustering by pair
| Variable | Result | Hazard ratio | P value | Adjusted hazard ratio | P value |
|---|---|---|---|---|---|
| No adjuvant radiation | 129 (50.0) | 1.00 (Ref) | – | 1.00 (Ref) | – |
| Adjuvant radiation | 129 (50.0) | 1.02 (0.73–1.44) | .867 | 0.99 (0.70–1.41) | .954 |
| No chemotherapy | 26 (10.1) | 1.00 (Ref) | – | ||
| Chemotherapy | 232 (89.9) | 0.56 (0.33–0.97) | .037 | 0.74 (0.41–1.36) | .340 |
| Age (y) | 66 (60–71) | 1.06 (1.04–1.09) | <.001 | 1.07 (1.04–1.10) | <.001 |
| Sex | |||||
| Male | 112 (43.4) | 1.00 (Ref) | – | ||
| Female | 146 (56.6) | 0.67 (0.47–0.95) | .026 | 0.68 (0.47–0.97) | .035 |
| Race/ethnicity | |||||
| Non-Hispanic white | 220 (85.3) | 1.00 (Ref) | – | ||
| Non-Hispanic black | 32 (12.4) | 0.86 (0.51–1.48) | .572 | 1.16 (0.67–1.99) | .602 |
| Hispanic | 5 (1.9) | 0.64 (0.15–2.77) | .550 | 0.45 (0.10–2.06) | .306 |
| Other or unknown | 1 (0.4) | 4.03 (0.46–35.5) | .209 | 1.73 (0.17–17.11) | .641 |
| Charlson-Deyo score | |||||
| 0 | 130 (50.4) | 1.00 (Ref) | – | ||
| 1 | 97 (37.6) | 1.38 (0.94–2.03) | .097 | 1.42 (0.96–2.12) | .082 |
| ≥2 | 31 (12.0) | 2.00 (1.18–3.40) | .011 | 2.16 (1.21–3.86) | .009 |
| Year of diagnosis | 2007 (2005–2010) | 1.02 (0.95–1.09) | .660 | 1.04 (0.97–1.12) | .265 |
| Median income ($) | |||||
| <38,000 | 48 (18.6) | 1.00 (Ref) | – | ||
| 38,000–47,999 | 60 (23.3) | 1.18 (0.67–2.06) | .570 | 1.02 (0.57–1.82) | .953 |
| 48,000–62,999 | 64 (24.8) | 1.25 (0.73–2.17) | .417 | 1.11 (0.60–2.06) | .748 |
| 63,000+ | 86 (33.3) | 1.03 (0.61–1.75) | .912 | 0.92 (0.51–1.68) | .798 |
| Facility location | |||||
| Urban | 236 (91.5) | 1.00 (Ref) | – | ||
| Nonurban | 22 (8.5) | 0.85 (0.45–1.62) | .628 | 0.68 (0.33–1.37) | .278 |
| Tumor size (mm) | 20 (12–30) | 1.01 (1.00–1.02) | .077 | 1.02 (1.01–1.03) | .005 |
| Hospital characteristic | |||||
| Academic and high volume | 8 (55.4) | 1.19 (0.44–3.20) | .730 | 0.66 (0.23–1.86) | .430 |
| Other academic | 112 (43.4) | 1.01 (0.69–1.48) | .939 | 0.96 (0.63–1.46) | .847 |
| Community | 3 (1.2) | 1.00 (Ref) | – | ||
| Distance to hospital (miles) | 9.2 (4.2–21) | 1.00 (1.00–1.01) | .197 | 1.00 (1.00–1.01) | .058 |
| Procedure performed | |||||
| Lobectomy | 112 (43.4) | 1.00 (Ref) | – | ||
| Sublobar or nonanatomic | 143 (55.4) | 1.44 (1.00–2.07) | .049 | 1.2 (0.80–1.79) | .378 |
| Pneumonectomy | 3 (1.2) | 0.41 (0.05–3.16) | .393 | 0.58 (0.07–4.83) | .616 |
Values are presented as n (%) or median (interquartile range). Ref, Reference category.
We also completed 2 sensitivity analyses (Table E2). First, we examined the number of lymph nodes sampled. In our analytic cohort of 1617 patients, 125 patients were missing data regarding lymph node sampling. Of those who did have information regarding lymph node sampling, 29.6% of patients had the recommended 10 or more lymph nodes sampled. Approximately 44% of patients had no lymph node sampling done. In our fully adjusted matched cohort, number of lymph nodes examined was not significant (P = .994), but when it was included in the model, patient sex was no longer a significant factor (P = .062). Second, we tested 2 additional time points in our adjustment to address immortal time bias. For the 90-day cutpoint, we excluded an additional 32 patients from the matched cohort who had died between 30- and 90-days postoperatively. In multivariable Cox proportional hazards modeling, our results were qualitatively equivalent whether we set the time mark at 90 days or 30 days. When we set the time mark at 0 days, the Kaplan-Meier curves cross and thus the assumption of proportional hazards was violated, indicating that Cox proportional hazards modeling was an inappropriate estimation for these data.
TABLE E2.
Comparison of matched (n = 258) and unmatched (n = 1359) patients
| Variable | Unmatched | Matched | P value |
|---|---|---|---|
| No adjuvant radiation | 1342 (98.8) | 129 (50.0) | <.001 |
| Adjuvant radiation | 17 (1.3) | 129 (50.0) | |
| No chemotherapy | 540 (39.7) | 26 (10.1) | <.001 |
| Chemotherapy | 819 (60.3) | 232 (89.9) | |
| Age (y) | 68 (62–74) | 66 (60–71) | .002 |
| Sex | .585 | ||
| Male | 615 (45.3) | 112 (43.4) | |
| Female | 744 (54.7) | 146 (56.6) | |
| Race/ethnicity | .343 | ||
| Non-Hispanic white | 1140 (84.2) | 220 (85.3) | |
| Non-Hispanic black | 164 (12.1) | 32 (12.4) | |
| Hispanic | 23 (1.7) | 5 (1.9) | |
| Other or unknown | 27 (2.0) | 1 (0.4) | |
| Charlson-Deyo score | .094 | ||
| 0 | 600 (44.2) | 130 (50.4) | |
| 1 | 536 (39.4) | 97 (37.6) | |
| ≥2 | 223 (16.4) | 31 (12.0) | |
| Year of diagnosis | 2009 (2007–2011) | 2007 (2005–2010) | <.001 |
| Median income ($) | .151 | ||
| <38,000 | 241 (18.2) | 48 (18.6) | |
| 38,000–47,999 | 349 (26.3) | 60 (23.3) | |
| 48,000–62,999 | 382 (28.8) | 64 (24.8) | |
| 63,000+ | 356 (26.8) | 86 (33.3) | |
| Facility location | .055 | ||
| Urban | 1218 (94.6) | 236 (91.5) | |
| Nonurban | 70 (5.4) | 22 (8.5) | |
| Tumor size (mm) | 20 (15–30) | 20 (12–30) | .066 |
| Hospital characteristic | .593 | ||
| Academic and high volume | 35 (2.6) | 8 (3.1) | |
| Other academic | 431 (31.8) | 89 (34.5) | |
| Community | 889 (65.6) | 161 (62.4) | |
| Distance to hospital (miles) | 11.2 (5.1–27.5) | 9.2 (4.2–21) | .024 |
| Procedure performed | <.001 | ||
| Lobectomy | 999 (73.5) | 112 (43.4) | |
| Sublobar or nonanatomic | 340 (25.0) | 143 (55.4) | |
| Pneumonectomy | 20 (1.5) | 3 (1.2) |
Values are presented as n (%) or median (interquartile range).
DISCUSSION
SCLC is an aggressive malignancy that is characterized by early lymphovascular invasion and dissemination to regional nodes and distant sites. When confined to the thorax, it has historically been treated with chemoradiation. However, more recent guidelines, supported by observational studies, suggest that surgical resection before or after chemotherapy can be curative for early stage, node-negative disease. We found that survival was good for those who did undergo surgery; additionally, there does not appear to be benefit (in terms of OS) to adjuvant RT after complete resection.
We present an analysis of those patients with SCLC who underwent complete resection. Because our cohort is based on pathologic staging and not clinical stage, we are unable to comment on the outcomes of potential surgical candidates who did not undergo resection. However, previous studies have suggested that surgery may be vastly underutilized: the rate of any surgical resection (R0, R1, or R2) varies from 20% to 30% of all patients with early stage disease.5,10 Although treatment guidelines do currently recommend surgery for early stage, node-negative disease, these findings suggest surgery was underutilized in this population most likely to have benefitted from resection. This may be due to a lack of high-quality evidence supporting improved survival after surgery or to an absence of surgical input in the development of multidisciplinary treatment strategies for patients with SCLC. The randomized controlled trials that have compared surgery to nonsurgical treatment for SCLC are older than age 40 years and included heterogeneous patient populations, with node-positive patients comprising the majority of the surgical cohort in these early trials.3,16 It is now more clearly understood that including these patients in the surgical cohort introduced an inherent bias against surgery in these trials.
More recently published large database analyses have been similarly limited by inadequate treatment data in the nonsurgical cohorts.17–19 One recent observational study addressed these concerns both by limiting their patient population to those most likely to benefit from surgical resection, and by only including nonsurgical patients treated with the maximum standard of care medical therapy.5 In this analysis, surgical resection was associated with a significantly improved 5-year OS.5 Similarly, an analysis comparing OS between patients treated with surgery versus nonsurgical treatment found an advantage to surgical therapy for stages I, II, and IIIA disease; with the greatest benefit seen in stage I patients and those with T1 or T2 N0 tumors.10
Although these data may shed light on the debate between operative and nonoperative management for SCLC, the role (and value) of adjuvant RT following surgical resection has not been adequately addressed. In our analysis, adjuvant RT did not improve survival in patients who had undergone complete resection. Additionally, in our propensity score-matched analysis, neither adjuvant nor neoadjuvant chemotherapy appeared to have an effect on OS. Similarly, an analysis of patient data from the Surveillance, Epidemiology, and End Results (SEER) program database found that the addition of RT to surgery had no significant effect on survival.7 An older study by Yu and colleagues20 also suggested that RT may not have a survival benefit. However, this study was limited by selection bias because the patient cohort included patients with positive margins. Another more recent analysis considered either chemotherapy or radiation as adjuvant therapy; in this study, the authors report an improved hazard ratio for patients treated with surgery and adjuvant therapy of any kind compared with surgery alone.10 Although Wakeam and colleagues21 reported that receipt of adjuvant mediastinal radiotherapy after sublobar resection for patients with node-negative disease was associated with improved survival, this was not statistically significant by log-rank analysis. Similarly, we found no difference in survival between sublobar and lobar resection in our fully adjusted model. Lastly, Yang and colleagues22 reported a survival benefit for patients who received adjuvant chemotherapy with or without radiation compared with patients who underwent surgery alone based on results from a Cox proportional hazards model of unmatched patients. Similarly, we found a survival benefit associated with adjuvant or neoadjuvant chemotherapy in our unmatched cohort, but this benefit was not present in our young, healthy cohort nor in our matched cohort. However, because it was outside the scope of our study, we did not evaluate key features of the chemotherapy regimen used that may influence effectiveness (eg, timing, dosing, and agents used). As such, no conclusions regarding the utility of chemotherapy should be made without additional analyses that are outside the scope of this study of local therapy options.
Comparing our results from unadjusted comparisons to a fully adjusted model further supports the need to carefully correct for confounding variables when observational data are used. In our analysis of the entire cohort of patients without limiting for those who might lack confounding characteristics and therefore be eligible for a clinical trial (our young, healthy cohort) and without propensity score matching to attempt to create more balanced groups, we found that treatment at a high-volume academic center was associated with worse survival, whereas black race was associated with improved survival. Typically, high-volume academic centers are associated with better outcomes and racial health disparities result in worse outcomes for African Americans across multiple disease entities. The finding that treatment at a high-volume center may be associated with worse outcomes was likely a result of referral bias, meaning that there was likely some unmeasured confounder influencing a physician’s decision to refer a patient to an academic center for more complex care. These findings did not persist in the young, healthy cohort nor did they persist in our propensity score-matched cohort. Obtaining this result on a minimally adjusted cohort indicates that the association was likely attributable to confounding. Because this association was not statistically significant in our fully adjusted model our results indicate that we were able to address the unmeasured confounders in the NCDB through our propensity score-matched analysis.
We performed a sensitivity analysis to assess the potential association between the number of lymph nodes sampled and overall survival. When nodal status was included in our fully adjusted propensity score-matched model, we did not find a significant association between number of nodes sampled and overall survival. This variable in the NCDB may be flawed because it did not account for the number of stations sampled and this variable is easily manipulated if, for example, a single node is removed in pieces and thus counted as multiple nodes. For this reason, we recommend against making conclusions regarding the importance of nodal sampling in SCLC resection based on our data.
Lastly, we found significantly improved survival for younger patients and those patients with smaller tumors in our matched analysis. These findings align with earlier analyses showing improved survival in younger patients and smaller tumors.5,7,22 Like our study, others have also found improved survival for female patients with lung cancer5,23–28 and for patients with lower comorbidity scores.5,22 Additionally, prior studies report improved survival for later year of diagnosis5,7 and after lobectomy (compared with nonanatomic resection).22
Our study does have some important limitations. First, data extracted from any database are subject to coding error. However, NCDB data are collected by trained and audited abstractors, improving reliability. Additionally, the data definitions are standardized. Thus, the effect of these differences is likely minimal. Second, all patients who were T1 or T2 N0 M0, and thus eligible for surgery based on stage, were evaluated. Due to the limitations of the NCDB data fields, we are unable to make further judgments on a patient’s eligibility for surgical intervention. For example, no data for performance status or pulmonary function tests were available. Additionally, although the overall Charlson-Deyo score was available, more granular details about individual comorbidities were lacking. Third, because the NCDB only contains data from CoC-accredited hospitals, these data may be limited by selection bias. However, this file contains more than 80% of all lung cancer cases from hospitals that vary in type and size.11 Fourth, because CoC accreditation may change from year to year for individual facilities, the characteristics of the facilities represented in the database in 2004 may be substantially different from those in 2013. However, because of the large number and range of facilities and the small number of patients per facility in our matched analysis, it is unlikely that treatment bias on the individual facility level would substantially influence our findings. Fifth, we limited our analysis to OS because other oncologic outcomes are not captured in the NCDB. Future studies should collect data for these outcomes to allow further detailed analysis. Sixth, it could be that patients who received adjuvant RT against current guidelines were treated at facilities with low-quality outcomes, introducing potential bias against this cohort. However, previous studies have shown wide variation in practice even within a center, so this limitation is unlikely to have influenced our results.29 Additionally, we adjusted for patient clustering at the facility level in all our multivariable models. And lastly, for a database study, we have a relatively small sample size of patients. This limitation speaks to the underuse of surgical therapy for early stage SCLC. Although the focus of our study is not to define the utilization rates of surgery for SCLC, recent studies estimate only 30% of eligible patients receive definitive surgical treatment for their cancer.5,10,29–31
Although previous studies have shown that surgery is a vastly underutilized local treatment for node-negative SCLC, survival after complete resection was good in our cohort. The addition of thoracic RT to complete resection does not appear to confer a survival benefit for these patients. Our study provides strong evidence to support this treatment algorithm. Although a randomized controlled trial in the modern era might be valuable to confirm our findings, a randomized controlled trial has many costs and logistical concerns. Our findings support the concept that surgical resection is sufficient local therapy in pN0 patients, avoiding the expense and potential morbidity of thoracic RT. Multidisciplinary teams, including a surgeon, should discuss each patient’s candidacy for surgery and adjuvant RT before initiating these treatments.
Central Message.
Surgery is underutilized for early stage small cell lung cancer; radiation therapy after complete resection may not be necessary.
Perspective.
Although historically considered a nonoperative disease, surgery can benefit patients with early stage small cell lung cancer. However, the role of adjuvant thoracic radiation after complete resection remains unclear. Our findings suggest that complete resection may obviate the need for adjuvant thoracic radiation.
Acknowledgments
The authors thank Jeanette W. Chung, PhD, for her assistance with the statistical methods.
The National Cancer Database is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The data used in the study are derived from a de-identified National Cancer Database file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigators.
Supported by the Northwestern Institute for Comparative Effectiveness Research in Oncology postdoctoral fellowship (Dr Englehardt). Dr Odell is supported by the National Cancer Institute of the National Institutes of Health (award No. K07CA216330), the Thoracic Surgery Foundation, the American Association for Thoracic Surgery Graham Foundation Oz Lemole Research Fellowship, and the American College of Surgeons.
Abbreviations and Acronyms
- CoC
Commission on Cancer
- NCDB
National Cancer Database
- OS
overall survival
- p
pathologic
- PUF
Participant Use File
- RT
radiation therapy
- SCLC
small cell lung cancer
Discussion

Dr Stephen C. Yang (Baltimore, Md). Thank you, Drs Jones, Donington, and Engelhardt for a great presentation and a very well-written manuscript. My appreciation to you and your colleagues for your many years of work advocating continually for the role of surgery in limited stage small cell carcinoma. It’s probably unusual for the Association or any thoracic conference to have 2 presentations on small cell on the same day, so hopefully the audience will buy into this concept of aggressive resection.
Your presentation is another series of patients with small cell lung cancer from the National Cancer Database (NCBD) comparing the benefit, or lack thereof, of adjuvant radiation after complete resection. This simulates the work that was done a few years ago looking at the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute database in 2 different articles.
However, as you point out, your cohort numbers are small. If we do some number crunching, we estimate about 35,000 patients are diagnosed with small cell lung cancer annually with about 5% with limited stage disease, or about 1800 patients a year should be operated on. So your cohort across 10 years is only about 1600 patients, so about 10% of what we should be operating on; further evidence that surgery is underutilized.
My questions are as follows. Do you have an idea, which may be a limitation with the NCBD again, of the target area of the radiation? Was it to the mediastinum, was it to the cranium, was it to the tumor, was it to the staple line, or to all of the above?

Dr Kathryn E. Engelhardt (Chicago, Ill). The NCDB allows us to determine some general location of radiation. We excluded patients who underwent percutaneous coronary intervention who had radiation to their mediastinum. These are really just patients who had thoracic radiation.
Dr Yang. And so this may be related to the treatment bias. We have suggested that lobectomy for limited-stage small cell is the proper cancer operation. Any comment why 30% did not get optimal surgical resection; that is, they had a sublobar resection? And even when you weed out the younger patients, that was only 25% who got lobectomies and no chemotherapy in 35%. So a significant number of patients did not get optimal therapy.
Dr Engelhardt. Yes, I agree. I think that’s an issue across all tumors that we are seeing. The presentation that was just given on mesothelioma points that out as well. There is obviously an issue with meeting guidelines across the nation with treatment of all different types of thoracic cancers, and all cancers in general.
I think that these studies that we do with the NCDB are really hypothesis-generating and allow us to point out where there may be concerns, and the next steps are really going to be drilling down and determining why those individual patients didn’t get the appropriate treatment. Was there some reason? Did the patient refuse? I’m not really sure what the answer is for each of those patients. But we are planning to initiate a qualitative study where we are going to try and talk with patients as well as providers to determine their thoughts and perspectives on guidelines and whether they follow them, why not, things like that.
Dr Yang. Well, we have already discussed the problems with the NCDB in getting appropriate data. I think a problem with the small cell cancer populations is that we don’t really know where the sites of recurrence are dependent on the treatment. So what database would you use to try to answer some of these other questions?
Dr Engelhardt. The NCDB was really designed as a quality improvement project, like American College of Surgeons National Surgical Quality Improvement Program and other, similar databases. It does have long-term survival, unlike the National Surgical Quality Improvement Program, but the best database probably to look at oncologic outcomes would be Surveillance, Epidemiology, and End Results Program. So that would be a place to look to validate some of these findings.
Dr Yang. But they have already done that with Surveillance, Epidemiology, and End Results Program; everybody has moved from that database to NCDB now.
Finally, in your manuscript you noted that high-volume academic centers have an adverse effect on survival. Why do you think that is so?
Dr Engelhardt. We did find that in our first analysis where we looked at all eligible patients before we adjusted for anything. Also, I think that that is most likely a factor of referral bias where the more complicated patients are getting referred to high-volume academic centers. And after we selected out patients and after we propensity score matched them, we no longer saw that association, which indicates that we had some success in adjusting for selection bias.
Dr Yang. So, again, thank you and your colleagues for your excellent work in small cell lung cancer. And I know you are looking for a cardiothoracic residency fellowship, so any program would be extremely lucky to have you, and thank you to your mentors for getting you into our specialty. I want to thank the program committee for their kind invitation to discuss this work.
Dr Engelhardt. Thank you.

Dr Michael T. Jaklitsch (Boston, Mass). It came out quite nice in the discussion there with Steve, and I just want to highlight again, not all databases are the same. We are seeing a lot of abstracts in this particular program for the NCDB, and in my personal opinion, that’s the worst of them all. So Surveillance, Epidemiology, and End Results Program has real strengths, the Society of Thoracic Surgeons is the Cadillac of them all, the National Surgical Quality Improvement Program gives us a lot of information, but the NCDB has no quality assurance step at the level where they are getting these data.
So if you go out to these local community hospitals, they need to meet certain criteria in order to be accredited as an American College of Surgeons cancer program, but these community hospitals are frequently laying off the data analysts they used to have. So, for instance, the staging form has to be filled out by the surgeon who did the operation, and there is no quality assurance step to say whether he or she filled it out correctly. So if you have an update in your staging system and you have a general surgeon who did a lobe and doesn’t know the upstaging of the staging system, he’s going to stage maybe by the old system or maybe by his best guess.
We have another community hospital where non–small cell lung cancer was being coded as large cell lung cancer. They had 80% of their cases were large cell lung cancers, the largest experience of large cell in the entire United States.
That’s what’s in the NCDB. We went to the NCDB to see if it was really true that the number of lymph nodes predict how long you live, and there are some people who are recording that they get 98 lymph nodes out of a wedge resection. I don’t know how you do that.
So there is no quality assurance piece to kick those out of the NCDB database, and I am concerned that so many abstracts we heard today are of NCDB data.
Dr Engelhardt. Well, I don’t represent the NCDB.
Unidentified Speaker. I also do not work for the NCDB, but I have used the NCDB, and I think the truth is somewhere in the middle. The NCDB does a wonderful job, actually, of having dedicated abstracters. And it is true that they do not do audits like the Society of Thoracic Surgeons does on 5% to 10% of cases, but these are trained abstracters. We just finished a Patient-Centered Outcomes Research Institute award where we worked with them online to abstract 10 patients per hospital who had lung cancer resection, and I think the quality of the data is actually quite high.
There are no perfect administrative data sets. The Society of Thoracic Surgeons, unfortunately, is not perfect either. And so I just wanted to say I do think that this is an excellent contribution, it’s very well done, so thank you.
Dr Engelhardt. I would just add that I agree that no database is perfect, and I think the most important thing to do is to validate our findings in multiple databases. Justin Dimick and his group at Michigan even showed that National Surgical Quality Improvement Program and administrative data sets don’t even overlap 50%. So I think that if we are able to find where those things do overlap and get down to some more accurate data, then we can make some real conclusions from these studies.

Dr Michael Lanuti (Boston, Mass). In an effort to make this the best that it could be, regardless of the idiosyncrasies of the NCDB, did your analysis include patients who received chemotherapy, because I didn’t see that in the data.
Dr Engelhardt. We did. Well, we didn’t focus on chemotherapy as a predictor for outcome. We did include patients with chemotherapy. We propensity score-matched patients whether they received neoadjuvant chemotherapy and also postoperative chemotherapy, and then we included chemotherapy as just a dichotomous yes/no variable in our Cox proportional hazards model.
Dr Lanuti. So those 2 cohorts have mixed populations of patients who did and did not get chemotherapy?
Dr Engelhardt. Correct.
Dr Lanuti. A practice pattern at our institution is that if we do a resection for an N0 small cell lung cancer, we often give adjuvant chemotherapy and then have a debate about adjuvant percutaneous coronary intervention. Your analysis excludes all patients who underwent percutaneous coronary intervention, which leaves us with the unanswered question of survival benefit. Intracranial recurrence is the most common place for small cell. If you can add more data or examine this subset, that would be of interest to readers.
Dr Engelhardt. Absolutely. Thank you.
Footnotes
Conflict of Interest Statement
Authors have nothing to disclose with regard to commercial support.
Webcast
You can watch a Webcast of this AATS meeting presentation by going to: https://aats.blob.core.windows.net/media/18Apr29/25ABC%20General%20Thoracic%20SS/S53/S53_6_webcast_043555803.mp4.
References
- 1.Bernhardt EB, Jalal SI. Small cell lung cancer In: Reckamp KL, ed. Lung Cancer: Treatment and Research. New York: Springer International Publishing; 2016:301–22. [DOI] [PubMed] [Google Scholar]
- 2.Byers LA, Rudin CM. Small cell lung cancer: where do we go from here? Cancer. 2015;121:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox W, Scadding JG. Medical research council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet. 1973;2:63–5. [DOI] [PubMed] [Google Scholar]
- 4.Barnes H, See K, Barnett S, Manser R. Surgery for limited-stage small-cell lung cancer. Cochrane Database Syst Rev. 2017;4:Cd011917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang CJ, Chan DY, Shah SA, Yerokun BA, Wang XF, D’Amico TA, et al. Longterm survival after surgery compared with concurrent chemoradiation for node-negative small cell lung cancer. Ann Surg. 2018;268:1105–12. [DOI] [PubMed] [Google Scholar]
- 6.Schreiber D, Rineer J, Weedon J, Vongtama D, Wortham A, Kim A, et al. Survival outcomes with the use of surgery in limited-stage small cell lung cancer: should its role be re-evaluated? Cancer. 2010;116:1350–7. [DOI] [PubMed] [Google Scholar]
- 7.Varlotto JM, Recht A, Flickinger JC, Medford-Davis LN, Dyer AM, DeCamp MM. Lobectomy leads to optimal survival in early-stage small cell lung cancer: a retrospective analysis. J Thorac Cardiovasc Surg. 2011;142:538–46. [DOI] [PubMed] [Google Scholar]
- 8.Weksler B, Nason KS, Shende M, Landreneau RJ, Pennathur A. Surgical resection should be considered for stage I and II small cell carcinoma of the lung. Ann Thorac Surg. 2012;94:889–93. [DOI] [PubMed] [Google Scholar]
- 9.Gaspar LE, McNamara EJ, Gay EG, Putnam JB, Crawford J, Herbst RS, et al. Small-cell lung cancer: prognostic factors and changing treatment over 15 years. Clin Lung Cancer. 2012;13:115–22. [DOI] [PubMed] [Google Scholar]
- 10.Wakeam E, Acuna SA, Leighl NB, Giuliani ME, Finlayson SRG, Varghese TK, et al. Surgery versus chemotherapy and radiotherapy for early and locally advanced small cell lung cancer: a propensity-matched analysis of survival. Lung Cancer. 2017;109:78–88. [DOI] [PubMed] [Google Scholar]
- 11.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The national cancer data base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Link BG, Phelan JC. Understanding sociodemographic differences in health—the role of fundamental social causes. Am J Public Health. 1996;86:471–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monfared ED, Mohseni M, Amanpour F, Jarrahi AM, Joo MM, Heidarnia MA. Relationship of social determinants of health with the three-year survival rate of breast cancer. Asia Pac J Cancer Prev. 2017;18:1121–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hyder O, Dodson RM, Nathan H, Schneider EB, Weiss MJ, Cameron JL, et al. Influence of patient, physician, and hospital factors on 30-day readmission following pancreatoduodenectomy in the United States. JAMA Surg. 2013;148: 1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reames BN, Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and operative mortality in the modern era. Ann Surg. 2014;260:244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lad T, Piantadosi S, Thomas P, Payne D, Ruckdeschel J, Giaccone G. A prospective randomized trial to determine the benefit of surgical resection of residual disease following response of small cell lung cancer to combination chemotherapy. Chest. 1994;106(6 Suppl):320s–33s. [DOI] [PubMed] [Google Scholar]
- 17.Brock MV, Hooker CM, Syphard JE, Westra W, Xu L, Alberg AJ, et al. Surgical resection of limited disease small cell lung cancer in the new era of platinum chemotherapy: its time has come. J Thorac Cardiovasc Surg. 2005;129:64–72. [DOI] [PubMed] [Google Scholar]
- 18.Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2:1067–77. [DOI] [PubMed] [Google Scholar]
- 19.de Hoyos A, DeCamp MM. Surgery for small cell lung cancer. Thorac Surg Clin. 2014;24:399–409. [DOI] [PubMed] [Google Scholar]
- 20.Yu JB, Decker RH, Detterbeck FC, Wilson LD. Surveillance epidemiology and end results evaluation of the role of surgery for stage I small cell lung cancer. J Thorac Oncol. 2010;5:215–9. [DOI] [PubMed] [Google Scholar]
- 21.Wakeam E, Giuliani M, Leighl NB, Finlayson SRG, Varghese TK, Darling GE. Indications for adjuvant mediastinal radiotherapy in surgically resected small cell lung cancer. Ann Thorac Surg. 2017;103: 1647–53. [DOI] [PubMed] [Google Scholar]
- 22.Yang C-FJ, Chan DY, Speicher PJ, Gulack BC, Wang X, Hartwig MG, et al. Role of adjuvant therapy in a population-based cohort of patients with early-stage small-cell lung cancer. J Clin Oncol. 2016;34:1057–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agarwal M, Brahmanday G, Chmielewski GW, Welsh RJ, Ravikrishnan KP. Age, tumor size, type of surgery, and gender predict survival in early stage (stage I and II) non–small cell lung cancer after surgical resection. Lung Cancer. 2010;68: 398–402. [DOI] [PubMed] [Google Scholar]
- 24.Batevik R, Grong K, Segadal L, Stangeland L. The female gender has a positive effect on survival independent of background life expectancy following surgical resection of primary non-small cell lung cancer: a study of absolute and relative survival over 15 years. Lung Cancer. 2005;47: 173–81. [DOI] [PubMed] [Google Scholar]
- 25.Chang JW, Asamura H, Kawachi R, Watanabe S. Gender difference in survival of resected non–small cell lung cancer: histology-related phenomenon? J Thorac Cardiovasc Surg. 2009;137:807–12. [DOI] [PubMed] [Google Scholar]
- 26.Hanagiri T, Sugio K, Uramoto H, So T, Ichiki Y, Sugaya M, et al. Gender difference as a prognostic factor in patients undergoing resection of non-small cell lung cancer. Surg Today. 2007;37:546–51. [DOI] [PubMed] [Google Scholar]
- 27.Shafer D, Albain K. Lung cancer outcomes in women. Semin Oncol. 2009;36: 532–41. [DOI] [PubMed] [Google Scholar]
- 28.Cerfolio RJ, Bryant AS, Scott E, Sharma M, Robert F, Spencer SA, et al. Women with pathologic stage I, II, and III non-small cell lung cancer have better survival than men. Chest. 2006;130:1796–802. [DOI] [PubMed] [Google Scholar]
- 29.Wakeam E, Byrne JP, Darling GE, Varghese TK. Surgical treatment for early small cell lung cancer: variability in practice and impact on survival. Ann Thorac Surg. 2017;104:1872–80. [DOI] [PubMed] [Google Scholar]
- 30.Engelhardt KE, Odell DD, DeCamp MM. Optimal local therapy for early-stage small cell lung cancer: surgery needs a seat at the table. Translat Cancer Res. 2017;S1248–52. [Google Scholar]
- 31.Engelhardt KE, Odell DD, DeCamp MM. Under-treatment of small cell lung cancer: the case for surgical resection. J Thorac Dis. 2017;9: 3509–13. [DOI] [PMC free article] [PubMed] [Google Scholar]